Abstract
Objective
To design a bundled case rate for Collaborative Care for Depression (CCD) that aligns incentives with evidence-based depression care in primary care.
Data Sources
A clinical information system used by all care managers in a randomized controlled trial of CCD for older primary care patients.
Study Design
We conducted an empirical investigation of factors accounting for variation in CCD resource use over time and across patients. CCD resource use at the patient-episode and patient-month levels was measured by number of care manager contacts and direct patient contact time and analyzed with count data (Poisson or negative binomial) models.
Principal Findings
Episode-level resource use varies substantially with patient's time in the program. Monthly use declines sharply in the first 6 months regardless of treatment response or remission status, but it remains stable afterwards. An adjusted episode or monthly case rate design better matches payment with variation in resource use compared with a fixed design.
Conclusions
Our findings lend support to an episode payment adjusted by number of months receiving CCD and a monthly payment adjusted by the ordinal month. Nonpayment tools including program certification and performance evaluation and reward systems are needed to fully align incentives.
Keywords: Collaborative Care for Depression, payment
Collaborative Care for Depression (CCD) in primary care redesigns care processes to incorporate evidence-based depression treatment guidelines and components of the Chronic Care Model (Wagner, Austin, and Von Korff 1996; Wagner 2000). A central element of the model is a care manager in the clinic who serves as a physician extender and conducts patient assessment, education, follow-ups, and care coordination, largely outside a patient's visits to his/her primary care physician (PCP) (Schulberg et al. 2001). More than 30 randomized controlled trials (RCTs) have shown that CCD improves depression outcomes, patient adherence to treatment, and satisfaction with care (Neumeyer-Gromen et al. 2004; Gilbody et al. 2006; Williams et al. 2007). The prevailing visit-based fee-for-service (FFS) physician payment system, however, constitutes a serious disincentive for providing this service (Berenson and Horvath 2003; Wolff and Boult 2005; Bachman et al. 2006; Unutzer et al. 2006).
Recognizing the importance of a payment mechanism, several recent large-scale implementation initiatives adopted case rate-based payment models to cover the cost of delivering evidence-based CCD. The Depression Improvement Across Minnesota, Offering a New Direction initiative adopted a flat monthly case rate (Institute for Clinical Systems Improvement 2008). This monthly rate was determined based on the average monthly cost of a 12-month CCD intervention tested in a RCT known as Improving Mood-Promoting Access to Collaborative Treatment (IMPACT) (Unutzer et al. 2002b). A more recent implementation of the IMPACT model to treat a broader set of behavioral health disorders among community health centers in Washington state adopted a lump sum payment to each clinic covering the cost of the yearlong program, with minimum caseload expectations and 25 percent of the payment retained to reward providers achieving prespecified depression treatment and outcome targets. The CCD payment in both initiatives is intended to cover CCD program cost to the clinic that is not covered by the visit-based physician payment.
The underlying goals of these pioneering efforts to pay for CCD are twofold: (1) to adequately cover the cost of providing CCD and (2) to align incentives with the delivery of CCD in a way that leads to better outcomes. The design of such a payment should reflect meaningful variation in resource requirement for evidence-based practice of CCD. In addition, it requires a consideration of incentives associated with a given design, and ways to address or ameliorate related issues.
In this paper, we used data from a large RCT, the IMPACT study (Unutzer et al. 2001, 2002b), to investigate salient and feasible properties of a bundled case rate payment design for CCD. In IMPACT, with close monitoring and guidance from the research team, the CCD intervention protocol was implemented with high fidelity. Meanwhile, participating practices came from diverse community and organizational settings (Unutzer et al. 2001). Delivery of CCD as seen in IMPACT thus represents probably the closest to evidence-based practice in “real-world” implementation where provider adoption of financial strategies is likely to be minimal. This unique feature of the data enables us to identify factors accounting for practically significant variation in resource use of CCD, which then guided our recommendation of two case rate-based payment models. We further compared implications for health care organizations in the IMPACT study of alternative payment models.
CONCEPTUAL FRAMEWORK
Our focus is on the design of an episode or monthly bundled case rate for CCD as an add-on to the visit-based, FFS physician payment system. Here, we define episode for payment purposes, which may or may not coincide with a depression episode based on clinical criteria. A CCD payment episode is a fixed time window (e.g., 1 year) following the start of the program. This case rate is intended to cover the cost of staffing the care manager and of regular consultation provided by a psychiatrist as well as other operational cost such as that of maintaining a registry tracking and reporting system. In this section, we discuss salient features of the CCD protocol that account for variation across patients and over time, provider financial strategies that are potential threats to evidence-based CCD, and possible remedies to be embedded in a payment design. Although our focus here is on CCD payment design, because resource utilization patterns and related issues we examine here are common to many chronic disease management programs, insights and conclusions we draw below may be generalizable to these other efforts.
The IMPACT protocol adopts the “stepped care” algorithm to tailor treatment to the need of individual patients over time (Unutzer et al. 2001). Specifically, patients receive intensive care management (in-person or phone contacts with the care manager on a weekly or biweekly basis) in the first 8–12 weeks. At the end of the initial phase, the algorithm calls for reduced intensity (monthly contacts) if patients show response to treatment and/or achieve remission, but sustained intensity if patients have persistent or recurrent depression. Starting from the seventh month, CCD intensity levels off to monthly contact regardless of patient response, because nonresponders would have been referred to mental health specialty care by then. Thus, one factor potentially accounting for variation in resource use of CCD is patient response and/or remission status in the interim. Meanwhile, regardless of treatment path, CCD activities are usually highly intensive in the initial phase, decline sharply in ensuing months, and level off starting from the seventh month. While the front-loaded nature of this approach to care is consistent with “stepped care,” time may have an independent effect on resource use (other than the effect of following a treatment algorithm). For example, it might be harder to maintain frequent CCD contacts in Month 3 than in Months 1 and 2 even if patients have not fully responded. Thus, for a monthly case rate design, one should consider, as potential adjustment factors, both interim response/remission status and a time indicator (e.g., whether this is the first, second, …, month of the program, or “the ordinal month” hereafter).
A direct implication of the front-loaded nature of CCD resource use is that, with a flat/fixed monthly rate based on the average monthly cost of a 12-month program, provider cost will be more adequately covered if patients remain in the program for as long as the program is designed for than if they terminate treatment early.
Several provider financial strategies are worth noting. The first is “cream skimming,” whereby CCD providers selectively enroll or attract patients whom they expect to require fewer resources to treat. This issue applies when payments are made per episode or per month. A partial remedy is case mix adjustment (such as by baseline severity of depression, comorbid conditions, or other factors associated with greater resource requirement) to adequately reflect expected variation in resource use. A second issue is “skimping,” referring to underprovision of CCD services compared with what is suggested by the clinical protocol once patients are enrolled. Under an episode payment, providers receive payment for every patient initiated into the CCD program, whereas, under a monthly payment, providers are paid for each month a patient receives some CCD service. Therefore, in the absence of quality oversight and performance incentives, an episode payment is more susceptible to skimping than a monthly design. The effect of skimping can be reduced by adjusting the episode payment with the length of time the patient receives service.
A third issue is “up-coding.” This could arise when providers up-code the case mix adjustment factor (e.g., patient baseline severity measure) in order to receive a higher case rate. Up-coding could also arise if the monthly case rate is adjusted by patient's interim response/remission status, which carries an incentive for providers to code up interim severity (i.e., code down response/remission). More detrimentally, adjusting by response/remission status provides disincentives for helping patients respond/remit, the ultimate goal of the CCD intervention. Payment design alone cannot address the up-coding issues, suggesting the need for other nonpayment mechanisms to align incentives. Examples of nonpayment tools include an accrediting/certifying process to ensure minimum level of fidelity to the program protocol to be eligible for payment and a pay-for-performance program to counteract some of the perverse incentives discussed above.
Our analysis of the IMPACT data served three purposes. First, we examined whether some of the assumptions/hypotheses made in the conceptual discussion had empirical support; these assumptions/hypotheses are (1) at both episode and monthly levels, baseline depression severity and comorbidity are associated with higher use of CCD; (2) at the episode level, resource use increases with patients' time in the program; and (3) at the monthly level, time since start of CCD (or the “ordinal month”) has an effect on monthly resource use independent from interim response/remission. Second, we examined to what extent CCD activities seen in a community trial reflected the evidence-based protocol. More specifically, we examined, to what extent, achieving response/remission (or failing to respond or remit) in the first 6 months was associated with reduced (or maintained) CCD use. Third, based on our empirical findings, we proposed alternative CCD payment designs and constructed payment rates, and assessed implications for health care organizations in the IMPACT study. The analysis will not, however, provide empirical evidence on how providers respond to incentives embedded in different designs.
METHODS
The IMPACT Registry Data
The IMPACT study was conducted in 18 primary care clinics from eight health care organizations (academic or HMO physician group practices, independent physician associations, and Veterans Affairs clinics) in five states (Unutzer et al. 2001). Because the health care organizations were responsible for implementation of CCD in IMPACT, we considered them (rather than the clinics or physicians) as the entities who would get paid and bear risks for costs of implementation had there been a payment mechanism. All depression care managers in the study used a web-based clinical information system to monitor patient response to treatment and to document and track care management activities (Unutzer et al. 2002a). This CCD registry provided data at the patient-contact level and recorded type of contact (in-person or phone), date and duration of contact, depression symptoms, and the Patient Health Questionnaire 9 (PHQ-9) score (Kroenke, Spitzer, and Williams 2001), patient's current treatment, changes in treatment plan, and care coordination with other providers. Eight hundred and eighty patients had at least one contact recorded in the registry; a total of 13,870 contacts were recorded.
All IMPACT participants met clinical diagnostic criteria for major depression or dysthymia at the time of the eligibility interview, but a small proportion took a long time (up to 1 year) to get engaged in CCD. As a result, 113 (13 percent) of the 880 CCD users had a PHQ-9 score below 5 at their initial visit to the care manager, indicating almost no depressive symptoms. Providers in real-world implementations are unlikely to engage patients who are no longer “acutely depressed.” We thus excluded these patients from the analysis below. Because this study aims to inform payment design for a time-limited (e.g., 12-month) CCD program, we excluded contacts beyond the first year. For monthly analysis, we further restricted to all patient-months with at least one CCD contact. This design enabled us to assess variation in monthly CCD resource use over the months when CCD payments would be made, and whether and to what extent certain factors accounted for such variation. Our analysis thus focused on 767 patient-episodes (for episode analysis) and 7,433 patient-months (for monthly analysis).
Measures
The two measures of CCD resource use are (1) number of CCD contacts and (2) care manager time (in minutes) in direct patient contact. For both measures, we did not distinguish between in-person and phone contacts because of our focus on costs to health care organizations in delivering CCD. Neither measure directly addresses care manager time outside patient contacts or time of psychiatric consultation, both of which are important components of the CCD. The registry data do not provide information for these two domains of provider costs. When deriving an estimate of total CCD cost associated with a given episode/month, we made the assumption that care manager time outside direct patient contact and time of psychiatric consultation was proportional to direct patient contact time (Harpole et al. 2003).
Patient depressive symptoms were measured by the PHQ-9, assessed and recorded at 93 percent of patient contacts. The baseline PHQ-9 score (assessed at the first contact) was categorized into 5–14 (mild to moderate, “moderate” hereafter) and 15+ (moderately severe to severe, “severe” hereafter) (Kroenke, Spitzer, and Williams 2001). For monthly analysis, we derived an indicator of response to treatment (defined as having achieved a 50 percent or greater reduction in PHQ-9 score compared with baseline) or remission (current PHQ-9 below 5) at the first contact of the month (“beginning of month” hereafter). If the first contact of an index month had a missing PHQ-9, we used the PHQ-9 recorded at the last contact of the previous month.
Statistical Analysis
The two outcomes, number of contacts and care manager time in direct patient contact, are positive count data. We estimated zero-truncated count data models (Long 1997) for both outcomes at the patient-episode and patient-month levels. The decision to use a Poisson versus a negative binomial model was based on a likelihood ratio test of overdispersion in the distribution of each outcome.
In the patient-episode analysis, predictors of CCD resource use included baseline depression severity, total number of months the patient used some CCD service, and interactions of the two. To allow for maximum flexibility in model specification, we included in the model 11 dichotomous indicators of months using CCD (1 month, 2 months, …, 11 months; 12 months being the reference category).
In the patient-month analysis, because beginning-of-month treatment response/remission does not apply to the first month, we separately estimated a model for first-month outcomes. Baseline depression severity was the only predictor in the first-month models. Analysis of Month 2–Month 12 outcomes included, as predictors, baseline severity, beginning-of-month response/remission indicator, indicators of ordinal month (3rd, 4th, …, 12th month; 2nd month being the reference), and interactions between baseline severity and ordinal month indicators, and between beginning-of-month response/remission and ordinal month indicators. The ordinal measure of months was preserved in the presence of no-contact months. For example, if a patient had no contact in the third month, but resumed contact in the fourth month, the exclusion of the third month from analysis did not change the ordinal month indicator for the fourth month. The two groups of interactions were added to allow the effects of baseline severity and of response/remission to vary by ordinal month.
Our initial analysis included patient demographics and comorbid conditions as additional predictors, but none of these predicted the outcomes in a statistically significant way. Therefore, we left these characteristics out of the final models. For each of the two outcomes, we thus estimated one model at the patient-episode level, and two models at the patient-monthly level—one for the first month in CCD, and the other for months 2–12.
RESULTS
Descriptive statistics at the health care organization, patient-episode, and patient-month levels (Table 1) indicated substantial variation in caseload across health care organizations as well as in CCD resource use. Based on estimated models, we report below predicted contacts and time, at the patient-episode and patient-month levels, conditional on factors hypothesized to affect CCD resource use. We present these results in figures. The full set of model output is provided in Appendix SA2.
Table 1.
Descriptive Statistics at the Health Care Organization, Patient-Episode, and Patient-Month Levels in the IMPACT Study
| Health Care Organizations (n = 8) | Patient-Episodes (n = 767) | Patient-Months (n = 7,433) | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of Patient-Episodes Per Organization | No. of Patient-Months Per Organization | Baseline PHQ-9 ≥15 (versus 5–14) | No. of Months in CCD | No. of Patient Contacts | Direct Patient Contact Time (minutes) | No. of Patient Contacts | Direct Patient Contact Time (minutes) | |
| Mean | 95.9 | 926 | 29.7% | 9.7 | 15.8 | 537.7 | 1.6 | 55.7 |
| SD | — | — | — | 2.5 | 5.9 | 285.7 | 0.9 | 45.1 |
| Minutes | 24 | 237 | — | 1 | 1 | 10 | 1 | 5 |
| Maximum | 124 | 1,290 | — | 12 | 40 | 1,530 | 6 | 330 |
CCD, Collaborative Care for Depression; IMPACT, Improving Mood-Promoting Access to Collaborative Treatment; PHQ-9, Patient Health Questionnaire-9.
Episode-Level CCD Resource Use
Overall, higher baseline severity was associated with greater CCD resource use, but the difference was not substantial and did not achieve statistical significance regardless of how long the patient remained in the program (Figure 1A and B). For patients with either moderate or severe depression at baseline, CCD resource use varied substantially with the number of months a patient received services in the program. A ninefold difference was observed between episodes that lasted for 1 month and those that lasted for 12 months, in terms of both total contacts and contact time. This finding is also consistent with the fact that marginal resource use diminishes over time.
Figure 1.
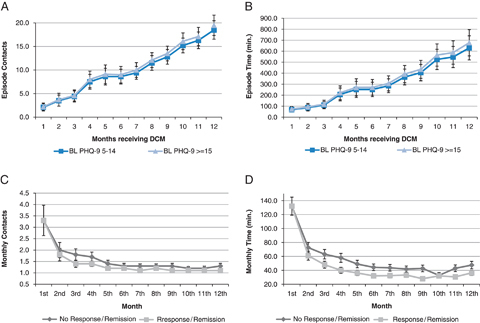
Factors Accounting for Variation in Episode and Monthly CCD Intensity. (A) Predicted Episode-Level Total Care Manager Contacts by Baseline PHQ-9. (B) Predicted Episode-Level Direct Patient Contact Time by Baseline PHQ-9. (C) Predicted Monthly Care Manager Contacts by Response/Remission at Beginning of Month. (D) Predicted Monthly Care Manager Time in Direct Patient Contact by Response/Remission at Beginning of Month. CCD, Collaborative Care for Depression; PHQ-9, Patient Health Questionnaire 9.
Note. Data shown are point predictions and confidence intervals based on estimated count data models.
Monthly CCD Resource Use
Depression severity made little difference in predicting monthly CCD resource use; we therefore eliminated that dimension in Figure 1C and D. Figure 1C and D show predicted monthly CCD resource use by ordinal month and patient response/remission at the beginning of month, for patients with moderate depression at baseline. Regardless of patient response/remission, CCD use declined sharply from the first to the second month (typically a one-third decline in both outcomes). It continued to decline, but more gradually, over Month 2–Month 6. Starting from the seventh month, CCD use remained fairly constant throughout the rest of the program period. (Resource use in the 12th/last month was consistently greater, e.g., on average 5–10 minutes longer in contact time, compared with the 11th month. This is consistent with the protocol that called for relapse prevention planning at the exit visit.)
Lack of a response/remission at the beginning of the month was associated with greater use of CCD. However, this difference achieved statistical significance for the contact time outcome only and for some but not all months. For example, among those with baseline moderate depression, the difference in monthly direct patient contact time peaked in Month 4 at about 18 minutes; in Month 10, the difference was reduced to 5 minutes and not statistically significant. Overall, variation in resource use associated with interim treatment response/remission seemed to be overwhelmed by variation associated with the ordinal month.
Based on these results, we assessed financial implications to health care organizations under four alternative payment designs, including (1) a fixed episode payment, (2) an episode payment adjusted by the number of months a patient received CCD services, (3) a fixed monthly payment, and (4) a monthly payment that is variable in the first 6 months, and flat for months 7–12. We calculated the profit margin, (total payment−total cost)/total cost, associated with each design for all eight health care organizations in the IMPACT trial. We further conducted the same calculation among high- versus low-users of CCD for each organization. (We describe details on how payment and costs were constructed, assumptions made, definition of high- versus low-users, and supplemental data sources in Appendix SA3.)
For both episode and monthly payments, because an adjusted design better matches payment to variation in provider cost both over time and across patients, the resulting profit margins have a much narrower range across all providers and are closer to zero compared with that under a fixed design (Figure 2). (Profit margins are largely above zero because we allowed for a 5 percent margin when calculating case rate based on predicted cost; see Appendix SA3.) Consistent with previous discussion, none of the payment designs will (or is intended to) exactly cover CCD cost on an individual patient basis: on average, high users of CCD were associated with “underpayment” (and thus a negative profit margin), and low users, “overpayment” (positive profit margin). However, the adjusted design is, in general, associated with a smaller under- or overpayment compared with the fixed design. One exception is that, under the monthly design, adjusted payment is associated with a slightly greater underpayment among high users. High users tended to remain in the program for a longer time. In this case, the fact that an adjusted design better matches payment with cost (thus resulting in a lower underpayment) is counteracted and dominated by the fact that a fixed monthly payment works in favor of patients who remain in the program for a longer time and an adjusted monthly payment does not, resulting in a greater underpayment.
Figure 2.
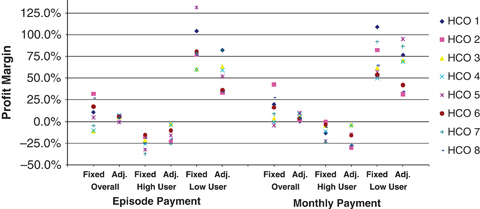
Profit Margins across Health Care Organizations in IMPACT under Alternative Payment Designs. HCO, Health Care Organization; IMPACT, Improving Mood-Promoting Access to Collaborative Treatment.
DISCUSSION
In this study, guided by a conceptual discussion, we conducted an empirical investigation using data from the IMPACT study to inform a case rate design for CCD. For an episode payment design, our findings supported an adjustment by time in the program to reflect the substantial differences in resource use. An alternative approach would be to mandate a minimum length of time receiving the service (e.g., 6 months) as precondition for receiving the first installment of an episode payment. For episodes exceeding the mandated minimum length, payment would be adjusted for additional time in the program (e.g., a flat monthly fee for every additional month in the program, making it a hybrid approach). For a monthly payment design, our findings supported an adjustment by the ordinal month in the first 6 months to reflect the front-loaded nature and sharp decline in resource use over time, but a fixed monthly rate starting from the seventh month. We concluded that patient case mix (such as baseline depression severity) and interim treatment response/remission did not warrant adjustment in either an episode or monthly payment design. This conclusion is based on the lack of empirical association between either of the two adjustment factors and variation in CCD resource use as well as to avoid perverse incentives associated with the two adjustments.
Several limitations pertaining to our data and analysis are worth noting. First, by choosing to participate in a research study, organizations in IMPACT may differ from the general population of primary care organizations in terms of leadership and staff buy-in for depression quality improvement. However, this kind of selection may be of minimal concern given our purpose, because we are not evaluating the effectiveness of the intervention in a general population of practices, but rather using the data to assess alternative payment models. Second, consistent with depression treatment guidelines, the IMPACT study used a 12-month treatment protocol regardless of patient response/remission status. To the extent that community implementation initiatives choose to adopt a protocol with varying length of follow-up conditional on patient treatment outcomes, our conclusions about payment designs may not be directly applicable, although the conceptual and empirical approaches we demonstrated here remain valuable. Third, we did not have patient-level data on care manager time outside direct patient contact. We made the proportionality assumption to estimate indirect time based on direct patient contact time. Given the importance of activities outside direct patient contact in CCD and other disease management/care coordination programs alike, future studies may consider specifically collecting this data. Fourth, our analysis focused on basic features of a case rate design and did not assess the risk/scale aspect of a payment, for example, the minimum caseload needed to mitigate the risk of random variation in cost that is not systematically reflected and covered by a given payment scheme.
While we have based the payment design on the cost of staffing the care manager and the cost of psychiatric consultation, it is worth considering the implications of CCD implementation to several major stakeholders: the PCPs/practices, the mental health specialists, and the patients. The CCD will likely require more care coordination time by the PCP that is not reimbursed under the FFS system. Although our analysis did not consider this additional cost, future payment designs may need to do so. CCD may increase or decrease the need for patient office visits to the PCP, although evidence from IMPACT points to a likely small reduction in nonmental health outpatient cost as a result of CCD (Katon et al. 2005; Unutzer et al. 2008). Many ongoing health care reform initiatives such as pay-for-performance, medical homes, and accountable care organizations may counteract these disincentives to PCPs by providing incentives for implementing proven models such as CCD. Implications for mental health specialty providers are also conceptually ambiguous: by more effectively managing depression in primary care, CCD may reduce the need for referral to specialty care; on the other hand, more depressed patients may be identified as a result of CCD, and the “stepped care” approach may facilitate referral of patients not responding to primary care-based treatment. Evidence from IMPACT indicates that the CCD intervention led to a 25 percent reduction in the cost of outpatient mental health care (not including CCD program cost) in the first 2 years (Katon et al. 2005), but little difference over a 4-year period (Unutzer et al. 2008). We are aware of no studies that have examined patients' willingness to pay for CCD. To the extent that future implementation may consider patient cost sharing, there is a need for research on implications of different levels of copayment on patient uptake and adherence behaviors.
Besides specific recommendations for the design of a case rate for CCD, our study generated a few insights that are applicable to similar efforts in general. First, the conclusions we drew regarding the time patterns of resource use and how payment could be designed to reflect these patterns while aligning incentives may be generalizable to other chronic disease management programs. Our findings may also be informative to policy makers contemplating payment reform initiatives such as the design of a per-patient-per-month payment to practices certified as a medical home. Second, an important insight is that a payment mechanism alone may fall short of adequately aligning incentives; nonprice mechanisms (such as a certification process to ensure minimum fidelity) may be necessary. Third, our study highlights the value of a careful examination of alternative designs—both opportunities and potential issues—before empirical investigation. Such conceptual discussion will provide a roadmap for analysis. It is also critical in identifying factors/information that may not be fruitfully applied to a payment design because of the perverse incentives they carry.
In conclusion, findings of our study suggested that two payment designs may best reflect variation in provider cost while carrying some incentives for evidence-based practice of CCD: an episode payment adjusted by time in the program and a monthly payment adjusted by the ordinal month. We also conclude that, to sufficiently align incentives, there is a need for nonprice tools in addition to a payment mechanism, including accreditation/certification and quality evaluation and reward systems.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: The authors acknowledge funding from the National Institute of Mental Health (Bao, K01 MH090087, Bao and Bruce, P30 MH085943, Solberg, R01MH080692). The IMPACT study was funded by grants from the John A. Hartford Foundation and the California Healthcare Foundation. The authors thank the following individuals for helpful comments on previous versions of the paper: Heather Taffet Gold, Ph.D., Dominic Hodgkin, Ph.D., John Mullahy, Ph.D., Harold Pincus, M.D., Andrew Ryan, Ph.D., and Bruce Schackman, Ph.D. Ming-Yu Fan, Ph.D., provided excellent support with the IMPACT data.
Disclosures: None.
Disclaimers: None.
Supporting Information
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Appendix SA2: Estimated Count Data Models for CCD Contacts and Care Manager Time in Direct Patient Contact at the Episode and Monthly Levels.
Appendix SA3: Calculation of Profit Margins for Health Care Organizations in the IMPACT Study under Alternative Payment Designs.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- Bachman J, Pincus HA, Houtsinger JK, Unützer J. Funding Mechanisms for Depression Care Management: Opportunities and Challenges. General Hospital Psychiatry. 2006;28(4):278–88. doi: 10.1016/j.genhosppsych.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Berenson RA, Horvath J. Confronting the Barriers to Chronic Care Management in Medicare. Health Affairs. 2003 doi: 10.1377/hlthaff.w3.37. W3: 37–53 [online] [DOI] [PubMed] [Google Scholar]
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative Care for Depression: A Cumulative Meta-Analysis and Review of Longer-Term Outcomes. Archives of Internal Medicine. 2006;166(21):2314–21. doi: 10.1001/archinte.166.21.2314. [DOI] [PubMed] [Google Scholar]
- Harpole LH, Stechuchak KM, Saur CD, Steffens DC, Unützer J, Oddone E. Implementing a Disease Management Intervention for Depression in Primary Care: A Random Work Sampling Study. General Hospital Psychiatry. 2003;25(4):238–45. doi: 10.1016/s0163-8343(03)00023-9. [DOI] [PubMed] [Google Scholar]
- Institute for Clinical Systems Improvement. The DIAMOND Initiative: Depression Improvement Across Minnesota, Offering a New Direction. Bloomington, MN: Institute for Clinical Systems Improvement; 2008. [Google Scholar]
- Katon WJ, Schoenbaum M, Fan M-Y, Callahan CM, Williams J, Hunkeler E, Harpole L, Zhou X-HA, Langston C, Unützer J. Cost-Effectiveness of Improving Primary Care Treatment of Late-Life Depression. Archives of General Psychiatry. 2005;62(12):1313–20. doi: 10.1001/archpsyc.62.12.1313. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a Brief Depression Severity Measure. Journal of General Internal Medicine. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JS. Regression Models for Categorical and Limited Dependent Variables. Thousand Oaks, CA: SAGE Publications Inc.; 1997. [Google Scholar]
- Neumeyer-Gromen A, Lampert T, Stark K, Kallischnigg G. Disease Management Programs for Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medical Care. 2004;42(12):1211–21. doi: 10.1097/00005650-200412000-00008. [DOI] [PubMed] [Google Scholar]
- Schulberg HC, Bryce C, Chism K, Mulsant BH, Rollman B, Bruce M, Coyne J, Reynolds CF. Managing Late-Life Depression in Primary Care Practice: A Case Study of the Health Specialist's Role. International Journal of Geriatric Psychiatry. 2001;16(6):577–84. doi: 10.1002/gps.470. [DOI] [PubMed] [Google Scholar]
- Unutzer J, Choi Y, Cook IA, Oishi S. Clinical Computing: A Web-Based Data Management System to Improve Care for Depression in a Multicenter Clinical Trial. Psychiatric Services. 2002a;53(6):671–78. doi: 10.1176/ps.53.6.671. [DOI] [PubMed] [Google Scholar]
- Unutzer J, Katon W, Callahan CM, Williams JW, Hunkeler E, Jr., Harpole L, Hoffing M, Della Penna RD, Noel PH, Lin EH, Arean PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C. Collaborative Care Management of Late-Life Depression in the Primary Care Setting: A Randomized Controlled Trial. Journal of American Medical Association. 2002b;288(22):2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- Unutzer J, Katon W, Williams JW, Callahan CM, Jr., Harpole L, Hunkeler EM, Hoffing M, Arean P, Hegel MT, Schoenbaum M, Oishi SM, Langston CA. Improving Primary Care for Depression in Late Life: The Design of a Multicenter Randomized Trial. Medical Care. 2001;39(8):785–99. doi: 10.1097/00005650-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Unutzer J, Katon WJ, Fan M-Y, Schoenbaum MC, Lin EHB, Della Penna RD, Powers D. Long-Term Cost Effects of Collaborative Care for Late-Life Depression. American Journal of Managed Care. 2008;14(2):95–100. [PMC free article] [PubMed] [Google Scholar]
- Unutzer J, Schoenbaum M, Druss BG, Katon WJ. Transforming Mental Health Care at the Interface with General Medicine: Report for the Presidents Commission. Psychiatric Services. 2006;57(1):37–47. doi: 10.1176/appi.ps.57.1.37. [DOI] [PubMed] [Google Scholar]
- Wagner EH. The Role of Patient Care Teams in Chronic Disease Management. British Medical Journal. 2000;320(7234):569–72. doi: 10.1136/bmj.320.7234.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EH, Austin BT, Von Korff M. Organizing Care for Patients with Chronic Illness. Milbank Quarterly. 1996;74(4):511–44. [PubMed] [Google Scholar]
- Williams JW, Gerrity M, Holsinger T, Dobscha S, Gaynes B, Dietrich A. Systematic Review of Multifaceted Interventions to Improve Depression Care. General Hospital Psychiatry. 2007;29(2):91–116. doi: 10.1016/j.genhosppsych.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Wolff JL, Boult C. Moving beyond Round Pegs and Square Holes: Restructuring Medicare to Improve Chronic Care. Annals of Internal Medicine. 2005;143(6):439–45. doi: 10.7326/0003-4819-143-6-200509200-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


