Abstract
Our purpose was to determine if high fat diet and treatment with a polyphenol regulates acetylation of lysine-382 of p53, the site regulated by sirtuin-1, and apoptosis in the endothelium of the atherosclerotic lesion-prone mouse aortic arch. In cultured endothelial cells two atherogenic stimuli, hydrogen peroxide and tumor necrosis factor-α, increased acetylation of p53 lysine-382, as well as caspase-3 cleavage, an indicator of apoptotic signaling. The polyphenol, S17834, significantly prevented these changes. In LDL receptor-deficient mice, a high fat diet increased, and treatment with S17834 attenuated early atherosclerotic lesions on the lesser curvature of the aortic arch. In wild type C57BL6 mice fed the same diet, no atherosclerotic lesions were observed in this lesion-prone area, but p53 acetylation and caspase-3 cleavage increased in the endothelium. In high fat fed mice, S17834 increased sirtuin-1 protein in the lesion-prone endothelium and prevented both the increase in p53 acetylation and caspase-3 cleavage without affecting blood lipids. These results indicate that high fat diet increases and S17834 decreases acetylation of p53 in lesion-prone aortic endothelial cells of normal mice independently of blood lipids, suggesting that the polyphenol may regulate endothelial cell p53 acetylation and apoptosis via local actions.
Introduction
Despite cardiovascular risk factors circulating in the blood, such as hyperlipidemia and hyperglycemia, atherosclerosis shows a predilection to areas of the aorta that are prone to lesion formation because of hemodynamic factors. The endothelium in one such area, the inner curvature of the aortic arch, shows increased permeability1,2, and altered inflammatory and antioxidant gene expression in the normal pig 3 and mouse 4,5 aorta. Further understanding of the local determinants of the altered phenotype of endothelial cells in lesion-prone areas and how they interact with circulating risk factors might lead to improved therapies.
Interest in polyphenols as a treatment for atherosclerosis has increased following the discovery that their therapeutic actions may be linked to their ability to directly activate sirtuin-1 (SirT1), an NAD+-dependent deacetylase and master regulator of cell division, metabolism, and longevity6. Indeed, endothelial specific overexpression of SirT1 protects against atherosclerosis in apolipoprotein E deficient mice7. Polyphenols and small molecule activators of SirT1 improve insulin resistance, body weight, and blood lipids in type 2 diabetic mice8. Whether or not they have local effects to stimulate SirT1 in endothelial cells is not known.
The polyphenol, S17834, has anti-inflammatory, hypolipidemic, and anti-atherogenic actions in non-diabetic9 Apo E deficient and type 1 diabetic LDL receptor deficient mice10. Although it does not scavenge superoxide anion directly, S17834 also inhibits the production of superoxide anion by vascular NADPH oxidase9. Its hypolipidemic effect is due at least in part to stimulation of SirT1 in hepatocytes, leading to increased phosphorylation of LKB1 and AMP-dependent protein kinase, resulting in inhibition of acetyl CoA carboxylase, fatty acid synthase, and lipid production11.
Although the dramatic inhibition of atherosclerosis caused by S17834 in type 1 diabetic mice can be explained by its actions in the liver that lower blood lipids10, we sought to determine if the polyphenol also regulates acetylation of the SirT1-dependent site on p53 in lesion-prone aortic endothelial cells independently of blood lipids that confound the interpretation of its local vascular effects that might influence atherogenesis in vivo. Employing a model of atherosclerosis induced by a type-2 diabetogenic diet in LDL receptor-deficient mice, we first demonstrated that S17834 decreased lipids as well as atherogenesis on the lesion-prone area on the inner curvature of the aortic arch. To determine if S17834 regulates acetylation of p53 in the endothelium of this lesion-prone area in the absence of a hypolipidemic effect, we studied wild type C57BL6 mice fed the same diabetogenic diet. We found that the diabetogenic diet enhanced, and S17834 decreased acetylation of p53 and cleavage of caspase-3 detected in the endothelial cells in the lesion-prone aortic arch of the normal mice, without evidence of atherosclerotic lesions or the large changes in blood lipids observed in the LDLr-/- mouse. Because the compound caused similar changes in cultured human aortic endothelial cells, these observations suggest that the in vivo effects of the polyphenol may be exerted locally in the aorta, as evidenced additionally by increased expression of SirT1 in the lesion-prone endothelium. These studies suggest that the polyphenol S17834, in addition to its potential hypolipidemic effect, may have local beneficial actions on vascular endothelium, decreasing apoptosis and influencing the progression of atherogenesis, by regulating the acetylation of p53.
Methods
Materials
S17834 [6,8-diallyl 5,7-dihydroxy 2-(2-allyl 3-hydroxy 4-methoxyphenyl)1-H benzo(b)pyran-4–one], a synthetic polyphenol, was obtained from the Institut de Recherche Servier (Suresnes, France). Antibodies (company; catalog #) : p53 acetylated lysine-382 (Cell Signaling Technology, Inc. Danvers, MA 01923; #2525), total p53 (Santa Cruz Biotechnology, Inc. Santa Cruz, CA. 95060; #sc-99, Pab 240), cleaved caspase-3 Asp175 (Cell Signaling Technology, Inc. Danvers, MA; #9661), SirT1 H-300 (Santa Cruz Biotechnology, Inc. Santa Cruz, CA. 95060; #sc-15404).
Male LDL receptor-deficient mice (LDLr-/-) with C57BL6 genetic background were obtained from Jackson Laboratories (#002207; Bar Harbor, ME); male wild type C57BL6 mice also were obtained from Jackson Laboratories (#0000664). Mice were obtained at 7 weeks of age, acclimatized for 1 week on chow diet, and at 8 weeks of age fed either chow diet containing 6 % fat (Harlan Teklad, Madison, WI 53744; # 2018), high fat high sucrose diet containing 35.5% fat (58% of calories, primarily as lard) and 36.6% carbohydrate (primarily sucrose) without added cholesterol12(Bioserve, # F1850, Frenchtown, NJ), or the same diet compounded with S17834 to deliver 130 mg/kg/d. This dose which was used in previous studies9,10 was chosen based on studies which showed that the bioavailability of oral doses in mice was low, and that doses in the range used here were necessary to inhibit vascular leaks in the mouse mesentery (TJ Verbeuren, personal communication). The administration of drug was calculated based on the average consumption of food (5 g/day) by a 23-g mouse. LDLr-/- mice and C57BL5 wild type mice were euthanized after 16 or 8 weeks on the diet, respectively, and were killed under isoflurane anesthesia, after which tissues were removed. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The study was approved by the Institutional Animal Care and Use Committee of Boston University Medical Center.
Preparation of aortas and immunohistochemistry
To evaluate atherosclerotic lesions in LDLr-/- mice, the base of the heart including the atria and aortic root was frozen and embedded in optimal cutting temperature (OCT) compound.13 Five μm sections were obtained every 50 μm from the base of the aortic leaflets, through the top of the aortic leaflets and extending to 800 μm above the base of the leaflets. The 16 sections included 4-5 from the aortic root within the valve leaflets and 11-12 above the valve leaflets. The serial sections were stained with Oil Red O, stained images were captured with a microscope with an attached digital camera, and the lesion area for each aortic ring was analyzed with Scion Image software.
For immunohistochemistry performed on the aortic arch of C57BL6 mice, the ascending thoracic aortic arch comprising the same area above the aortic leaflets examined in the frozen sections from LDLr-/- mice was isolated and immediately placed in 10% buffered formalin acetate overnight, and then processed and embedded in paraffin. A notch in the greater curvature of the ascending aortic arch was cut at the level of the transverse arch in order to locate the beginning of the lesser curvature when sectioning. The apparent thickening of the aortic wall along the tangentially sectioned inner curvature of the arch provided a marker of the location of the lesion-prone arch (Supplemental Figure 1). After removal of paraffin and rehydration, 5-μm thick sections were treated with 10 mmol/L citric acid buffer (pH 6.0) and then heated to recover antigenicity in a microwave oven (2 min, 3 times at 700 W). Nonspecific binding was blocked with 10% normal goat serum or mouse IgG blocking reagent in PBS (pH 7.4) for 60 min before incubation with primary antibodies in PBS with 1% BSA overnight at 4°C. Tissue sections were then incubated with a biotinylated anti-rabbit or anti-mouse IgG secondary antibody (Vectastain ABC kit, Vector Laboratories, Burlingame, CA). Vector Red alkaline phosphatase substrate (Vector) was used to visualize positive immunoreactivity. All positive staining was confirmed by ensuring that no staining occurred under the same conditions with non-immune rabbit or mouse isotype control IgG antibodies (Vector). Immuno-fluorescent staining of acetylated p53 and cleaved caspase-3 was chosen for the improved sensitivity it provided. Cellular staining was enhanced by subtracting uniform background levels of fluorescence. Semi-quantitative analysis of tissue immuno-reactivity was done by 4-5 observers blinded to the identity of the samples using an arbitrary grading scale from 0 to 4 to estimate the degree of positive staining. From 6 – 13 individual mouse aortas were examined in each group of mice reported in figures 3-5.
Figure 3.
S17834 prevents the increase in acetylated p53 caused by HFHS diet in the lesion-prone aortic endothelium of C57BL6 wild type mice. Examples of immuno-fluorescent staining for p53 acetylation in lesion-prone aortic endothelial cells are shown at left. The green fluorescence of the FITC-tagged secondary antibody used to detect the acetylated p53 antibody produced yellow color in the endothelial cell nuclei that were stained red with propidium iodide. (A) Bar graph at right shows the mean ± sem of the average staining scored on each aortic cross section from each of 8-10 mice per group. Each mouse is represented by a diamond point. (B) Staining for total p53 (see supplemental figure 4) was performed and scored in a subset of mice, and for those mice a ratio was calculated for the staining scored for p53 acetylation to that for total p53 (6 mice each group). The asterisks indicate the statistically significant increase caused by HFHS diet compared with chow (*P<0.05), or the significant decrease caused by S17834 treatment of mice fed HFHS diet (†P<0.05).
Figure 5.
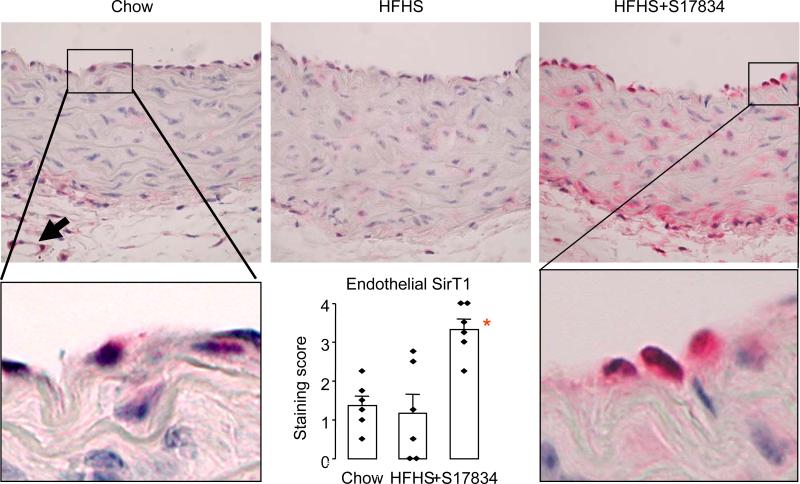
S17834 increases endothelial SirT1 protein in lesion-prone aortic endothelial cells in C57BL6 wild type mice fed HFHS diet. Examples are shown of SirT1 staining in control chow-fed, HFHS diet-fed, and S17834-treated, HFHS diet-fed mice. Distinct cellular staining in endothelial cells was observed and specifically scored by observers, and average scores for each mouse are shown in the bar graph inset. The asterisk indicates that S17834 treatment significantly increased scores for staining of SirT1 in endothelial cells (P<0.05, 6 mice per group).
Human aortic endothelial cells (HAEC)
HAECs were obtained from Cambrex Bio Science Walkersville, Inc. (Walkersville, MD 21793) as cryopreserved cell suspensions and were cultured in endothelial cell growth medium-2 containing 2% fetal bovine serum and Single-Quots EGM-2 BulletKit (Lonza Walkersville, Inc. , Maryland, 21793 USA #CC-3162 (CC-3156 & CC-4176). Cells between the fifth and eighth passages were used for experiments. After they reached confluence, the cells were cultured with 0.5% fetal bovine serum endothelial cell basal medium-2 overnight. Cells were treated for 1 h with S17834 solubilized in DMSO at a final concentration of DMSO that did not exceed 0.1%. The DMSO did not affect cell viability, and control data were obtained in cells treated with vehicle containing the same concentration of DMSO. When indicated, the cells were treated simultaneously with the polyphenol and either H2O2 (500 μM, Sigma-Aldrich, St. Louis, Missouri 63178; #H1009) or TNFα (50 ng/mL, Calbiochem, Gibbstown, NJ 08027; #654205) for indicated times prior to scraping into lysis buffer (Cell Signaling Technology, Inc. Danvers, MA 01923; #9803). The sub-micromolar concentrations of S17834 used here in HAEC (0.25 - 0.625 μM) are lower than the 3 – 10 μM concentration shown previously to inhibit TNFα-induced adhesion molecule expression9, and were chosen so that for the duration of the assays, the compound did not affect basal p53 acetylation or caspase-3 cleavage in the absence of H2O2 or TNFα. Also, in a concentration range of 10 - 100 μM, S17834 failed to stimulate the activity of a wide variety of protein kinase C isoforms, mitogen-activated protein kinases, and AMP kinase in in vitro kinase assays (data not shown). At a concentration of 50 μM, S17834 stimulated SirT1 deacetylase activity in a commercial in vitro assay11.
Cells were washed with PBS and lysed at 4°C in lysis buffer (20 mmol/L Tris-HCl, pH 8.0, 1% [vol/vol] Nonidet P-40, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L sodium orthovanadate, 1 mmol/L dithiothreitol, 1 mmol/L phenylmethylsulfonyl fluoride, 2 μg/mL aprotinin, 2 μg/mL leupeptin, and 1 μg/mL pepstatin), followed by centrifugation at 14,000 rpm for 10 min at 4°C. Protein concentrations in cell lysates were measured using a Bio-Rad protein assay kit. The cell lysates (20–50 μg protein) were combined with the appropriate amount of 6 × SDS sample buffer (0.32 mol/L Tris-HCl, pH 6.8, 30% [vol/vol] glycerol, 12% [wt/vol] SDS, 5% [vol/vol] β-mercaptoethanol, and bromophenol blue) and then heated at 95°C for 5 min. Samples were subjected to 8% SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride membranes by wet transfer at 30 V overnight. The membranes were blocked with 5% (wt/vol) nonfat dry milk in Tris-buffered saline with Tween-20 (TBST) (20 mmol/L Tris-HCl, pH 7.6, 0.138 mol/L NaCl, and 0.1% [vol/vol] Tween 20) and subsequently blotted with the appropriate antibodies in TBST containing 1% (wt/vol) BSA. Primary antibodies were used at the following concentrations overnight at 4°C: anti–p53-Ac-K382 (1:1000 dilution), anti-total p53 (1:1000), anti–cleaved caspase-3 (1:1000), anti-SirT1 (1:1000). The membranes were incubated with HRP-conjugated secondary antibodies at a 1:5,000 dilution in TBST containing 5% (wt/vol) nonfat dry milk for 1 h, and the bound antibodies were visualized by an enhanced chemiluminescence system. Bands were quantified using a GS-700 Imaging Densitometer (Bio-Rad) and normalized to the band density obtained in control cells in the same experiment.
Annexin-V-FITC labeling and fluorescence-activated cell sorting analysis
Cells were exposed to FITC-conjugated annexin V and to the fluorescent dye propidium iodide (PI). The cells were harvested, washed with ice-cold PBS, and resuspended with annexin V-fluorescein isothiocyate (FITC) and PI according to the protocol of an apoptosis detection kit (BD 556570, Becton-Dickinson). The final mixture of annexin V-FITC/PI stain was then applied to a FACScalibur flow cytometer and CellQuest software for analysis (approximately 10,000 events). The results were interpreted as follows: cells negative for both PI and annexin-V-FITC staining were considered live cells; PI-negative, annexin-V-FITC-positive stained cells were considered in early apoptosis; PI-positive, annexin-V-FITC negative were considered necrotic cells, and PI positive, annexin-V-FITC-positive-stained cells were considered in late apoptosis or secondary necrosis.
Statistics
Data are means±sem. Scores for immuno-histochemical staining, densitometry of immunoblot bands, flow cytometry, and atherosclerotic lesion area were analyzed by ANOVA and post-hoc Tukey's test. P< 0.05 was considered statistically significant.
Results
S17834 decreases oxidant- and cytokine-induced increases in p53 acetylation and apoptotic signaling in cultured human aortic endothelial cells
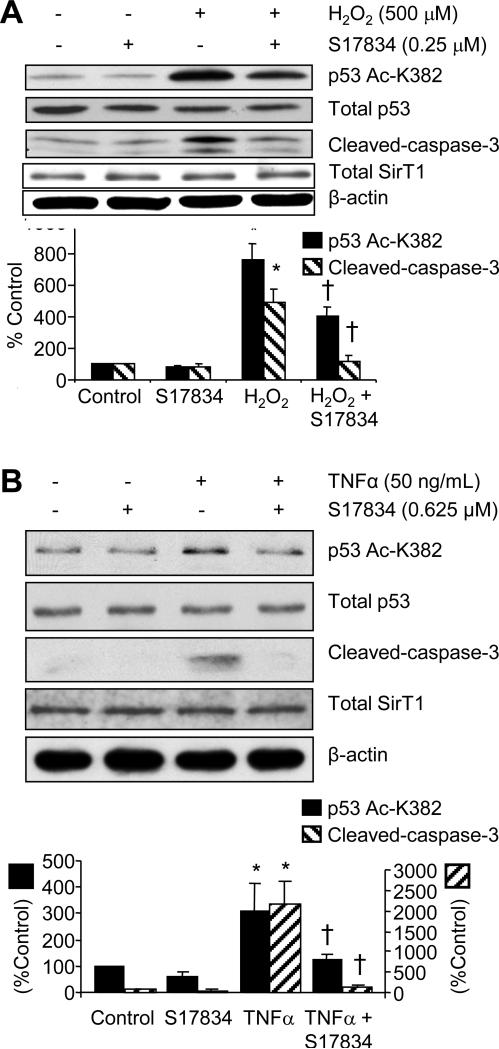
Increased levels of both oxidants and cytokines, such as hydrogen peroxide and TNFα, increase endothelial apoptosis. To determine if these pro-atherogenic stimuli increase acetylation of p53 lysine-382 and promote apoptotic signaling, human aortic endothelial cells were exposed for 4-6 h to hydrogen peroxide (500 μm, Figure 1A) or TNFα (50 ng/mL, Figure 1B). These stimuli both increased acetylation of p53 lysine-382, without changing total p53 expression (Figure 1A and B). The relevance of the increased acetylation of p53 caused by hydrogen peroxide or TNFα to endothelial cell turnover was indicated by increased cleaved caspase-3. S17834 significantly prevented the increase in acetylated p53, as well as that of cleaved caspase-3 in response to both hydrogen peroxide and TNFα. There was no change in total SirT1 protein expression that could explain the altered p53 acetylation in these 6 h studies in cultured HAEC. These studies indicate that S17834 can counter pro-apoptotic atherogenic stimuli directly in endothelial cells.
Figure 1.
S17834 inhibits hydrogen peroxide (H2O2)- and TNFα-induced increases in p53-lysine 382 acetylation and cleaved caspase-3 in HAEC. Cells were treated with S17834 for 1 h prior to treating with H2O2 (A) or TNFα (B). p53 acetylation was evaluated 4 h after H2O2 and 6 h after TNFα. Caspase-3 acetylation was evaluated at 6 h. Representative immunoblots are shown above bar graphs summarizing results from 3 experiments. Total p53 was unchanged by H2O2, TNFα, or S17834. Total SirT1 was unchanged by TNFα or S17834 and α-actin, used as a loading control, was unchanged. The bar graphs indicate that H2O2 or TNFα significantly increased (*P<0.05) both p53 acetylation (black bar) and cleaved caspase-3 (striped bar), and S17834 significantly prevented the increase (†P<0.05). S17834 did not affect either p53 acetylation or caspase-3 cleavage in cells that were not treated with H2O2 or TNFα. Note that the bar graph in Figure 1B has two ordinates denoting p53 acetylation and caspase-3 cleavage separately.
To determine if endothelial cell changes in caspase-3 and p53 acetylation were accompanied by apoptotic cell death, HAEC were stained for Annexin-V and propidium iodide, indicative of early apoptotic cell death and necrotic cell death, respectively. TNFα significantly increased the number of cells that were positively stained for Annexin-V, but unstained with propidium iodide, indicating increases in apoptotic cell death. S17834 (0.625 μM), but not two lower doses of the polyphenol, prevented the TNFα-induced increase in apoptosis (Supplemental Figure 2). Under the same conditions, neither TNFα nor S17834 significantly affected the number of cells that were stained with propidium iodide but unstained with Annexin-V, indicating no differences in necrotic cell death (ANOVA, P>0.9, data not shown). These data establish the effective concentration of S17834 in preventing endothelial cell apoptosis caused by atherogenic stimuli.
S17834 inhibits atherogenesis in high fat fed LDLr-/- mice
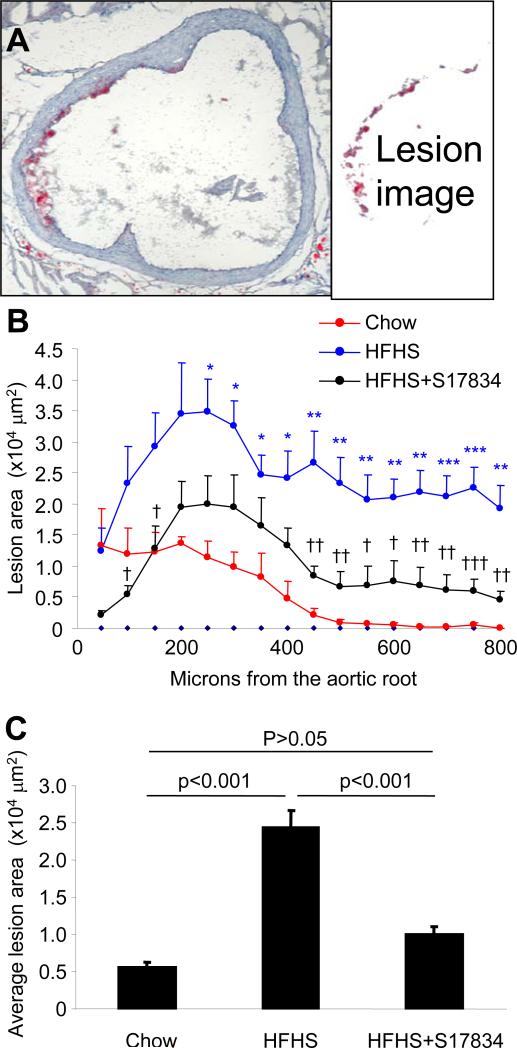
HFHS diet administered to LDLr-/- mice from age 8 to 24 weeks of age significantly increased body weight and fasting serum lipids, but not glucose as previously reported12. The increased body weight and serum cholesterol and triglycerides in LDLr-/- mice fed HFHS diet were significantly improved in mice treated with S17834 (130 mg/kg/d, Table 1). The average area of lipid stained lesions throughout the aortic root was significantly greater in HFHS diet-fed LDLr-/- mice compared with those fed normal chow, but the increase caused by the HFHS diet was particularly marked distal to the aortic valve where the lesions extended onto the inner curvature of the aortic arch. In LDLr-/- mice fed normal chow for the same duration there were no significant lesions on this lesion-prone area of the arch (Figure 2). S17834 significantly prevented the increase in average lesion area caused by the HFHS diet, decreasing the lesion area throughout the aortic root and ascending arch (Figure 2). The aortic root lesions of normal diet fed LDLr-/- mice showed staining for acetylated lysine-382 of p53 and caspase-3 cleavage within atherosclerotic lesions and in endothelial cells within the lesion prone areas around the valve cusps, consistent with increased cell turnover (Supplemental Figure 3). Because effects on these parameters in LDLr-/- mice fed HFHS diet or treated with S17834 could be affected by the large changes in blood lipids in the LDLr-/- mouse, further studies were performed in wild type C57BL6 mice.
Table 1.
The effect of HFHS diet and S17834 on metabolic parameters in wild type and LDLR-/- mice
| Wild Type C57BL6 Mice (n = 11-15) | LDLR -/- Mice (n = 8) | |||||
|---|---|---|---|---|---|---|
| Normal Chow (8 weeks) | HFHS (8 weeks) | HFHS+S17834 (8 weeks) | Normal Chow (16 weeks) | HFHS (16 weeks) | HFHS+S17834 (16 weeks) | |
| Body Weight (g) | 30.0 ± 0.3 | 34.5 ± 0.9 | 35.9 ± 1.4 | 30.5 ± 1.6 | 42.4 ± 1.6* | 33.8 ± 2.1# |
| Blood glucose (mmol/L) | 9.50 ± 0.56 | 9.89 ± 0.5 | 9.89 ± 0.44 | 8.17 ± 0.50 | 8.46 ± 0.67 | 8.12 ± 0.44 |
| Total Cholesterol (mmol/L) | 2.22 ± 0.06 | 3.25 ± 0.08* | 3.72 ± 0.10 | 8.32 ± 0.41 | 19.56 ± 2.12* | 13.78 ± 0.91# |
| Triglyceride (mmol/L) | 0.97 ± 0.01 | 1.08 ± 0.01 | 1.14 ± 0.01 | 1.22 ± 0.12 | 2.04 ± 0.17* | 1.36 ± 0.25# |
P< 0.05 compared to normal chow.
† P< 0.05 compared to HFHS diet.
Figure 2.
S17834 prevents the increase in atherosclerotic lesions in the aortic root in LDLr -/- mice fed HFHS diet. Serial cryosections through the aortic valve and the ascending aortic arch were obtained every 50 μm over a span of 800 μm and stained with Oil Red O. The aortic leaflets spanned from the first section for approximately 250 – 300 μm; sections 300 – 800 μm were from the ascending aortic arch. An aortic cross section 800 μm from the base of the aortic valve leaflets from a HFHS-fed LDLr-/- stained is shown on the left in (A), together with the extracted image of the lipids stained by Oil Red O shown to the right, the area of which was quantified for lesion area. Quantified lesion areas at 50 μm intervals are shown in B. Compared with normal chow diet (red line), HFHS diet (blue line) significantly increased average lesion area (asterisks, *P<0.05, **P<0.01, ***P<001). S17834 treatment (black line) resulted in lesions that were significantly less compared with mice fed HFHS diet (crosses, †P<0.05, ††P<0.01, †††P<0.001). For additional analysis, the average lesion area was calculated over the 800 μm extending from the base of the aortic valve leaflets to the ascending aortic arch for each animal (panel C). P values for the changes in average lesion area are shown above the bars. Data are from 4 mice fed control chow diet, 8 fed HFHS diet, and 8 fed HFHS diet and treated with S17834.
p53 acetylation, caspase-3 cleavage, and SirT1 expression in lesion-prone endothelium of C57BL6 mice fed HFHS diet
HFHS diet administration to wild type C57BL6 mice from age 8 to 16 weeks induced obesity with minimal changes in serum lipids, and no atherosclerotic lesions as previously reported12 (Table 1 and Supplemental Table). Although positive staining for macrophages with F4/80 antibody was present in the lesions along the inner curvature of the aortic arch in LDLr-/- mice fed HFHS diet, no lesions or macrophages were evident in the lesion-prone area of HFHS diet-fed C57BL6 mice (data not shown).
Staining for p53 acetylated lysine-382 was restricted to the lesion-prone endothelium on the inner curvature of the aortic arch and was minimally detected in C57BL6 mice fed normal diet (Figure 3). In mice fed HFHS diet a 3-fold increase occurred in staining of endothelial acetylated p53 on the lesion prone aortic arch, but in mice treated with S17834 the staining was not significantly different from that in normal mice (Figure 3). Staining for total p53 was observed diffusely in the aortic wall including endothelial cells, and was not different from normal in mice fed HFHS diet, whether or not they were treated with S17834 (Supplemental Figure 4). The ratio of endothelial staining scores for acetylated p53 to those for total p53 was also significantly increased in mice fed HFHS diet, and decreased in those treated with S17834 (Figure 3).
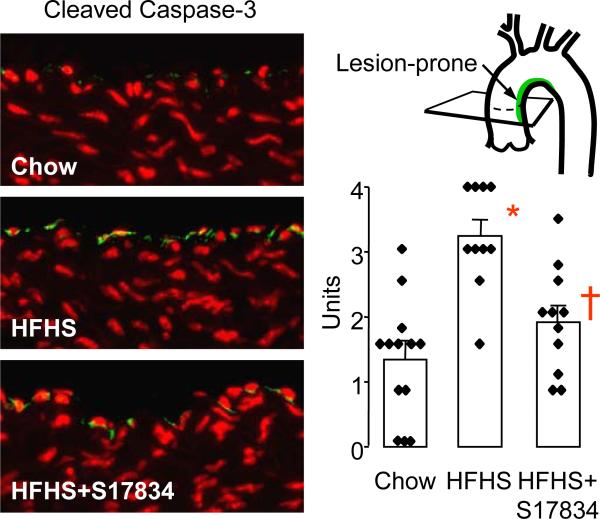
As in studies in cultured HAEC, the pro-apoptotic actions of p53 were assessed by staining for cleaved caspase-3. Staining for cleaved caspase-3 was also primarily restricted to endothelial cells in the lesion-prone inner curvature of the aortic arch (Figure 4). Only rare endothelial cells were stained in mice fed normal diet, but a significantly greater than 2-fold increase in cleaved caspase-3 staining occurred in the endothelium of mice fed HFHS diet. Treatment with S17834 significantly decreased cleaved caspase-3 staining of the lesion-prone endothelium to near control levels (Figure 4).
Figure 4.
S17834 prevents the increase in cleaved caspase-3 caused by HFHS diet in the lesion-prone aortic endothelium of C57BL6 wild type mice. Examples of immuno-fluorescent staining for cleaved caspase-3 in lesion-prone aortic endothelial cells are shown at left. The FITC-tagged secondary antibody was used to detect the cleaved caspase-3 antibody (green) in the endothelial cells. Nuclei were counterstained with propidium iodide (red). The bar graph at right shows the mean ± sem of the average scores obtained for each aortic cross section from each of 10-13 mice in each group. A statistically significant increase in staining for cleaved caspase-3 was caused by HFHS diet compared with chow (*P<0.05), and S17834 treatment resulted in a significant decrease compared with mice fed HFHS diet (†P<0.05).
SirT1 protein was stained diffusely in the aortic wall of both chow diet and HFHS diet fed C57BL6 mice, with no significant difference in intensity in the endothelium (Figure 5). However, in mice treated with S17834, SirT1 staining increased throughout the aortic wall, and there was a significant increase in staining of endothelial cells in the lesion-prone aortic arch (Figure 5).
Discussion
SirT1 directly deacetylates lysine-382 of p53, thereby regulating its function14, and p53 in turn regulates cell turnover, apoptosis, and senescence15,16. Our novel data showing that HFHS diet administered to normal mice increases endothelial cell acetylation of p53 and caspase-3 cleavage in the atherosclerotic lesion-prone aortic arch is consistent with acetylation of the SirT1-dependent site on p53 contributing to increased turnover of the endothelium in that site. Although systemic risk factors like hyperlipidemia and hyperglycemia affect their extent, the location of atherosclerotic lesions is dictated primarily by local shear forces exerted by turbulent blood flow patterns 17,3,18. The inner curvature of the aortic arch is one such site where disturbed flow has been implicated in increasing inflammatory and antioxidant genes in both pigs 17,3,4,18 and mice 4 fed a normal diet. In the mouse fed normal diet, the inner curvature of the aortic arch also has been noted to have increased expression of NFκB p655 protein and adhesion molecule mRNA 4, as well as decreased expression of endothelial nitric oxide synthase mRNA and protein 19. In pigs fed a normal diet, the lesion-prone area has been demonstrated to be a site of increased permeability1,18 and increased endothelial cell turnover2. Consistent with increased cell turnover, the endothelium of the lesion-prone pig aortic arch has an almost 3-fold higher expression of caspase-3 mRNA compared to low prone areas3.
Our observations in normal mice fed HFHS diet imply that increased acetylation of the SirT1-dependent site on p53 activity accelerates these changes in endothelial cell phenotype in lesion-prone areas of the aorta, and also suggest that similar mechanisms may contribute to the increased atherogenesis observed in these aortic sites in hyperlipidemic LDLr-/- mice fed the same diet. The fact that hydrogen peroxide and TNFα caused similar changes in HAEC in culture not only indicates that these atherogenic mediators increase acetylation of the SirT1-dependent site on p53 and caspase-3 cleavage, but is also consistent with their potential role in causing the same changes in vivo in lesion-prone aortic endothelium of normal mice fed HFHS diet.
Our previous findings indicate that the polyphenol, S17834, activates AMPK in hepatocytes, accounting for a decrease in lipid synthesis 11,10. AMPK phosphorylation was decreased in the liver of type 1 diabetic mice, and treatment with S17834 increased its phosphorylation, explaining the decrease in hepatic and serum lipids10. We have found that AMPK phosphorylation is also decreased in the livers of the type-2 diabetic LDLr -/- mice fed HFHS diet studied here, and the decrease is prevented by S17834 treatment (data not shown). This suggests that a decrease in plasma cholesterol, which has been shown previously to be primarily in the LDL fraction in LDL receptor -/- mice fed HFHS diet12, is caused by hepatic effects of the polyphenol and explains the decrease in atherosclerotic lesions. The large changes in plasma lipids caused by HFHS diet and S17834 treatment in the LDLr-/- mouse are likely due to the lack of hepatic reuptake of LDL via the LDL receptor, and therefore reflect changes in hepatic lipid synthesis12.
Because of these systemic effects, we sought to determine what local effects the polyphenol treatment may have on atherogenic events in the aortic endothelium. This was the principal reason why we studied wild type mice in which changes in blood lipids were minimal and where there were no inflammatory cells observed in the aortic arch of animals fed HFHS diet. The polyphenol did not decrease serum lipids in normal mice fed HFHS diet, excluding an effect on serum lipid levels as a cause for the improvements observed. We focused on p53 acetylation and caspase-3 cleavage, because of the recognized increase in endothelial cell turnover of endothelial cells in the lesion prone aortic arch in atherogenesis20,21, and showed that both apoptotic signaling components are detectable in endothelial cells of both LDLr-/- and C57BL6 mice fed HFHS diet. We found that S17834 prevented the effects of the HFHS diet, decreasing p53 acetylation at lysine-382 and caspase-3 cleavage in the lesion prone arch of the C57BL6 mice. Because of the confounding influence of changes in plasma lipids, we did not perform a detailed analysis of changes in apoptotic cell signaling in the LDLr-/- mice, but we speculate that similar directional changes accompanied the changes in lesions. Increased apoptosis of fibroblasts, smooth muscle cells, or macrophages can conceivably be pro- or anti-atherosclerotic, and apoptotic cell clearance may regulate atherosclerotic lesion size21. It is more accepted that increased endothelial cell apoptosis and turnover in the lesion prone aortic arch is an early precursor of atherosclerosis21,20. This makes more relevant our findings that the polyphenol decreases the degree of apoptotic signaling in endothelial cells in the lesion prone aortic arch of normal mice fed HFHS diet.
Unlike with short-term treatment of HAEC, the polyphenol increased SirT1 protein expression in aortic endothelial cells in chronically treated mice, further supporting that the decreased p53 acetylation and cleaved caspase-3 are accounted for by an increase in SirT1 deacetylase activity in the endothelium. We have not excluded the possibility that activity of histone acetylases or acetyltransferases might affect p53 acetylation in HAEC or the mouse aortic arch which could account for our findings, although in the case of the aortic arch in vivo, the increased protein expression is consistent with an increase in SirT1 deacetylase activity. While this work was in progress, it was reported that endothelial specific overexpression of SirT1 inhibits development of atherosclerosis in apolipoprotein E-deficient mice7, providing direct evidence that increased endothelial SirT1 expression can attenuate atherogenesis. It is also possible that sumoylation 22, phosphorylation 23, or S-glutathiolation 24 post-translational modifications could modify SirT1 deacetylase activity independently of changes in expression. In addition, the effects of SirT1 may be exerted via stimulation of nitric oxide synthase activity 25 which might also affect endothelial cell turnover.
As with any pharmacological study, the dose used in vivo and the concentrations used in vitro are potential limitations. Plasma levels of S17834, which is largely metabolized in the liver, have not been measured in mice administered the dose given in this study, but we were able to show direct affects of the compound on p53 acetylation, caspase-3 cleavage and apoptosis in submicromolar concentrations applied to HAEC in culture suggesting that the in vivo effects were exerted locally. In addition, studies of polyphenols in particular are complicated because they affect many metabolic regulators including PPAR's, AMP kinase, and mitochondrial electron transport, making it difficult to identify specific effects. Whether polyphenols activate SirT1 in vivo is an intense area of current interest. This underscores the importance of our observation that acetylation of the SirT1-dependent lysine on p53 is increased by HFHS diet and that this can be explained by a local effect of S17834 to increase SirT1 expression at atherosclerotic lesion prone sites.
Conclusion
Our results indicate that HFHS diet increases acetylation of the SirT1-dependent site on p53 and increases cleavage of caspase-3 in lesion-prone aortic endothelial cells in normal mice. Furthermore, treatment with the polyphenol, S17834, may exert local effects independently of changes in blood lipids on endothelial cell SirT1 expression, deacetylation of p53, and caspase-3 cleavage to prevent the increases in cell turnover and responses to atherogenic stimuli that may contribute to early lesion formation.
Supplementary Material
Acknowledgements
The authors wish to thank Karlene Maitland-Toolan, Robert M. Weisbrod, and Nathalie Bitar for technical assistance. Funding for the studies came from a Strategic Alliance between the Vascular Biology Section at Boston University Medical Center and the Institut de Recherche Servier; as well as by the National Institutes of Health Grants [P01- HL068758, R37- HL104017 and R01 DK076942].
Funding: This work was supported by funding from the National Institutes of Health.
Footnotes
Potential Conflicts of interest: Dr. Verbeuren is an employee and Dr. Cohen is a consultant to Institut de Recherche Servier.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caplan BA, Gerrity RG, Schwartz CJ. Endothelial cell morphology in focal areas of in vivo Evans blue uptake in the young pig aorta. I. Quantitative light microscopic findings. Exp Mol Pathol. 1974;21:102–117. doi: 10.1016/0014-4800(74)90082-3. [DOI] [PubMed] [Google Scholar]
- 2.Caplan BA, Schwartz CJ. Increased endothelial cell turnover in areas of in vivo Evans Blue uptake in the pig aorta. Atherosclerosis. 1973;17:401–417. doi: 10.1016/0021-9150(73)90031-2. [DOI] [PubMed] [Google Scholar]
- 3.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr., Davies PF. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci U S A. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203:2073–2083. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Wang Z, Chen H, Zhou S, Zheng W, Liu G, Wei Y, Cai H, Liu D, Liang C. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le CD, Shaw RJ, Navas P, Puigserver P, Ingram DK, de CR, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cayatte AJ, Rupin A, Oliver-Krasinski J, Maitland K, Sansilvestri-Morel P, Wierzbicki M, Verbeuren TJ, Cohen RA. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets NADPH oxidase. Arterioscler Thromb Vasc Biol. 2001;21:1577–1584. doi: 10.1161/hq1001.096723. [DOI] [PubMed] [Google Scholar]
- 10.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 11.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreyer SA, Lystig TC, Vick CM, LeBoeuf RC. Mice deficient in apolipoprotein E but not LDL receptors are resistant to accelerated atherosclerosis associated with obesity. Atherosclerosis. 2003;171:49–55. doi: 10.1016/j.atherosclerosis.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Cayatte AJ, Du Y, Oliver-Krasinski J, Lavielle G, Verbeuren TJ, Cohen RA. The thromboxane receptor antagonist S18886 but not aspirin inhibits atherogenesis in apo E-deficient mice evidence that eicosanoids other than thromboxane contribute to atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:1724–1728. doi: 10.1161/01.atv.20.7.1724. [DOI] [PubMed] [Google Scholar]
- 14.Vaziri H, Dessain SK, Ng EE, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 15.Cheng J, Cui R, Chen CH, Du J. Oxidized low-density lipoprotein stimulates p53-dependent activation of proapoptotic Bax leading to apoptosis of differentiated endothelial progenitor cells. Endocrinology. 2007;148:2085–2094. doi: 10.1210/en.2006-1709. [DOI] [PubMed] [Google Scholar]
- 16.Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 2007;43:571–579. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Passerini AG, Shi C, Francesco NM, Chuan P, Manduchi E, Grant GR, Stoeckert CJ, Jr., Karanian JW, Wray-Cahen D, Pritchard WF, Davies PF. Regional determinants of arterial endothelial phenotype dominate the impact of gender or short-term exposure to a high-fat diet. Biochem Biophys Res Commun. 2005;332:142–148. doi: 10.1016/j.bbrc.2005.04.103. [DOI] [PubMed] [Google Scholar]
- 18.LaMack JA, Himburg HA, Friedman MH. Distinct profiles of endothelial gene expression in hyperpermeable regions of the porcine aortic arch and thoracic aorta. Atherosclerosis. 2007;195:e35–e41. doi: 10.1016/j.atherosclerosis.2007.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Won D, Zhu SN, Chen M, Teichert AM, Fish JE, Matouk CC, Bonert M, Ojha M, Marsden PA, Cybulsky MI. Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow. Am J Pathol. 2007;171:1691–1704. doi: 10.2353/ajpath.2007.060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Q. Disturbed flow-enhanced endothelial turnover in atherosclerosis. Trends Cardiovasc Med. 2009;19:191–195. doi: 10.1016/j.tcm.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Thorp EB. Mechanisms of failed apoptotic cell clearance by phagocyte subsets in cardiovascular disease. Apoptosis. 2010;15:1124–1136. doi: 10.1007/s10495-010-0516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia SV, Bhalla K, Bai W. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007;9:1253–1262. doi: 10.1038/ncb1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sasaki T, Maier B, Koclega KD, Chruszcz M, Gluba W, Stukenberg PT, Minor W, Scrable H. Phosphorylation regulates SIRT1 function. PLoS ONE. 2008;3:e4020. doi: 10.1371/journal.pone.0004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zee RS, Yoo CB, Pimentel DR, Perlman DH, Burgoyne JR, Hou X, McComb ME, Costello CE, Cohen RA, Bachschmid MM. Redox Regulation of Sirtuin-1 by S-Glutathiolation. Antioxid Redox Signal. 2010;13:1023–1032. doi: 10.1089/ars.2010.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.