Abstract
Prolactin (PRL) is a hormone and a neuromodulator. PRL sensitizes TRPV1 responses in sensory neurons, but it is not clear whether peripheral inflammation results in the release of endogenous PRL, or whether endogenous PRL is capable of acting as an inflammatory mediator in a sex-dependent manner. To address these questions, we examined inflammation-induced release of endogenous PRL, and its regulation of thermal hyperalgesia in female and male rats. PRL is expressed in several types of peripheral neuronal and non-neuronal cells, including TRPV1-positive nerve fibers, preadipocytes and activated macrophages/monocytes localized in the vicinity of nerves. Evaluation of PRL levels in hindpaws and plasma indicated that complete Freund’s adjuvant (CFA) stimulates release of peripheral, but not systemic PRL within 6–48h in both ovariectomized females with estradiol replacement (OVX-E) and male rats. The time course of release varies in OVX-E and male rats. We next employed the prolactin receptor (PRL-R) antagonist, Δ1-9-G129R-hPRL to assess the role of locally-produced PRL in nociception. Applied at a ratio of 1:1 (PRL:Δ1-9-G129R-hPRL; 40nM each), this antagonist was able to nearly (≈80%) reverse PRL-induced sensitization of capsaicin responses in rat sensory neurons. CFA-induced inflammatory thermal hyperalgesia in OVX-E rat hindpaws was significantly reduced in a dose-dependent manner by the PRL-R antagonist at the 6h, but not the 24h time point. In contrast, PRL contributed to inflammatory thermal hyperalgesia in male rats at 24h, but not 6h. In summary, these findings indicate that inflammation leads to accumulation of endogenous PRL in female and male rats. Further, PRL acts as an inflammatory mediator at different time points for female and male rats.
Keywords: prolactin, sensory neurons, TRPV1, prolactin antagonist, inflammation
Introduction
PRL, a member of the family of class I cytokines (Boutin et al., 1988), functions as both a hormone and a neuromodulator. The functions of PRL are diverse and include: water and electrolyte balance, growth and development, endocrinology and metabolism, brain and behavior, reproduction, immunoregulation and tissue protection (Bole-Feysot et al., 1998). Many of these effects are mediated by PRL released from the pituitary gland. PRL is also expressed and released from extrapituitary tissues including the brain, reproductive organs, mammary gland, immune cells, and skin (Ben-Jonathan et al., 1996).
Clinical research has reported elevated PRL levels in several human conditions that are associated with increased pain (Wennbo & Tornell, 2000; Dugan et al., 2004; Lissoni et al., 2005; Bosco et al., 2008). Treatment of sensory neurons with exogenous PRL rapidly (5–15 min) sensitizes TRPV1-mediated responses (Diogenes et al., 2006), which is a critical component in the development of thermal hyperalgesia (Caterina et al., 2000; Davis et al., 2000). In addition, PRL can be released by stimulated sensory neurons (Diogenes et al., 2006). These findings are consistent with the hypothesis that PRL plays a role in inflammatory thermal hyperalgesia. However, the regulation of PRL levels by inflammation has not been evaluated and the roles of endogenous PRL during inflammation are unclear. Finally, it is well documented that pituitary release of PRL and its effect on receptors is functionally different in males and females (Yokoyama et al., 2002; Colao et al., 2006; Bernichtein et al., 2010; Jacobson et al., 2010). However, differences in the actions of endogenous PRL in inflammatory thermal hyperalgesia in females and males have not been examined.
The present studies examined whether CFA mediated inflammation evokes peripheral release of PRL. Released PRL was evaluated in both female (OVX-E) and male rats. Furthermore we investigated whether CFA-induced, locally-produced PRL acts as an inflammatory mediator and contributes to thermal hyperalgesia in both OVX-E and male rats.
Materials and Methods
Experimental Animals and Hormone Manipulations
Adult male or female Sprague-Dawley rats (200–250g, Charles River Laboratories, Wilmington, MA) were housed three per cage under a 12-h light/12-h dark cycle with food and water available ad libitum. All animal study protocols were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and conformed to the International Association for the Study of Pain and federal guidelines. Where appropriate, ovariectomy was performed by Charles Rivers Inc., approximately one week prior to arrival at our lab. Hormonal replacement conditions commenced the day following delivery and experiments were performed within 7–21 days of capsule insertion. Capsules contained a low dose of 17βE2 equivalent to physiological non-estrous levels in female rats (one pellet at 0.4 mg/pellet generates 15–20 ng/ml serum) (Mannino et al., 2005) and were implanted s.c. under brief isoflurane anesthesia. CFA-induced inflammation was initiated by i.p.l. injection of 50μl of 1:1 saline:CFA (Sigma-Aldrich, St. Louis, MO) under light isoflurane anesthesia.
Interstitial Fluid Collection and ELISA
Rat paw samples were collected with 6 mm biopsy punches (HealthlinkR®, Fray Corp., Buffalo, NY); two samples from each paw were placed on a filter (5 ml polystyrene round-bottom tube with cell-strainer cap; BD Falcon, Franklin Lakes, NJ) and samples were centrifuged for 10 min at 240 g, as described previously, with recovery of the interstitial fluid (Bletsa et al., 2006). Serum samples were collected in blood collection tubes with sodium citrate (BD Biosciences). Samples were stored at −20° C until assay with a commercially available rat PRL EIA kit (SPIbio, Montigny le Bretonneux, France, distributed by Cayman Chemical).
Protein Isolation and Western Blotting
Total protein from interstitial fluid was lyophilized and dissolved in 20 μl of Western loading buffer. The amount of protein was determined using a BCA kit (Sigma-Aldrich). A sample of 45 μg total protein (2–3.5 μl) was loaded for SDS-PAGE using 12.5% polyacrylamide gels. Protein was transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA), blocked in 5% fat-free milk in TBS-Tween 20, and visualized using a previously validated antibody to PRL (C17, Santa Cruz, Santa Cruz, CA) (Diogenes et al., 2006) and β-actin (C17, Santa Cruz, Santa Cruz, CA) (Jeske et al., 2006). Appropriate HRP-conjugated secondary antibodies were applied and enhanced chemoluminescence detecting reagent (GE Healthcare, Piscataway, NJ) was used according to manufacturer instructions. Blots were then exposed to X-Ray film and developed using a Mini-Medical-90 X-Ray film processor (AFP Imaging, Elmsford, NY). Developed films were scanned and quantified using NIH ImageJ software.
Immunohistochemistry
Rats were deeply anesthetized and underwent transcardial perfusion with 4% paraformaldehyde (PFA) and 0.2% picric acid in 0.1 M phosphate buffer (PB). Tissue biopsies were collected from hindpaws with 6mm biopsy punches and tissue was post-fixed in 4% PFA in PB for 20 min before being submerged in 30% sucrose in PB overnight. Tissue samples were then embedded in Neg-50 (Richard Allan Scientific, Kalamazoo, MI), frozen on dry ice and transferred to −80°C before being cut into 30μm thick sections and mounted on glass slides. Slides were incubated at room temperature with primary antibodies against PRL, TRPV1, ED-1, neurofilament 200kD subunit, and growth associated protein-43. The specificity of the rabbit anti-PRL serum (1:250, AFP425-10-91, kindly provided by Dr A.F. Parlow, Director, Pituitary Hormones and Antisera Center, NIDDK) was assessed by Dr. Parlow and analysis shows absence of binding to other pituitary hormones (product datasheet). Staining with this widely used antiserum shows expression of PRL in nerve fibers and this finding is consistent with expression of PRL mRNA seen in trigeminal sensory neurons (Diogenes et al., 2006). The guinea pig anti-TRPV1 serum (1:2000, GP14100, Neuromics, Bloomington, MN, USA) recognizes a single band at 95–100 kDa in extracts of dorsal root ganglia (DRG) (Guo et al., 1999) and labels the same population of DRG cells as seen with the rabbit Neuromics TRPV1 antibody (Guo et al., 1999) and with the rabbit antibody from the Julius group (Kiasalari et al., 2010). The mouse anti ED-1 monoclonal antibody (1:50, MAB1435, Millipore) recognizes a 90–100 kD protein expressed on the lysomal membrane of myeloid cells that is expressed by most tissue macrophages (manufacturer’s information). Staining of positive control tissue with CFA-induced inflammation showed increased numbers of stained cells as previously shown (Ji et al., 2002). The mouse anti-neurofilament kD 200 (N52 clone) monoclonal antibody (1:2000, N0142, Sigma) recognizes phosphorylated and nonphosphorylated 200 kD neurofilaments in rat spinal cord extract and does not identify other filaments (manufacturer’s information). Our results using this antibody are consistent with other studies in rat skin (Kang et al., 2010). The mouse anti-growth associated protein (GAP43) monoclonal (clone 9-1E12) antibody (1:5000, MAB347, Millipore) recognizes a single band at ≈ 45 kD in rat brain and nerve tissue (Schreyer & Skene, 1991) and membrane fractions derived from maintained neurons (manufacturer’s information); this antibody labels fibres in rat skin as previously described (Pare et al., 2007). The N52 and GAP43 antibody cocktail (both of mouse origin) allowed the identification of both small fibers with GAP43 and larger fibers with N52 (Pare et al., 2007). Sections were then incubated with species-specific AlexaFluor secondary antibodies (Molecular Probes, Eugene, OR) as previously described (Henry et al., 2006). Finally, dry slides were coverslipped with Vectashield containing the DNA stain, 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA). Images were acquired with a Nikon C1si laser scanning confocal imaging microscope.
Electrophysiology
All recordings were performed in whole-cell voltage-clamp (Vh= −60 mV) configuration at 22–24°C, from the somata of neurons (15–45 pF) using an Axopatch200B amplifier and pCLAMP9.0 software (Axon Instruments, Union City, CA). Data were filtered at 0.5 kHz and sampled at 2 kHz. Standard external solution (SES) had the following composition in mM: 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose and 10 HEPES, pH 7.4. The pipette solution consisted of (in mM): 140 KCl, 1 MgCl2, 1 CaCl2, 10 EGTA, 10 D-glucose, 10 HEPES, 0.2 Na-GTP and 2.5 Mg-ATP, pH 7.3. Drugs were applied using a fast, computer controlled pressure-driven 8-channel system (ValveLink8; AutoMate Scientific, San Francisco, CA).
Behavioral Studies
Behavioral studies were conducted by a blinded observer. Animals were housed for at least one week prior to handling and habituated to the testing environment for at least 1 hr prior to testing. CFA-induced inflammation was initiated by ipsilateral i.p.l. injection of 50μl of 1:1 saline:CFA (Sigma-Aldrich, St. Louis, MO) under light isoflurane anesthesia. Thermal responses were assessed as previously described (Hargreaves et al., 1988). In brief, animals were placed on a glass surface with temperature held constant at 20°C. Following habituation, thermal withdrawal latencies to a radiant heat beam were recorded at each time point (3X measurements at each time point, averaged to obtain the data value used in analyses). In order to prevent tissue damage, the stimulus was terminated after ≈20 seconds if the animal did not withdraw the hindpaw. The PRL-R antagonist Δ1-9-G129R-hPRL was synthesized and chromatography-purified as previously described (Bernichtein et al., 2003; Goffin et al., 2005). Δ1-9-G129R-hPRL was dissolved in a 5 μM sodium bicarbonate solution at a starting concentration of 1mg/ml. In the time-course experiments, withdrawal latencies were assessed in each rat prior to injection of vehicle or antagonist (identified as baseline). Indicated concentrations of the PRL antagonist were injected ipl into the hindpaw or sc into the lower back of the animals. 50 μl was injected with a 30G needle while animals were lightly restrained. The antagonist was delivered 30 min prior to measurement of thermal responses.
Data Analyses
Statistical analyses were carried out according to “the Editorial published in EJN issue 28/12”. GraphPad Prism 4.0 (GraphPad, La Jolla, CA) was used for statistical analyses. The data in Figs were given as mean ± standard error of the mean (SEM), with the value of n referring to the number of analyzed cells or animals for each group. All experiments were performed at least in triplicate. When only two groups were evaluated, the statistical differences were analysed using unpaired t-test. Differences between groups were assessed by one-way or two-way analysis of variance (ANOVA) with Bonferroni’s multiple comparison post-hoc tests. The test statistic values (t, F), degrees of freedom (df) and exact p values for each test are calculated. Specific analyses are indicated in the legend to figure section. No corrections for violations of normality or sphericity were required. A difference was accepted as statistically significant when p<0.05. Significance levels of p<0.05, <0.01 and <0.001 are marked by *, ** and ***, respectively. For the behavioral study, the percent possible maximum effect (%MPE) was calculated as [(test latency−baseline latency)/(cut-off time−baseline latency)], as previously described (Lichtman, 1998).
Results
Expression pattern of PRL in peripheral nerve fibers and non-neuronal cells
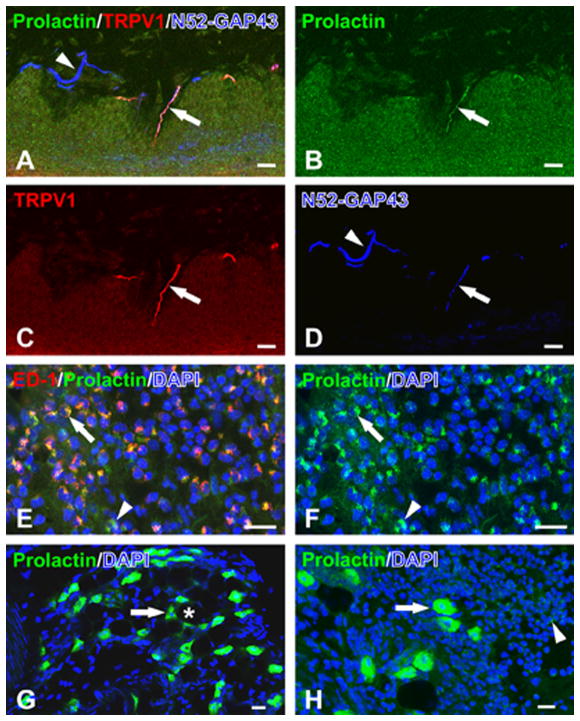
We first investigated whether peripheral cells express PRL following induction of inflammation. Tissues were qualitatively analyzed at the 6h post-CFA time point. PRL is co-localized with TRPV1 in N52/GAP43-identified nerve fibers innervating inflamed hindpaw skin from female OVX-E rats (Fig. 1A–D). Non-neuronal sources of PRL included ED-1-identified macrophages/monocytes (Fig. 1E–F), which were commonly observed within the inflamed hindpaw 6 hours after CFA injection. Other non-neuronal sources of PRL included preadipocyte-like cells in uninflamed (Fig. 1G) as well as inflamed (Fig. 1H) hindpaw skin. The relative expression of PRL appeared greater in preadipocyte-like cells when compared to the expression seen in inflammatory cells (Fig. 1H). Altogether, our data indicate that PRL protein is expressed in both peripheral neurons, including TRPV1-positive sensory neuron fibers, and in non-neuronal peripheral cells located in samples from inflamed hindpaw of OVX-E rats.
Figure 1. Expression pattern for PRL in peripheral tissues of inflamed OVX-E rat hindpaw.
Inflammation was generated by hindpaw CFA injection. Samples were collected 6h post-CFA. All presented images were acquired with a confocal microscope (Nikon). (A) PRL (green; B) is co-localized with TRPV1 (red; C) in a N52/GAP43-identified nerve fiber (blue; D) in inflamed OVX-E rat hindpaw skin. The PRL fiber is located in a dermal papilla while other neighboring N52/GAP43-identified nerve fibers lacked both PRL and TRPV1 (arrowhead). (E) PRL (green; F) is localized within ED-1-identified monocytes/macrophages (red) in the inflamed hindpaw skin of an OVX-E rat. The nuclear stain, DAPI (blue), identifies a dense inflammatory cell infiltrate where many of the cells with ED-1 staining also show PRL staining (arrow). (G) PRL (green) is expressed within preadipocyte-like cells (arrow) in the uninflamed subcutaneous hindpaw tissues of an OVX-E rat. Preadipocytes are typically located at the periphery of the larger adipocytes (*) as seen in areas mainly lacking the nuclear stain, DAPI (blue). (H) PRL (green) is also strongly expressed in preadipocytes (arrow) in the subcutaneous inflamed t issues of the OVX-E rat hindpaw. The relative expression of PRL within the inflammatory cells appears less than that seen in the preadipocyte-like cells. All scale bars = 20 μm.
Peripherally released PRL levels are regulated by inflammation
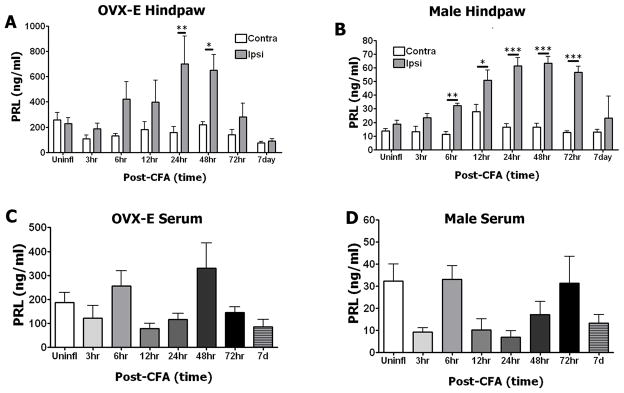
Previous studies have demonstrated that cultured sensory neurons contain a pool of PRL that can be released by capsaicin challenge (Diogenes et al., 2006). Here, we addressed the issue of whether CFA-induced inflammation leads to release of PRL at the periphery as well as systemically. To evaluate local PRL levels, interstitial fluid (i.e. extracellular fluid) was collected from punch biopsies of uninflamed (Contra) and inflamed (Ipsi) hindpaws of OVX-E and intact male rats. Elevations in PRL levels were detected at 6–72h post-CFA injection in hindpaw biopsies from both OVX-E females and male rats (Fig 2A and 2B, respectively, p≤ 0.05–0.001). Local endogenous PRL levels returned to baseline by 7 days post-CFA. There were notable sex differences in PRL basal expression levels and inflammation-induced PRL release. First, basal and inflammation-evoked PRL levels were dramatically higher in OVX-E females (n=5–9/group) compared to intact male rats (n=3–6/group). Thus, interstitial fluid from OVX-E female rats had mean values ranging from 75–700 ng/ml, whereas in male rats PRL means ranged from 11–63ng/ml (Fig 2A and 2B). Second, in hindpaws from intact males, levels of released PRL were significantly higher in inflamed (i.e. Ipsi) tissues at 6–72h post-CFA (Fig 2B). In contrast, inflamed hindpaw samples from OVX-E female rats exhibited significantly greater released PRL levels compared to uninflamed Contra samples at 24–48h post-CFA (Fig 2A). In addition, inflammation-induced up-regulation of released PRL levels were also observed at the 6, 12 and 72h post-CFA time points in the OVX-E samples, though these differences did not reach significance (Fig 2A).
Figure 2. Endogenous PRL levels are regulated by hindpaw inflammation in OVX-E and intact male rats.
(A) Time-course of PRL levels from CFA-inflamed (Ipsi) and uninflamed (Contra) hindpaws of OVX-E rats. Data were analyzed by two-way ANOVA (F=17.61; df=7). 24h time point post-CFA, ** p=0.007, t=3.657. 48h time point post-CFA, *p=0.02, t=3.175; n=5–9. (B) Time-course of released PRL levels from CFA-inflamed (Ipsi) and uninflamed (Contra) hindpaws of intact male rats. Results of two-way ANOVA between Ipsi and Contra groups across time points are indicated by horizontal bars connecting groups that showed significant differences (F=7.186; df=7). 6h time point post-CFA, ** p=0.007, t=3.404. 12h post-CFA, *p=0.03; t=3.062. 24h post-CFA, ***p<0.001; t=6.932. 48h post-CFA, ***p<0.001, t=7.29. 72h post-CFA, ***p<0.001; t=7.139. n=3–9. (C and D) Time course of PRL levels in serum from CFA-inflamed (injected into hindpaw) OVX-E (C) and intact male (D) rats. One-way ANOVA was performed comparing the uninflamed group to the other time points. n=4–9
In order to assess whether systemic changes in PRL levels occur with hindpaw CFA administration, we examined PRL levels in serum at each inflammatory time point (Fig 2C and 2D). One-way ANOVA comparing post-inflammation time points to the uninflamed group indicated that there were no significant changes in serum levels of PRL over the presented time course (3h–7d; p>0.05). It can also be noted that serum PRL levels in OVX-E females were ~10-fold greater than in intact males. The observed minor variations in serum PRL likely resulted from slight differences in the time of day that samples were collected, and fall within the expected range of values (Yuan & Pan, 2002). Diurnal variation in systemic endogenous PRL levels predicts an afternoon surge that necessarily falls at sample collection for some time points. In summary, hindpaw inflammation up-regulates local, but not systemically released PRL levels in both OVX-E and male rats. However, the concentration and time course of released PRL differ in OVX-E female versus male rat interstitial fluid.
Inflammation regulates local PRL protein levels
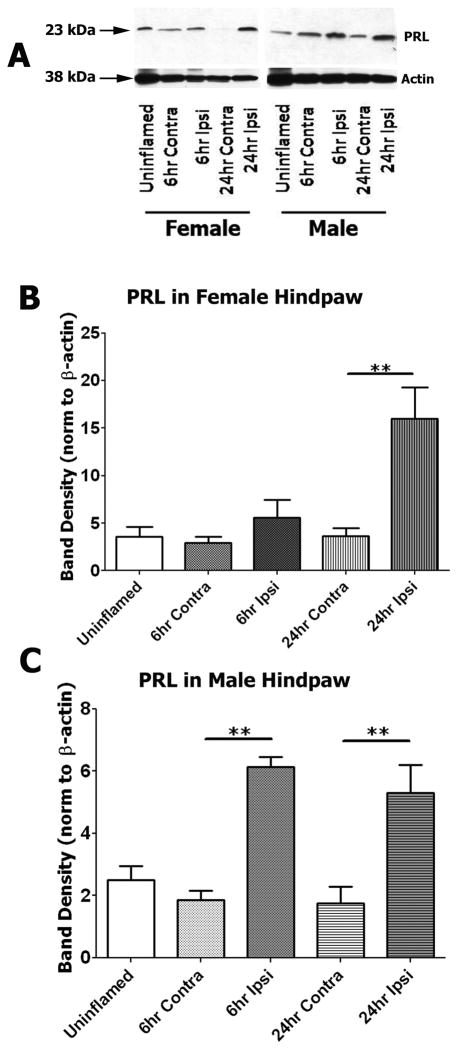
We next employed Western blot analysis to evaluate PRL protein in interstitial fluid collected from uninflamed and inflamed hindpaws of OVX-E and male rats. Western blots indicate that the inflammation-induced PRL is the 23 kDA form of PRL (Fig 3A). Representative bands shown in Figure 3A were obtained with equal sample amount (45 μg) of total protein across wells. The blots were re-probed with β-actin antibody (Fig 3A). Data were analyzed as PRL band densities normalized to β-actin, using one-way ANOVA comparing all groups. Figure 3B and 3C demonstrate that PRL protein is up-regulated by inflammation at 6h (males) and 24h (males and OVX-E females). Normalization against β-actin assumes that β-actin is not regulated by inflammation. Therefore, we also performed a normalization to sample volume. Such normalization demonstrated similar results to the β-actin normalization (data not shown). Altogether, these analyses confirmed a similar pattern of effects to those observed by ELISA, with increased PRL found in the interstitial fluid of inflamed hindpaws from both female and male rats.
Figure 3. Inflammation induces increases in the 23 kDA form of PRL in interstitial fluid.
(A) Representative Western blots show PRL-specific and β-actin bands (23 kDa and 38 kDa; marked with arrows) obtained with interstitial fluid collected from uninflamed and inflamed hindpaws of OVX-E and male rats. 45 μg total protein (2–3.5 μl) was loaded in each well, resolved in a 12.5% SDS-PAGE gel and transferred to a PVDF membrane. The membrane was probed with a specific antibody against rat PRL and β-actin (see “Material and Methods”). (B and C). Bar graphs show the relative amount of PRL protein at 6h and 24h post-CFA time points in interstitial fluid collected from uninflamed (Contra) and inflamed (Ipsi) OVX-E (B) and male (C) rats. PRL protein was quantified as values of band intensities (ImageJ software) normalized to β-actin. One-way ANOVA was carried out for all possible group pairs (For OVX-E, F=9.066; df=4. For males, F=14.54; df=4). Statistical significance between groups is indicated above horizontal bars connecting compared groups. For OVX-E 24h time point, ** p=0.002, t=4.805; n=3–4. For male 6h time point, **p=0.002; t=5.6; n=3–5. For male 24h time point, **p=0.003, t=4.646.
The PRL-R antagonist, Δ1-9-G129R-hPRL, reverses PRL-induced sensitization of rat TRPV1
The actions of a full PRL-R antagonist, Δ1-9-G129R-hPRL, have been characterized for long-term trophic effects of exogenous rat and human PRL in various in vitro cell culture assays, including involvement of Jak/STAT and MAP kinase pathways (Bernichtein et al., 2003; Eyal et al., 2007). This receptor antagonist also successfully inhibited actions triggered by autocrine PRL, thereby assessing the functional impact of the latter in various experimental cell models (Dagvadorj et al., 2007; Eyal et al., 2007). It was previously demonstrated that PRL sensitizes TRPV1-mediated responses (Diogenes et al., 2006). Furthermore, this action of PRL is transient (<5–15 min), which implies that a PRL/PRL-R/TRPV1 pathway in sensory neurons could involve other cellular signaling cascades. Thus, it has been reported that PKC and PI3-kinase can be activated by PRL (Bole-Feysot et al., 1998; Clevenger & Kline, 2001). These kinases are closely involved in the regulation of TRPV1 activities by certain inflammatory mediators (Bonnington & McNaughton, 2003). Therefore, we evaluated the action of Δ1-9-G129R-hPRL on PRL-induced sensitization of the TRPV1 channel.
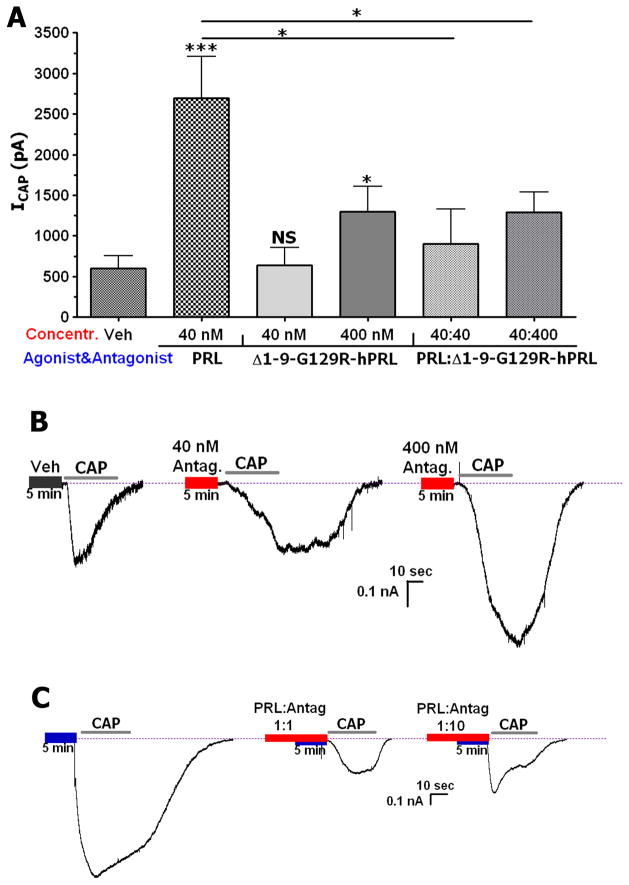
Figure 4A and corresponding traces (Fig 4B) demonstrate that the PRL-R antagonist exhibits weak partial agonistic activity at high (400nM), but not low concentrations (40 nM). We next examined blockade of exogenous PRL’s sensitizing effects by Δ1-9-G129R-hPRL at different ratios of the antagonist to PRL. Figure 4A and representative traces (Fig 4C) illustrate that at a PRL:PRL-R antagonist ratio of 1:1 (40 nM each), Δ1-9-G129R-hPRL almost completely reverses PRL-induced sensitization of the capsaicin-activated current (ICAP). Moreover, at a ratio of 1:10 when the antagonist (400 nM) exhibited partial agonistic properties, Δ1-9-G129R-hPRL still substantially reversed PRL effects. Altogether, our data support the conclusion that Δ1-9-G129R-hPRL can act as an effective antagonist in assays involving acute actions of PRL, which includes PRL-induced sensitization of TRPV1 responses.
Figure 4. Effects of a PRL-R antagonist on PRL-induced sensitization of TRPV1.
(A) Effects of different concentrations of the PRL-R antagonist, Δ1-9-G129R-hPRL, on PRL-induced sensitization of ICAP (100 nM). Sensory neurons were maintained with NGF (100ng/ml) and recorded at 3–5 days. Concentrations of PRL and Δ1-9-G129R-hPRL administered at their molar ratios are indicated. One-way ANOVA with post-hoc Bonferroni correction was performed to compare all groups. Statistical significance obtained using one-way ANOVA is shown by corresponding horizontal bars, or by asterisks above bars to compare with vehicle (F=8.24; df=3). Veh vs 400 nM, *p =0.04; t=2.257; Veh vs 40 nM, ***p<0.001, t=4.726; n=8–12. 40 vs 40:40, *p=0.02; t=3.03. 40 vs 40:400, *p=0.048; t=2.202 (B–C) Representative traces showing voltage clamp recordings of ICAP from TG sensory neurons. Sensory neurons were pre-treated for 5 min with either vehicle (Veh), 40 nM or 400 nM of Δ1-9-G129R-hPRL (B), PRL (40nM) or the indicated ratio of PRL and antagonist (C). The horizontal bar on the x-axis labels indicates application of CAP. Concentrations and ratios of PRL and Δ1-9-G129R-hPRL are noted.
Effects of a PRL-R antagonist on CFA-induced thermal hyperalgesia in OVX-E female and intact male rats
Inflammation by CFA or carrageenan leads to peripheral release of a wide range of inflammatory mediators (Bueno & Fioramonti, 2002; Schaible et al., 2002; Linley et al., 2010). These mediators act at different time points of inflammation (Costello & Hargreaves, 1989; Ferreira et al., 1993a; Ferreira et al., 1993b). Moreover, release of various inflammatory mediators may be sex dependent, as there are differences in inflammatory pain between males and females (Manson, 2010). Here, several questions were addressed. First, we examined whether endogenous PRL could be considered an inflammatory mediator. Second, we evaluated whether PRL acts differently at various time points of inflammation. Third, we investigated whether PRL contributes to inflammatory thermal hyperalgesia in both females and males.
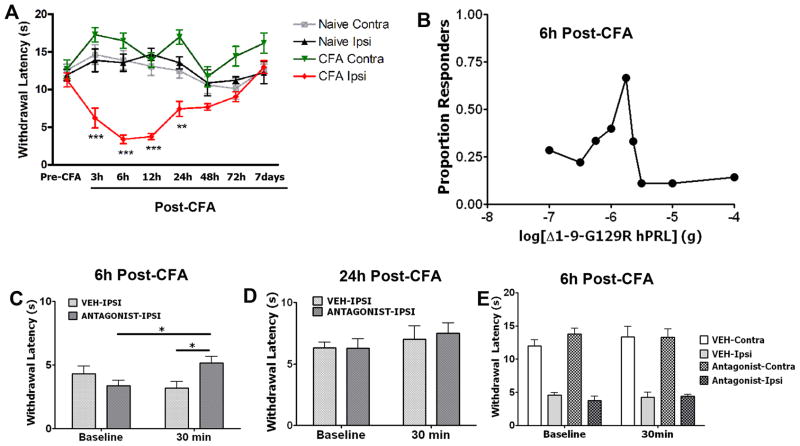
To examine the role of endogenous PRL in CFA-induced inflammatory thermal hyperalgesia, a standard pharmacological approach (Costello & Hargreaves, 1989; Ferreira et al., 1993b; Sommer et al., 1997) utilizing a PRL-R antagonist (Δ1-9-G129R-hPRL) was employed. Figure 5A shows that thermal nociceptive responses peak at 6–24h post-CFA, and then gradually decreases over a 7 day period. Accordingly, we chose to evaluate the anti-hyperalgesic effects of Δ1-9-G129R-hPRL at the 6h and 24h time points. A dose-response relationship in OVX-E rats at the 6h post-CFA time point for the antagonist (0.1–10μg) showed a biphasic relationship with peak effects of the antagonist at the 1.78μg dose (Fig. 5B). Therefore, all subsequent experiments were conducted with the 1.78μg dose of Δ1-9-G129R-hPRL. At the 6h post-CFA time point, CFA-induced thermal hyperalgesia in OVX-E rats was significantly reduced by injection of the 1.78μg Δ1-9-G129R-hPRL (Fig. 5C). In contrast, at the 24h post-CFA time point, inflammatory thermal hyperalgesia in OVX-E rats was not altered upon administration of the antagonist (Fig 5D). Finally, we evaluated whether the reduction in thermal hyperalgesia with the PRL-R antagonist is a locally-mediated effect. The PRL antagonist was only able to reverse CFA-induced thermal hyperalgesia when injected into the local (ipsilateral) hindpaw, but not with systemic administration (Fig. 5E). This finding indicates that the effects of the antagonist on inflammatory heat hyperalgesia in OVX-E female rats are locally-mediated at a site in the inflamed tissue. In summary, our findings demonstrate that endogenous PRL substantially contributes to thermal hyperalgesia at particular points of inflammation in OVX-E female rats.
Figure 5. Effects of the PRL-R antagonist Δ1-9-G129R-hPRL on CFA-induced thermal hyperalgesia in OVX-E female rats.
All experiments presented in this figure were conducted with OVX-E female rats. (A) Time course of CFA-induced thermal hyperalgesia. CFA was injected into Ipsi hindpaws. Naive animals were injected with vehicle. Two-way ANOVA shows statistical differences between pre-CFA recordings and each of 3, 6, 12 and 24h time points (F=14.15; df=6). *** (p <0.001 for 3, 6 and 12h , ** p=0.004 for 24 h time point, n=6). (B) Qualitative dose-response relationship for Δ1-9-G129R-hPRL. Proportion of responders was calculated based on 25% MPE, indicating peak effects of the antagonist with a 1.78μg dose. (C) Withdrawal latency (sec) to radiant heat stimulus 6h following intraplantar administration of CFA. Following testing at 6h post-CFA (marked as baseline), animals were injected with veh or 1.78μg of Δ1-9-G129R-hPRL and withdrawal latencies were assessed 30 min later. Two-way ANOVA results are shown for groups with significant differences by horizontal bars connecting compared groups (F=9.97; df=1). Between groups * (p =0.03; t=2.693; n=6) and within group (p =0.02; t=5.152; n=6). (D) Withdrawal latency to radiant heat stimulus 24h following intraplantar administration of CFA. Following testing at 24h post-CFA (baseline), animals were injected with veh or 1.78μg of Δ1-9-G129R-hPRL and withdrawal latencies were assessed 30 min later. Two-way ANOVA was performed, comparing all groups. n=6–8 (E) Following testing at 6h post-CFA (baseline), vehicle or the PRL-R antagonist were administrated s.c. to the backs of the animals. The withdrawal latencies were measured from Ipsi and Contra hindpaws at 30 min post-antagonist. Two-way ANOVA was performed, comparing all groups. n=6–7.
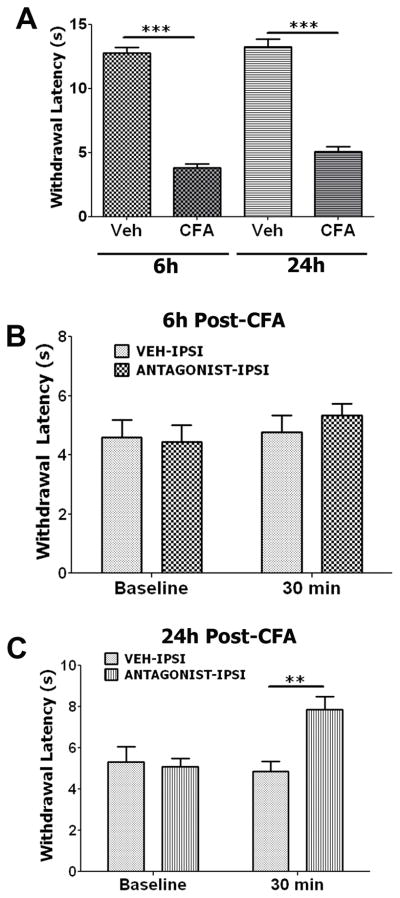
We next examined the role of endogenous PRL in inflammatory thermal hyperalgesia in intact males, as PRL release of a lesser magnitude is also stimulated by inflammation in male rats (Fig 2B). To begin with, we confirmed baseline CFA-induced thermal hyperalgesia in male rats at 6h and 24h post-CFA (Fig 6A). Unlike in OVX-E female rats, endogenous PRL contributes to CFA-induced thermal hyperalgesia at 24h, but not 6h in intact male rats (Fig 6B and 6C). This observation implies that PRL acts as a functional inflammatory mediator at differing time points in males and females.
Figure 6. Effects of the PRL-R antagonist Δ1-9-G129R-hPRL on CFA-induced thermal hyperalgesia in intact male rats.
All experiments presented in this figure were conducted on intact male rats. (A) CFA produces profound thermal hyperalgesia in Ipsi hindpaws at 6h and 24h post-CFA. Unpaired t-test results are shown by horizontal bars connecting groups with statistically significant differences. *** (for 6h, p<0.001; t=17.06; df=6; df=6; n=5–6; and for 24h, p<0.001; t=11.38; df=6; n=5–6). (B) Withdrawal latency (sec) to radiant heat stimulus 6h following intraplantar administration of CFA. Following testing at 6h post-CFA (marked as baseline), animals were injected with veh or 1.78μg of Δ1-9-G129R-hPRL and withdrawal latencies were assessed 30 min later. Two-way ANOVA did not indicate differences between groups. n=4–6. (C) This experiment was conducted at 24h post-CFA, with otherwise identical procedures described in panel B. Two-way ANOVA results are illustrated by horizontal bars connecting groups showing statistically significant differences (F=5.777; df=1). ** p=0.005, t=3.682; n=5–6.
Discussion
Exogenous PRL sensitizes TRPV1-mediated responses in cultured trigeminal sensory neurons (Diogenes et al., 2006). However, the physiological function of endogenous PRL in nociceptive transmission in both females and males had not previously been investigated. The present research addressed the role of endogenous PRL in peripheral inflammatory thermal hyperalgesia in both female and male rats. Initially, we showed that local endogenous PRL can be supplied by a variety of neuronal and non-neuronal cells that contain the PRL protein. Thus, PRL expression was detected in TRPV1-positive sensory neuron fibers, preadipocyte-like cells, and macrophages/monocytes located in the vicinity of nerve fibers. Since large numbers of macrophages/monocytes are observed following CFA-induced inflammation, the expression of PRL within these immune cells represents one possible source for the increased PRL levels observed in inflamed tissue. Secretion of PRL by activated monocytes (macrophages and/or macrophage precursor cells) as early as 6h following CFA injection could support a role for local PRL in contributing to the early inflammatory response. Even though the transcription of PRL by immune cells in the naïve rat has been described as weak or even absent under basal conditions (Ben-Jonathan et al., 2008), this response may increase in the CFA model of inflammation. Indeed, the production and secretion of PRL by activated human macrophages was previously reported (Sabharwal et al., 1992). PRL also regulates the secretion of other inflammatory mediators from immune cells (Wang et al., 1997; Brand et al., 2004). Therefore, increased PRL at this early time point may be important in the initiation of a cascade of immune responses. As a result, PRL possibly contributes indirectly to nociception at later time points, in addition to the rapid direct effects of PRL (within 30 min). Also, given the newly recognized pro-inflammatory functions of adipocytes (Wozniak et al., 2009), it is quite possible that PRL could be involved in this process.
As a next step, we evaluated whether peripheral inflammation regulates the amount of local endogenous PRL in female and male rats. This is a critically important research objective, since PRL would be an unlike contributor to inflammatory hyperalgesia if there is no PRL release initiated by inflammation. The presented data indicate that there is an approximately 3–4 fold increase in PRL levels in inflamed tissue. Although PRL levels are greater in females, the increase in PRL levels post-CFA occurs in both female and male rats. In males, statistically significant differences between the ipsilateral and contralateral hindpaws were observed at the 6–72h post-CFA time points; while in females, differences were evident at the 24–48h post-CFA time points. Taken together, inflammation-induced increases in endogenous local PRL could potentially lead to sensitization of the TRPV1 channel in vivo and contribute to nociceptive responses during inflammation.
Tissue inflammation promotes the generation of a wide range of inflammatory mediators (Ferreira et al., 1993b). They are generated at differing concentrations and time courses from the time when inflammation is initiated (Costello & Hargreaves, 1989; Hargreaves et al., 1989; Hargreaves & Costello, 1990; Ferreira, 1993; Ferreira et al., 1993a; Ferreira et al., 1993b; Asfaha et al., 2002). Accordingly, there might be little-to-no correlation between the actual amount of released PRL and the magnitude of effects on inflammatory thermal hyperalgesia, because this association depends on the amount of other inflammatory mediators that likely vary across time. Thus, in oral surgery patients, bradykinin release was peaked at 3h post-surgery time point. Comparison of released bradykinin levels to concurrent pain revealed a counter clockwise hysteresis, suggesting a delay between peak levels of bradykinin in the effect compartment and pain (Hargreaves & Costello, 1990). Some inflammatory mediators are capable to trigger release of other mediators. Thus, kinins possess the capacity to release a second wave of mediators such as IL-1, IL-8, TNFα, prostaglandins and leukotrienes (Bhoola et al., 1992). PGE2 release in isolated rat dura mater can also be enhanced by ATP (Zimmermann et al., 2002). This chain of events could be reasoning behind delay in peak pain responses and kinin release. Further, the diverse pattern of production for inflammatory mediators can also be sex dependent (Manson, 2010). In this respect, inflammation-induced PRL release could contribute to the difference in male and female CFA-induced thermal hyperalgesia at defined time points. With these points taken into account, it is clear that contributions of inflammation-induced released PRL to CFA-induced thermal hyperalgesia can only be defined by direct experiments.
To identify in vivo roles of released PRL in thermal hyperalgesia, we implemented previously described pharmacological approaches for additional inflammatory mediators (Costello & Hargreaves, 1989; Asfaha et al., 2002). The PRL-R antagonist Δ1-9-G129R-hPRL was selected for these experiments. It was designed as a full competitive antagonist of the human and rat PRL receptor. As such, it was shown to block the effects of endogenous or exogenous PRL in various human cell bioassays involving, among others, Jak/STAT and MAP kinase signaling pathways (Dagvadorj et al., 2007; Eyal et al., 2007). However, nociceptive signaling by PRL is transient (Diogenes et al., 2006) and may recruit other signaling pathways associated with a variety of kinases, such as protein kinase C and PI3-kinase, which can be activated by the PRL-R (Buckley et al., 1988; Goupille et al., 2000). Therefore, to proceed with this PRL-R antagonist, the effects of Δ1-9-G129R-hPRL were evaluated in an assay that is relevant to studying acute effects in thermal hyperalgesia such as the blockade of PRL-induced sensitization of capsaicin responses (Diogenes et al., 2006). The results reflected in Figure 4 suggest that in this particular assay, Δ1-9-G129R-hPRL may act as a partial agonist on rat PRL-R at high (400 nM), but not low (40nM) concentrations. Furthermore, in behavioral experiments, Δ1-9-G129R-hPRL is also effective at defined doses, and the dose-response curve is bell-shaped, which reflects previous findings reported for partial agonists of the PRL-R (Goffin et al., 2005) (Fig 5B). Interestingly, this evidence for a partial agonist effect at the PRL-R is particularly promising given that partial agonists often have fewer side effects than full antagonists, yet still maintain effectiveness for the desired target effect (Hogg & Bertrand, 2007).
Our data demonstrate about a 30% reduction in hyperalgesia following administration of the PRL-R antagonist to OVX-E female rat hindpaws at a 6h post-CFA time point (Fig 5C and 5D), and at the 24h post-CFA time point in intact male rats (Fig 6B and 6C). The anti-hyperalgesic effect of a PRL-R antagonist establishes the relevance of endogenous local PRL in behavioral nociception. However, the relative contribution of neuronal and non-neuronal sources of PRL to nociception has yet to be established. Furthermore, the magnitude of the effect was unexpected since numerous other inflammatory mediators such as arachidonic acid metabolites, bradykinin, prostaglandins, a variety of cytokines and chemokines and growth factors including TNFα, IL-1β, and NGF contribute to thermal hyperalgesia (McMahon et al., 2010). In addition, the present findings demonstrate that endogenous PRL has an anticipated sex-dependent influence on inflammatory thermal hyperalgesia, as released PRL and its receptor function differently in males and females (Yokoyama et al., 2002; Colao et al., 2006; Bernichtein et al., 2010; Jacobson et al., 2010). Of note, the 25–30% magnitude of this effect is generally thought to be predictive of clinically relevant findings (Kelly, 2001; Rowbotham, 2001). Moreover, clinical studies have found increases in PRL levels in painful conditions such as burn injury (Brizio-Molteni et al., 1984; Dugan et al., 2004), migraine headache (Bosco et al., 2008), and breast and prostate cancers (Wennbo & Tornell, 2000; Lissoni et al., 2005). Altogether, these findings support the notion that PRL-R antagonists may have clinical utility in a variety of pain states. Collectively, our findings provide evidence for a novel role of endogenous PRL in behavioral inflammation- induced nociception. In this respect, PRL may be a useful alternative target for analgesic drug development.
Acknowledgments
The authors would like to thank Gaby Helesic, Griffin Perry, Jie Li, Debbie Gass, and Paul Chen for technical assistance. This project was supported by NIH DE17696 to A.N.A and a Canadian NSERC PGS-D award to P.E.S.
References
- Asfaha S, Brussee V, Chapman K, Zochodne DW, Vergnolle N. Proteinase-activated receptor-1 agonists attenuate nociception in response to noxious stimuli. Br J Pharmacol. 2002;135:1101–1106. doi: 10.1038/sj.bjp.0704568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr Rev. 2008;29:1–41. doi: 10.1210/er.2007-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects. Endocr Rev. 1996;17:639–669. doi: 10.1210/edrv-17-6-639. [DOI] [PubMed] [Google Scholar]
- Bernichtein S, Kayser C, Dillner K, Moulin S, Kopchick JJ, Martial JA, Norstedt G, Isaksson O, Kelly PA, Goffin V. Development of pure prolactin receptor antagonists. J Biol Chem. 2003;278:35988–35999. doi: 10.1074/jbc.M305687200. [DOI] [PubMed] [Google Scholar]
- Bernichtein S, Touraine P, Goffin V. New concepts in prolactin biology. J Endocrinol. 2010;206:1–11. doi: 10.1677/JOE-10-0069. [DOI] [PubMed] [Google Scholar]
- Bhoola KD, Elson CJ, Dieppe PA. Kinins--key mediators in inflammatory arthritis? Br J Rheumatol. 1992;31:509–518. doi: 10.1093/rheumatology/31.8.509. [DOI] [PubMed] [Google Scholar]
- Bletsa A, Berggreen E, Fristad I, Tenstad O, Wiig H. Cytokine signalling in rat pulp interstitial fluid and transcapillary fluid exchange during lipopolysaccharide-induced acute inflammation. J Physiol. 2006;573:225–236. doi: 10.1113/jphysiol.2006.104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco D, Belfiore A, Fava A, De Rose M, Plastino M, Ceccotti C, Mungari P, Iannacchero R, Lavano A. Relationship between high prolactine levels and migraine attacks in patients with microprolactinoma. J Headache Pain. 2008;9:103–107. doi: 10.1007/s10194-008-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin JM, Jolicoeur C, Okamura H, Gagnon J, Edery M, Shirota M, Banville D, Dusanter-Fourt I, Djiane J, Kelly PA. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988;53:69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- Brand JM, Frohn C, Cziupka K, Brockmann C, Kirchner H, Luhm J. Prolactin triggers pro-inflammatory immune responses in peripheral immune cells. Eur Cytokine Netw. 2004;15:99–104. [PubMed] [Google Scholar]
- Brizio-Molteni L, Molteni A, Warpeha RL, Angelats J, Lewis N, Fors EM. Prolactin, corticotropin, and gonadotropin concentrations following thermal injury in adults. J Trauma. 1984;24:1–7. doi: 10.1097/00005373-198401000-00001. [DOI] [PubMed] [Google Scholar]
- Buckley AR, Crowe PD, Russell DH. Rapid activation of protein kinase C in isolated rat liver nuclei by prolactin, a known hepatic mitogen. Proc Natl Acad Sci U S A. 1988;85:8649–8653. doi: 10.1073/pnas.85.22.8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno L, Fioramonti J. Visceral perception: inflammatory and non-inflammatory mediators. Gut. 2002;51(Suppl 1):i19–23. doi: 10.1136/gut.51.suppl_1.i19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Clevenger CV, Kline JB. Prolactin receptor signal transduction. Lupus. 2001;10:706–718. doi: 10.1191/096120301717164949. [DOI] [PubMed] [Google Scholar]
- Colao A, Di Sarno A, Guerra E, De Leo M, Mentone A, Lombardi G. Drug insight: Cabergoline and bromocriptine in the treatment of hyperprolactinemia in men and women. Nat Clin Pract Endocrinol Metab. 2006;2:200–210. doi: 10.1038/ncpendmet0160. [DOI] [PubMed] [Google Scholar]
- Costello AH, Hargreaves KM. Suppression of carrageenan-induced hyperalgesia, hyperthermia and edemaby a bradykinin antagonist. Eur J Pharmacol. 1989;171:259–263. doi: 10.1016/0014-2999(89)90118-0. [DOI] [PubMed] [Google Scholar]
- Dagvadorj A, Collins S, Jomain JB, Abdulghani J, Karras J, Zellweger T, Li H, Nurmi M, Alanen K, Mirtti T, Visakorpi T, Bubendorf L, Goffin V, Nevalainen MT. Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway. Endocrinology. 2007;148:3089–3101. doi: 10.1210/en.2006-1761. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, Hargreaves KM. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci. 2006;26:8126–8136. doi: 10.1523/JNEUROSCI.0793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan AL, Malarkey WB, Schwemberger S, Jauch EC, Ogle CK, Horseman ND. Serum levels of prolactin, growth hormone, and cortisol in burn patients: correlations with severity of burn, serum cytokine levels, and fatality. J Burn Care Rehabil. 2004;25:306–313. doi: 10.1097/01.bcr.0000124785.32516.cb. [DOI] [PubMed] [Google Scholar]
- Eyal O, Jomain JB, Kessler C, Goffin V, Handwerger S. Autocrine prolactin inhibits human uterine decidualization: a novel role for prolactin. Biol Reprod. 2007;76:777–783. doi: 10.1095/biolreprod.106.053058. [DOI] [PubMed] [Google Scholar]
- Ferreira SH. The role of interleukins and nitric oxide in the mediation of inflammatory pain and its control by peripheral analgesics. Drugs. 1993;46(Suppl 1):1–9. doi: 10.2165/00003495-199300461-00003. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Lorenzetti BB, Cunha FQ, Poole S. Bradykinin release of TNF-alpha plays a key role in the development of inflammatory hyperalgesia. Agents Actions. 1993a;38:C7–9. doi: 10.1007/BF01991120. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Lorenzetti BB, Poole S. Bradykinin initiates cytokine-mediated inflammatory hyperalgesia. Br J Pharmacol. 1993b;110:1227–1231. doi: 10.1111/j.1476-5381.1993.tb13946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin V, Bernichtein S, Touraine P, Kelly PA. Development and potential clinical uses of human prolactin receptor antagonists. Endocr Rev. 2005;26:400–422. doi: 10.1210/er.2004-0016. [DOI] [PubMed] [Google Scholar]
- Goupille O, Barnier JV, Guibert B, Paly J, Djiane J. Effect of PRL on MAPK activation: negative regulatory role of the C-terminal part of the PRL receptor. Mol Cell Endocrinol. 2000;159:133–146. doi: 10.1016/s0303-7207(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Costello A. Glucocorticoids suppress levels of immunoreactive bradykinin in inflamed tissue as evaluated by microdialysis probes. Clin Pharmacol Ther. 1990;48:168–178. doi: 10.1038/clpt.1990.132. [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Dubner R, Costello AH. Corticotropin releasing factor (CRF) has a peripheral site of action for antinociception. Eur J Pharmacol. 1989;170:275–279. doi: 10.1016/0014-2999(89)90550-5. [DOI] [PubMed] [Google Scholar]
- Henry MA, Freking AR, Johnson LR, Levinson SR. Increased sodium channel immunofluorescence at myelinated and demyelinated sites following an inflammatory and partial axotomy lesion of the rat infraorbital nerve. Pain. 2006;124:222–233. doi: 10.1016/j.pain.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Bertrand D. Partial agonists as therapeutic agents at neuronal nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;73:459–468. doi: 10.1016/j.bcp.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Jacobson EM, Hugo ER, Tuttle TR, Papoian R, Ben-Jonathan N. Unexploited therapies in breast and prostate cancer: blockade of the prolactin receptor. Trends Endocrinol Metab. 2010;21:691–698. doi: 10.1016/j.tem.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid WIN 55,212-2 regulates TRPV1 phosphorylation in sensory neurons. J Biol Chem. 2006;281:32879–32890. doi: 10.1074/jbc.M603220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Kang S, Wu C, Banik RK, Brennan TJ. Effect of capsaicin treatment on nociceptors in rat glabrous skin one day after plantar incision. Pain. 2010;148:128–140. doi: 10.1016/j.pain.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18:205–207. doi: 10.1136/emj.18.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiasalari Z, Salehi I, Zhong Y, McMahon SB, Michael-Titus AT, Michael GJ. Identification of perineal sensory neurons activated by innocuous heat. J Comp Neurol. 2010;518:137–162. doi: 10.1002/cne.22187. [DOI] [PubMed] [Google Scholar]
- Lichtman AH. The up-and-down method substantially reduces the number of animals required to determine antinociceptive ED50 values. J Pharmacol Toxicol Methods. 1998;40:81–85. doi: 10.1016/s1056-8719(98)00041-0. [DOI] [PubMed] [Google Scholar]
- Linley JE, Rose K, Ooi L, Gamper N. Understanding inflammatory pain: ion channels contributing to acute and chronic nociception. Pflugers Arch. 2010;459:657–669. doi: 10.1007/s00424-010-0784-6. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Bignami A, Frontini L, Manganini V, Dapretto E, Gardani GS, Vigano P, Strada G. Possible involvement of prolactin in endocrine-resistant metastatic prostate cancer. Int J Biol Markers. 2005;20:123–125. [PubMed] [Google Scholar]
- Mannino CA, South SM, Inturrisi CE, Quinones-Jenab V. Pharmacokinetics and effects of 17beta-estradiol and progesterone implants in ovariectomized rats. J Pain. 2005;6:809–816. doi: 10.1016/j.jpain.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Manson JE. Pain: sex differences and implications for treatment. Metabolism. 2010;59(Suppl 1):S16–20. doi: 10.1016/j.metabol.2010.07.013. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Bennett DLH, Bevan S. Inflammatory mediators and modulators of pain. In: Wall PMR, editor. Textbook of Pain (online) 2010. [Google Scholar]
- Pare M, Albrecht PJ, Noto CJ, Bodkin NL, Pittenger GL, Schreyer DJ, Tigno XT, Hansen BC, Rice FL. Differential hypertrophy and atrophy among all types of cutaneous innervation in the glabrous skin of the monkey hand during aging and naturally occurring type 2 diabetes. J Comp Neurol. 2007;501:543–567. doi: 10.1002/cne.21262. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC. What is a “clinically meaningful” reduction in pain? Pain. 2001;94:131–132. doi: 10.1016/S0304-3959(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Sabharwal P, Glaser R, Lafuse W, Varma S, Liu Q, Arkins S, Kooijman R, Kutz L, Kelley KW, Malarkey WB. Prolactin synthesized and secreted by human peripheral blood mononuclear cells: an autocrine growth factor for lymphoproliferation. Proc Natl Acad Sci U S A. 1992;89:7713–7716. doi: 10.1073/pnas.89.16.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible HG, Ebersberger A, Von Banchet GS. Mechanisms of pain in arthritis. Ann N Y Acad Sci. 2002;966:343–354. doi: 10.1111/j.1749-6632.2002.tb04234.x. [DOI] [PubMed] [Google Scholar]
- Schreyer DJ, Skene JH. Fate of GAP-43 in ascending spinal axons of DRG neurons after peripheral nerve injury: delayed accumulation and correlation with regenerative potential. J Neurosci. 1991;11:3738–3751. doi: 10.1523/JNEUROSCI.11-12-03738.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C, Schmidt C, George A, Toyka KV. A metalloprotease-inhibitor reduces pain associated behavior in mice with experimental neuropathy. Neurosci Lett. 1997;237:45–48. doi: 10.1016/s0304-3940(97)00813-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, O’Neal KD, Yu-Lee L. Multiple prolactin (PRL) receptor cytoplasmic residues and Stat1 mediate PRL signaling to the interferon regulatory factor-1 promoter. Mol Endocrinol. 1997;11:1353–1364. doi: 10.1210/mend.11.9.9982. [DOI] [PubMed] [Google Scholar]
- Wennbo H, Tornell J. The role of prolactin and growth hormone in breast cancer. Oncogene. 2000;19:1072–1076. doi: 10.1038/sj.onc.1203349. [DOI] [PubMed] [Google Scholar]
- Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci. 2009;54:1847–1856. doi: 10.1007/s10620-008-0585-3. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Schwacha MG, Samy TS, Bland KI, Chaudry IH. Gender dimorphism in immune responses following trauma and hemorrhage. Immunol Res. 2002;26:63–76. doi: 10.1385/ir:26:1-3:063. [DOI] [PubMed] [Google Scholar]
- Yuan ZF, Pan JT. Involvement of angiotensin II, TRH and prolactin-releasing peptide in the estrogen-induced afternoon prolactin surge in female rats: studies using antisense technology. Life Sci. 2002;71:899–910. doi: 10.1016/s0024-3205(02)01773-3. [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Reeh PW, Averbeck B. ATP can enhance the proton-induced CGRP release through P2Y receptors and secondary PGE(2) release in isolated rat dura mater. Pain. 2002;97:259–265. doi: 10.1016/S0304-3959(02)00027-1. [DOI] [PubMed] [Google Scholar]