Abstract
Objective
Blood C-Reactive Protein (CRP) is routinely measured to gauge inflammation and in rheumatoid arthritis (RA), heightened CRP is predictive of a poor outcome and lowered CRP indicative of a positive response to therapy. CRP interacts with the innate and adaptive immune systems in ways that suggest it may be causal in RA and, although this is not proven, it is widely assumed CRP makes a detrimental contribution to the disease process. Paradoxically, animal studies indicate CRP might be beneficial in RA.
Methods
We compared the impact of CRP deficiency versus transgenic over-expression on the inflammatory and immune responses using CRP deficient mice (Crp−/−) versus human CRP transgenic mice (CRPTg), respectively, and we compared the susceptibility of wild type, Crp−/−, and CRPtg to collagen-induced arthritis (CIA), a disease that resembles RA in humans.
Results
CRP deficiency significantly altered the inflammatory cytokine response evoked by challenge with endotoxin or anti-CD3 antibody, and heightened some immune responses. Compared to CIA in wild type mice, CIA in Crp−/− progressed more rapidly and was more severe whereas CIA in CRPTg was dramatically attenuated. Despite these disparate clinical outcomes, anti-collagen autoantibody responses during CIA did not differ among the genotypes.
Conclusion
CRP exerts an early and beneficial effect in mice with CIA. The mechanism of this effect remains unknown but does not involve improvement of the autoantibody profile. In humans the presumed detrimental role of heightened blood CRP during active RA might be balanced by a beneficial effect of baseline CRP manifest during the pre-clinical stages of disease.
Rheumatoid arthritis (RA) is a chronic, debilitating disease characterized by systemic inflammation and erosive destruction of the joints [1,2]. The hands and feet are the most commonly affected sites, but the disease can affect other joints such as the elbow, shoulder, knee, and hip [3]. Several theories have been proposed to explain the underlying mechanisms of RA, but none has been universally accepted nor conclusively demonstrated. Since the discovery of rheumatoid factor (RF; antibodies against the Fc portion of immunoglobulin G) it has been postulated that RA is an autoimmune disease [1]. It is thought that RF interacting with the Fc portion of IgG promotes formation of immune complexes that activate the complement system and bind to various Fc receptors (FcRs), thereby contributing to inflammation associated with RA [1,2, 4]. In concert with the autoimmune model, various kinds of inflammatory cells (macrophages, dendritic cells, etc) infiltrate the synovium of patients with RA [1,2] and are also thought to exert influence on the disease’s onset and clinical course. A critical role of T-cells is postulated – their interaction with macrophages, fibroblasts, and other cells thought to contribute to the production of deleterious cytokines (eg. IL-2, IL-4, IL-10, and IFN-γ) [1].
C-reactive protein (CRP) is a widely used blood marker of inflammation [5], and growing evidence indicates it plays an active role in host defense [6] and certain cardiovascular diseases [7]. It has long been recognized that in RA patients the concentration of CRP in the blood correlates positively with disease severity and progression [8]. Like RF, CRP can form immune complexes that activate complement [9, 10] and bind to FcRs [11, 12], so it is not unreasonable to predict CRP also participates in the RA disease process. Indeed, although many of CRP’s functions arguably are effected in the fluid phase [13], CRP is found within the arthritic joint [13, 14] and synovial fluid [15], and its presence there can be used to differentiate inflammatory from non-inflammatory arthritis [15]. CRP blood level has also been incorporated into clinical algorithms used to measure RA disease activity [16]. Despite all of this “guilt by association” still little is known about the biology of CRP in the context of arthritis. In fact no human study to date has directly investigated the contribution of CRP to RA, and the animal studies performed so far have had mixed results. For instance early studies of experimentally induced arthritis in rabbits established that the serum was the source of synovial CRP [17], and that intra-articular injection of (rabbit) CRP elevated knee joint temperature if arthritis was present but not if the joint was healthy [18]. These findings, pointing to CRP as a potentiator of already existing inflammation in RA, are in alignment with the clinical observations. In contrast, a more recent study of experimentally induced arthritis using rabbit CRP transgenic mice [19] showed that (rabbit) CRP was protective with the protective effect being exerted during a short time at the very beginning of disease initiation. The potential relevancy of this observation to the pre-clinical stages of RA has still not been investigated.
To gain new evidence for a contribution of CRP to RA, for the first time we used human CRP transgenic mice (CRPTg) [20, 21] in tandem with a newly engineered CRP deficient strain (Crp−/−) [this report] to examine the strength and direction of CRP’s contribution to inflammation, immunity, and collagen-induced arthritis (CIA). We found that compared to wild type mice; (a) Crp−/− expressed less blood TNF-α and IL-10 and more IL-6 following i.p. endotoxin challenge and less blood IFNγ, IL-2, and IL-4 after i.v. injection of anti-CD3 antibody, (b) Crp−/− had an enhanced antibody response to vaccination with the thymus independent antigen TNP-ficoll, and (c) Crp−/− exhibited more rapid clinical progression and more severe clinical symptoms of CIA. Conversely, CRPTg, which we previously showed make less IL-10 [22], had a weaker immune response to TNP-ficoll, mounted a robust human CRP acute phase response during the inductive phase of CIA, and had only mild symptoms of disease. Surprisingly, the aggressiveness of CIA in Crp−/− and its mildness in CRPTg was not because of heightened or depressed autoantibody responses, respectively, as the anti-collagen II responses of Crp−/− and CRPTg were not different from that of wild type. We do not dispute that CRP might have a detrimental effect during active RA, but we caution that this can only be achieved in the context of already established disease. Our new observations, which align best with earlier ones made by others [19], indicate that CRP is probably beneficial during the early stages of experimentally induced arthritis in mice and during the pre-clinical stage of RA in humans.
Materials and Methods
Animals
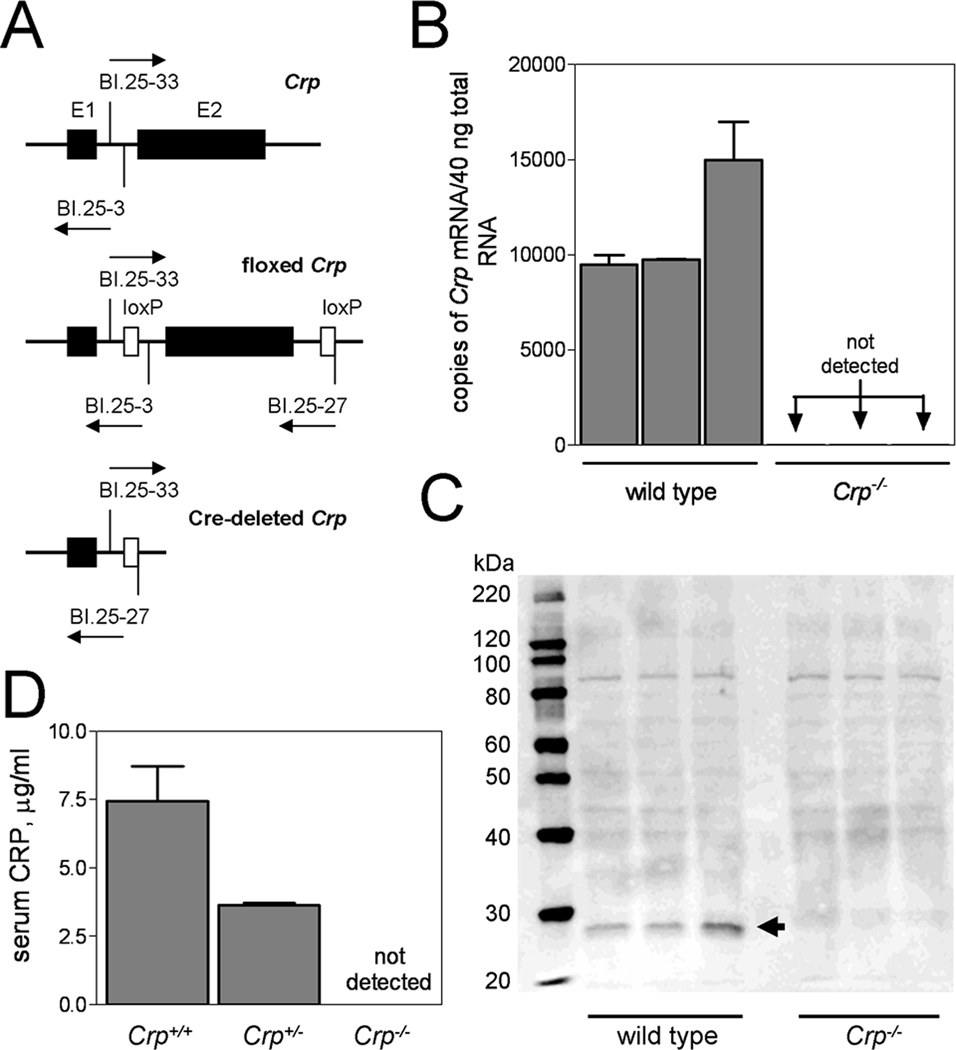
Transgenic mice carrying lox P targeted Crp alleles (floxed Crp), wherein exon 2 of Crp was flanked by lox P sites (Fig. 1A), were generated using a conditional targeting vector derived using the Lambda KOS system (Lexicon Pharmaceuticals, Inc.). Floxed Crp mice were mated with protamine-Cre recombinase transgenic mice to generate hybrid offspring carrying one Cre-deleted Crp allele (Fig. 1A). These were intercrossed to generate CRP deficient mice (Crp−/−) and backcrossed to the C57BL/6 strain (The Jackson Laboratories). Quantitative real-time PCR (qRT-PCR) and Western blot assays performed using standard methods confirmed absence of CRP mRNA (Fig 1B) and protein (Fig 1C) in Crp−/−. ELISA done on acute-phase sera (collected 24hr after i.p. injection of endotoxin) confirmed absence of blood CRP in Crp−/− and approximately 50% reduction of CRP in Crp+/− (Fig 1D). The human CRP transgene, its detection by PCR, and its human-like expression in CRPTg (also strain C57BL/6) have been fully described elsewhere [20,21]. Human CRP is present in the blood of CRPTg at concentrations relevant to humans [5] i.e., low levels under steady-state conditions (<1 to 30 µg/ml) and high levels during an endotoxemia or infection-induced acute phase response (100 to 500 µg/ml). Mouse CRP is still expressed in CRPTg, but mouse CRP is not a major acute phase protein [23]. For certain experiments Crp−/− were reconstituted (by breeding) with CRPTg to generate mice that express only the human form of CRP.
Figure 1. Targeted deletion of the mouse Crp gene.
CRP deficient mice were generated by Cre-Lox recombination wherein exon 2 (E2) of the Crp gene was flanked by loxP sites to direct Cre-recombinase mediated deletion (A). The arrows indicate positions and directions of elongation of sense and anti-sense primers (BI.25-3, BI.25-33, BI.25-27) used to discriminate Crp from floxed Crp and Cre-deleted Crp. The resultant CRP deficient mice (Crp−/−) do not express Crp mRNA (B; quantitative real-time PCR of liver total RNA from 3 wild type mice and 3 Crp−/− mice) nor do they express the CRP protein (C; Western blot of plasma proteins from mice shown in panel B and D; ELISA done on sera from n = 5 mice of the indicated genotype). The arrow in panel C points to the position of migration of the mouse CRP monomer.
Mice were housed at constant humidity (60 ± 5%) and temperature (24 ± 1°C) with a 12 hour light cycle (6 AM to 6 PM), and maintained ad libitum on sterile bottled water and regular chow (Harlan Teklad). Mice were 8–12 weeks old when used and both sexes were combined unless specifically noted. All animal use protocols were approved by the Institutional Animal Care and Use Committees at the University of Alabama at Birmingham and Boehringer Ingelheim Pharmaceuticals and were consistent with the Guide for the Care and Use of Laboratory Animals (NIH publication 96-01, revised 1996).
Measurement of inflammatory and immune responses
To cause sterile peritonitis, 0.5 ml of zymosan A (Sigma-Aldrich; 2 mg/ml in sterile 0.9% NaCl) was injected i.p. into each animal. Mice were sacrificed 4 h later and their peritoneal cavities lavaged with ice-cold phosphate buffered saline (PBS). The total leukocyte count was determined for the peritoneal exudates and differential cell counts performed using Giemsa-stained cytospin preparations. Aseptic endotoxemia was induced by i.v administration of 200 µl pyrogen-free 0.9% NaCl containing 200 ng Escherichia coli lipopolysaccharide (LPS) serotype 055:B5 (Sigma) plus 1 mg D-galactosamine (D-GalN; Sigma). One hour later mice were anesthetized and blood collected. To activate T-cells, mice received 10 µg of hamster anti-mouse CD3 antibody (eBioscience) i.p., and blood was collected 3 hours later. To measure the delayed-type hypersensitivity (DTH) response, mice were immunized s.c. with 100 µg of ovalbumin (OVA fraction V) emulsified with 50 µl complete Freund's adjuvant (both from Sigma) in 50 µl. Six days later, each mouse was challenged by injection (into one ear pinnae) of 200 µg OVA in 10 µl of saline. The DTH response was assessed by measuring the thickness of the ear prior to and 24 h after challenge using an engineer’s micrometer (Mitutoyo 2804F-10); the difference between the two measurements giving an index of ear swelling. Carrageenan induced paw edema was induced by giving an intraplantar injection of 0.3% carrageenan (Sigma) in a 20 ul volume into the left hind paw using a 26 gauge needle. Paw volume was measured by determining fluid displacement upon immersion in water, and the difference in volume measured prior to and 3 h following carrageenan administration is reported. To measure the antibody response mice were immunized via i.p. injection of 10 ug TNP-Ficoll (Biosearch Technologies), and pre- and post-immunization plasma was obtained to determine anti-TNP antibody titers. The methodology used to measure in vitro proliferative responses and IFNγ–producing ability of isolated mixed splenocytes, induction of dinitrofluorobenzene (DNFB) and fluorescein isothiocyanate (FITC) induced contact hypersensitivity (CH), and induction of cutaneous anaphylaxis are detailed in the Supplementary Information.
Collagen-induced arthritis
Collagen-induced arthritis was elicited using a previously described protocol [24]. Briefly, complete Freund’s adjuvant (CFA) containing 4mg/ml Mycobacterium tuberculosis (M.tb) was emulsified 1:1 with a 4 mg/ml solution of chicken type-II collagen (CII). CFA and CII were from Chondrex, Inc. (Redmond, WA). At the start of each experiment (day 0), 100 µl of a freshly prepared emulsion was injected intradermally using a 23 gauge needle at a site toward one side of the base of the tail. On day 21, a booster injection (100 µl of CII emulsified in incomplete Freund’s adjuvant, IFA) was administered at a site contralateral to the primary injection site. Thrice weekly thereafter, and until day 50, the clinical signs of arthritis were recorded for each paw. The clinical scoring system we used was described by Brand et al. [25] where 0 = no evidence of erythema and swelling, 1 = erythema and mild swelling confined to the tarsals or ankle joint, 2 = erythema and mild swelling extending from the ankle to the tarsals, 3 = erythema and moderate swelling extending from the ankle to the metatarsal joints, 4 = erythema and severe swelling encompassing the ankle, foot, and digits, or ankylosis of the limb. We used multiple individuals to score mice (NRJ, MAP, AJS) and we verified the accuracy of our scoring by micro computerized tomography (micro-CT) and histological analysis of representative arthritic limbs [26] (see Supplemental Information, Fig S1). For statistical analysis a mouse was considered to have presented with CIA on the day its clinical score reached 2, and a mouse was considered to have full blown CIA only if its symptoms were sustained thereafter. The rate of progression of disease was estimated by calculating the slope of the linear regression of days since clinical presentation (abscissa) versus clinical score (ordinate). This regression was done on data collected for the first week following disease onset.
Measurement of CRP, cytokines, and antibodies
Anti-TNP ELISA used TNP-BSA coated plates (Biosearch Technologies), biotinylated goat anti-mouse IgM (Caltag), and streptavidin-HRP (Caltag). Plasma cytokines were measured using ELISA kits from R&D systems. Serum mouse CRP was measured using the mouse C-Reactive Protein kit from Life Diagnostics, Inc. according to the manufacturer’s instructions, and human CRP was measured using an ELISA developed in our laboratory [21]. The latter does not detect mouse CRP and has a lower limit of detection of approximately 20 ng of human CRP per ml of mouse serum. Anti-CII IgG was measured using ELISA grade CII and mouse anti-CII IgG standards from Chondrex, Inc (Redmond, WA).
Statistical Analysis
All pooled data are expressed as the mean ± SEM, without transformation, and the sample size is given. Group comparisons were done using unpaired Student’s t-tests or with one-way ANOVA followed by post-hoc pairwise least-squared difference (PLSD) tests or Dunnett’s analysis for multiple comparisons. Differences were considered significant when the p value was <0.05. Statistical analyses were performed using Graphpad Prism 3.02 or Statview 5.0.1.
Results
CRP deficiency alters inflammatory and immune responsiveness
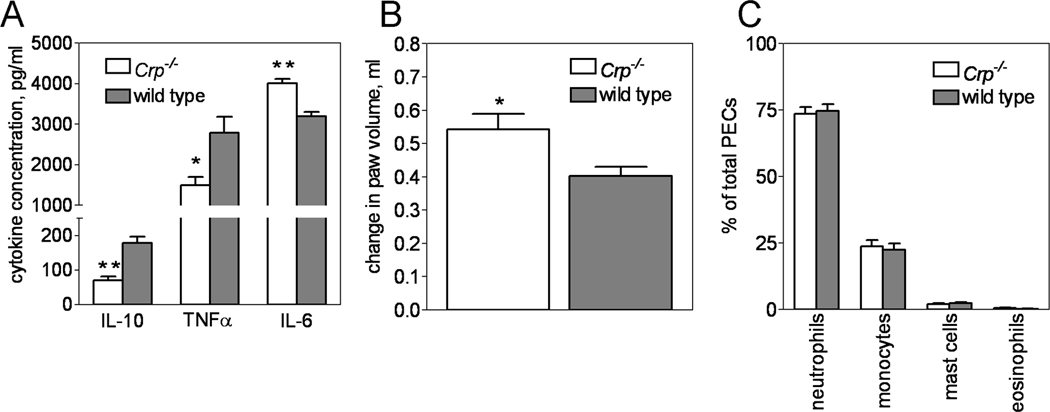
Crp deletion altered the inflammatory response to endotoxin challenge; Crp−/− having a significantly weakened serum IL-10 and TNFα response compared to wild type (71 ± 11 versus 179 ± 17 pg/mL IL-10 and 1498 ± 201 versus 2786 ± 395 pg/mL TNF-α, respectively) and a significantly strengthened IL-6 response (4007 ± 117 versus 3193 ± 109 pg/mL IL-6, respectively) (Fig. 2A). Carrageenan induced paw inflammation was also affected, being significantly worsened in Crp−/− compared to wild type (0.54 ± 0.05 ml of swelling versus 0.40 ± 0.03 ml, respectively) (Fig 2B). However, Crp deletion had no effect on the total number of inflammatory cells recruited to the body cavity during zymosan-induced peritonitis (data not shown) nor the proportion of each type of inflammatory cell (neutrophils, monocytes, mast cells, eosinophils) recruited to the body cavity (Fig 2 C). Likewise, deletion of Crp had no effect on FITC or DNFB induced contact hypersensitivity and OVA induced active cutaneous anaphylaxis (Supplementary Information, Fig S2). These results suggest that under certain circumstances, mouse CRP dampens the inflammatory response.
Figure 2. Targeted deletion of mouse Crp alters the inflammatory response.
Compared to wild type mice (dark bars), Crp−/− mice (open bars) had significantly less serum IL-10 and TNF-α and significantly more serum IL-6 after endotoxin challenge (A; n = 23 mice of each genotype. Note break in y-axis.) and a significantly greater inflammatory response to carageenan injection (B; n = 13 Crp−/− and n = 20 wild type). In contrast, recruitment of peritoneal exudates cells (PECs) into the peritoneal cavity during zymosan-induced peritonitis was unaffected. (C; n = 12 mice per genotype). The results shown in each panel are from 2 separate experiments. In panel A, * and ** indicates p < 0.006 and p < 0.0001, respectively, for PLSD tests. In panel B, * indicates p = 0.01 for Students t-test
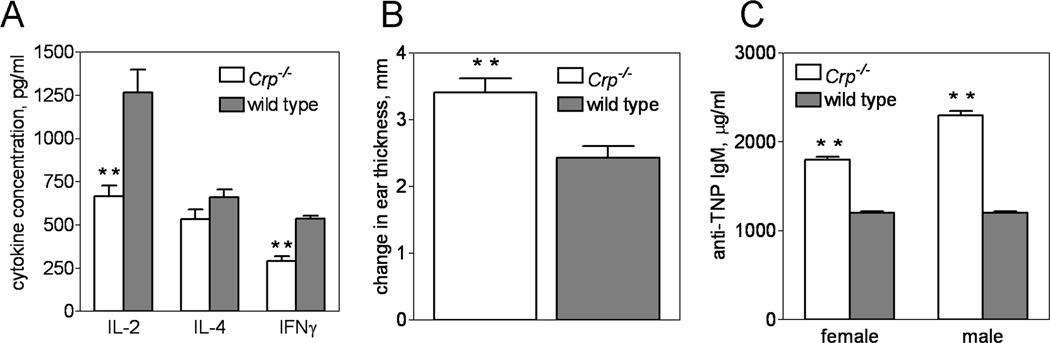
For mice treated with a T cell targeting anti-CD3 antibody, the plasma IL-2 and IFN-γ responses of Crp−/− were significantly impaired compared to wild type (666 ± 63 versus 1267 ± 134 pg/mL IL-2 and 293 ± 28 versus 538 ± 16 pg/mL IFN-γ, respectively) (Fig. 3A), whereas the IL-4 response was not. The impaired production of these cytokines in Crp−/− given anti-CD3 was likely due to a trans-effect of FcγR-bearing cells, and not because of an intrinsic defect in T cells, as stimulation of Crp−/− mixed splenocytes with anti-CD3 in vitro induced less proliferation and less IFN-γ production than wild type splenocytes only if the anti-CD3 was presented in soluble form (see Supplementary Information, Fig S3). Further, T cell proliferation and cytokine production by Crp−/− versus wild type splenocytes did not differ if splenocytes were directly stimulated with the superantigen SEB or the mitogen ConA (see Supplementary Information, Fig. S3). In accordance with this interpretation, Crp deletion significantly strengthened the DTH response to OVA (Fig. 3B), a response thought to be T cell initiated but macrophage driven [27]. Interestingly, the antibody response of Crp−/− immunized with the thymus independent antigen TNP-Ficoll was significantly stronger than that of wild type (Fig. 3C) whereas that of CRPTg was weaker (Fig. S4). These results suggest that under certain circumstances mouse CRP can influence both T cell and B cell responses, albeit indirectly.
Figure 3. Targeted deletion of Crp alters the immune response.
The serum cytokine response to i.p. administered anti-CD3 antibody was reduced for Crp−/− (open bars) compared to wild type (dark bars) (A; n = 21 mice per genotype), whereas the ovalbumin induced delayed-type hypersensitivity response (B; n = 10 mice per genotype) and the TNP-Ficoll elicited anti-TNP antibody response (C; IgM antibodies measured on day 7 after immunization, n = 4 females and 5 males of each genotype) were significantly enhanced. The results shown in each panel are from 2 separate experiments. **; p < 0.002 for PLSD tests (A, C) or t-test (B).
CRP deficiency exacerbates CIA
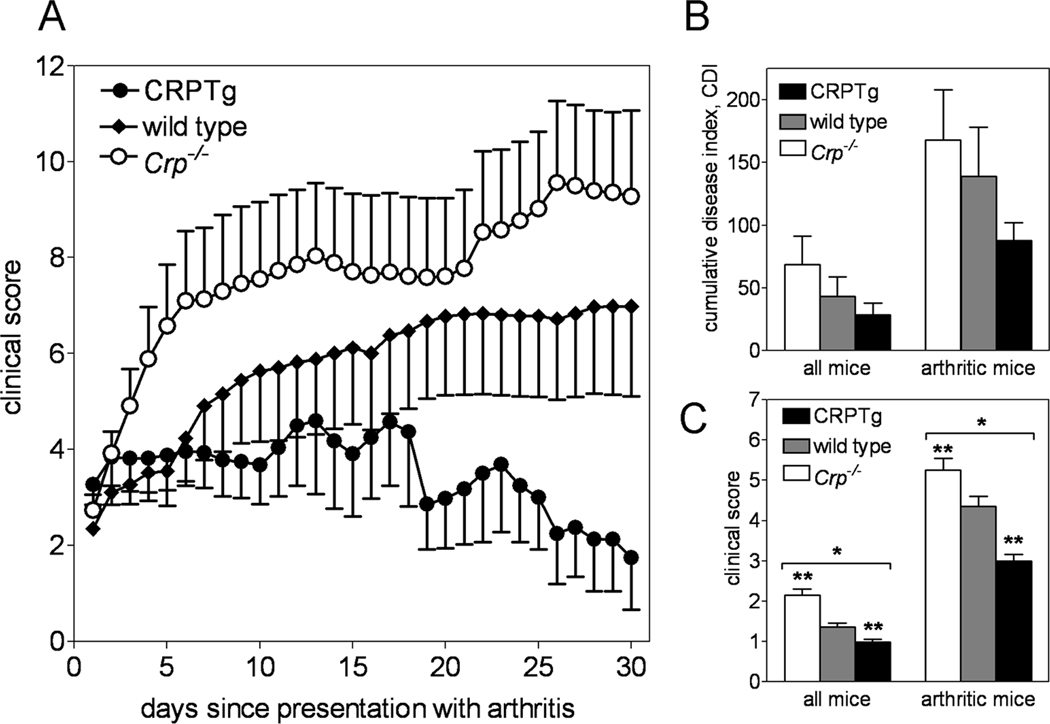
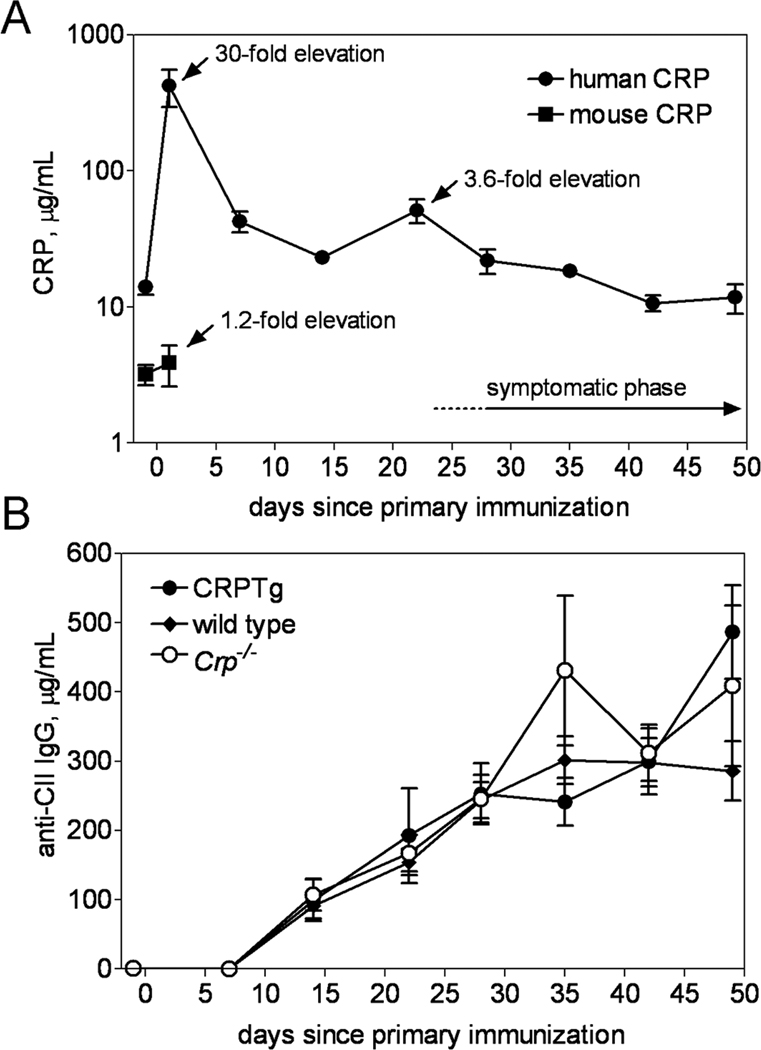
Given the observed pleiotropic effects of mouse CRP deficiency and human CRP expression on inflammatory and immune responses in mice, the clinical data linking rise and fall of blood CRP to worsened and improved RA severity, respectively, and the paradoxical reports of CRP mediated benefits in mouse models of RA, we investigated the impact mouse CRP deficiency and human CRP excess each have on the incidence, onset, and progression of CIA. Micro-CT and histopathological analysis (Fig. S1) revealed gross pathology that was typical of mouse CIA, with no readily observable differences among the genotypes. Likewise incidence of CIA was similar among the 3 genotypes and its clinical onset was uniformly manifest at approximately 4 weeks after induction (Table 1). Importantly however, following its clinical presentation the progression of CIA was fastest (Table 1, Fig 4A) and its severity was greatest (Fig. 4, B and C) for Crp−/−. Conversely for CRPTg, in which elevation of human CRP well above baseline values was observed (Fig. 5A), the tempo of CIA was slowest (Table 1, Fig 4A) and its severity lowest among the three genotypes (Table 1, Fig. 4,A–C). Importantly, an experiment done using Crp−/− mice reconstituted (by breeding) with the human Crp transgene showed that replenishment with human CRP restores resistance to CIA (Fig S5). Despite the disparate effects, the anti-CII IgG autoantibody responses of wild type, Crp−/−, and CRPTg were not different (Fig. 5B). These findings show that CRP exerts a significant and beneficial effect on the development of arthritis in mice – an effect seemingly unrelated to the autoantibody response.
Table I.
Clinical traits of collagen-induced arthritis in CRP deficient (Crp−/−), wild type, and human CRP transgenic (CRPTg) mice.
| n | Onset (mean ± sem days) |
Incidence % |
progression* (slope, r2) |
|
|---|---|---|---|---|
| Crp−/− | 27 | 28.18 ± 2.53 | 40.7 | 0.757, 0.945 |
| wild type | 36 | 28.18 ± 1.87 | 30.8 | 0.365, 0.924 |
| CRPTg | 25 | 27.50 ± 2.35 | 32.0 | 0.083, 0.570 |
The slope and r2 value for the linear regression of days since presentation with arthritis versus clinical score, calculated for days 1 through 7 (see Figure 4A).
Figure 4. CIA is exacerbated by targeted deletion of the mouse Crp gene and dampened by transgene-expressed human CRP.
CIA was induced in wild type (♦, n = 36), Crp−/− (○, n = 27), and CRPTg (●, n = 25) mice and arthritis symptoms were monitored for 50 days, as described in the Materials and Methods. Following initial clinical presentation, Crp−/− had the most rapid progression of disease, whereas disease was nearly static for CRPTg (A; see also Table 1). Furthermore compared to wild type mice, disease symptoms were significantly worse in Crp−/− (open bars) and significantly better for CRPTg (black bars) than for wild type (gray bars) (B and C). The results shown are from 3 separate experiments. *; p < 0.0001 for ANOVA and **; p < 0.0001 for PLSD tests.
Figure 5. Mouse Crp deletion and human CRP expression do not alter the anti-CII IgG response during CIA.
(A) Robust elevation of serum human CRP level (filled circle) was observed in CRPTg one day after initial immunization with CII and CFA (30-fold increase above baseline) and again one day following the booster injection (3.6-fold incrase), and levels of human CRP slowly returned to normal during the symptomatic phase. In comparison, in the same animals mouse CRP (open circle) was only modestly elevated. (B) Serum anti-CII IgG levels did not differ between Crp−/− (open circle), wild type (filled diamond), and CRPTg (filled circle).
Discussion
Our comprehensive comparison of wild type, Crp−/−, and CRPTg mice reveals that CRP exerts a significant influence on both the inflammatory and immune responses. At least in mice, CRP actively participates in these processes and is not simply associated with them. CRP has been shown to have numerous effects in vitro that indicate it should be able to interact with the inflammatory and immune systems on multiple levels in vivo. For example, the protein’s interaction with phosphocholine [9, 28] on biologically relevant ligands [6, 7, 29] could allow CRP to target the sources of inflammation, whereas its interaction with the complement proteins C1q and Factor H [30] could allow it to influence immune complex formation and its interaction with various FcRs [11, 12, 21] could allow it to influence antigen presentation and thereby cellular and humoral immunity. Based on these known abilities of CRP it is perhaps not so surprising that Crp deletion influenced the cytokine response evoked by challenge with both endotoxin and anti-CD3 antibody, altered the delayed type hypersensitivity response to OVA, and strengthened the antibody response to immunization with the thymus independent antigen TNP-Ficoll. In fact we showed earlier that transgenic expression of human CRP had the opposite effects on some cytokine and immune responses, for example leading to increased production of IL-10 [31] and decreased production of IgM autoantibodies [32], and we showed here that the immune response to TNP-Ficoll was weaker in CRPTg than wild type (Fig. S4).
As expected based on its effect on inflammatory and immune responsiveness, CRP deletion did influence the course of CIA. In alignment with an earlier report showing that elevation of rabbit CRP during induction of antigen-induced arthritis suppressed disease in rabbit CRP transgenic mice [19], our data from Crp−/− and CRPTg together suggest an early and beneficial effect of CRP in CIA. Notably compared to wild type mice, Crp−/− showed more rapid progression of disease with more severe symptoms, whereas CRPTg showed slower progression of disease with milder symptoms. As expected replenishment of Crp−/− with the human Crp transgene restored their resistance to CIA. In their sum these findings strongly suggest that CRP confers benefit during the early inductive phase of CIA. We think this beneficial effect requires accessory cells. Indeed, in preliminary experiments wherein we induced arthritis by direct injection of arthritogenic monoclonal antibodies, thereby bypassing the need for accessory cells and T cells, we have observed no difference in onset, progression, or severity of arthritis in wild type versus Crp−/− mice (data not shown).
At first glance our findings might seem to be in conflict with the large body of evidence showing that higher blood CRP level associates positively with worsening of symptoms in RA patients. With one important caveat, i.e. that our observations in mice with CIA might not extrapolate to humans with RA, we believe this apparent paradox can be resolved. We propose that during health, baseline blood CRP is sufficient to enable tonic suppression of inflammation that would otherwise predispose to autoimmunity. Thus in mice, deletion of Crp renders animals prone to a more rapidly evolving and more severe form of CIA because the inflammatory response is de-suppressed, whereas transgenic overexpression of Crp has the opposite effect. It is important to understand that in the mouse model we employed, the influence of CRP (or lack of CRP) is exerted (or not) already prior to disease induction. In humans this beneficial, tonic-suppressive effect of CRP would go unrecognized as it would be manifest during the pre-clinical stage of RA. In the context of clinically diagnosed, active arthritis on the other hand, levels of blood CRP could be raised either in response to worsening of the associated inflammation, as most assume, or in an effort to dampen it, as we propose. This model fully accounts for our findings and the positive association of elevated blood CRP level with symptoms of ongoing RA.
In this study we provide the first direct evidence that CRP deficiency alters the course of experimentally induced arthritis in mice. Others recently described a separately generated CRP deficient C57Bl/6 mouse strain [35], but whether that strain – like ours – is susceptible to collagen-induced arthritis or not remains to be tested. Regardless, our observation of worsened CIA in the CRP deficient mouse suggests that baseline CRP maintains health rather than promotes disease. Further study is needed to identify the means by which baseline CRP provides tonic suppression of inflammation and autoimmunity in CIA, but based on observations we made in another model of experimentally induced autoimmunity [31,33] we think the beneficial effect of CRP is likely mediated by FcγRIIB expressing cells. The disruption of a protective CRP → FcγRIIB pathway may be one reason why FcγRIIB deficient mice also show increased susceptibility to CIA [28]. Our findings suggest we may have to rethink CRP epidemiology as it relates to CRP biology in the context of arthritis. Since patients seldom visit rheumatologists before symptoms of RA manifest, much of the data pointing to a positive correlation of higher blood CRP level with worsening disease is from patients with active RA rather than pre-clinical RA. Our model predicts that in healthy people baseline blood CRP level should be inversely correlated with future RA risk. In people whose baseline level of CRP is insufficient to maintain the tonic-suppressive effect there is progression of disease. Ultimately an observational study will be needed to validate these predictions. In the future we hope to illuminate CRP’s mechanisms of action in arthritis and point the way toward novel therapies.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank members of the UAB Comparative Pathology Laboratory for their help with histology and tissue analysis, and the UAB Small Animal Phenotyping Core (funded by NIH grant P30DK056336) for assisting with micro-CT analysis.
This study was funded in part by NIH grant 1R21DA026914 (to AJS), a pilot and feasibility award (to AJS) from the NIH-funded UAB Clinical Nutrition Research Unit (5P30DK056336), and a training grant (to NRJ) from the NIH-funded UAB training program in Rheumatic Diseases Research (5T32AR007450). Boehringer Ingelheim International, SWK, HJ, and JBM hold a patent on the Crp−/− strain.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Goronzy JJ, Weyand CM. Developments in the scientific understanding of rheumatoid arthritis. Arthritis Res Ther. 2009;11:249. doi: 10.1186/ar2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapetanovic MC, Lindqvist E, Saxne T, Eberhardt K. Orthopaedic surgery in patients with rheumatoid arthritis over 20 years: prevalence and predictive factors of large joint replacement. Ann Rheum Dis. 2008;67:1412–1416. doi: 10.1136/ard.2007.086710. [DOI] [PubMed] [Google Scholar]
- 4.Newkirk MM. Rheumatoid factor avidity in patients with rheumatoid arthritis: identification of pathogenic RFs which correlate with disease parameters and with the gal(0) glycoform of IgG. J clin immunol. 1995;15:250. doi: 10.1007/BF01540882. [DOI] [PubMed] [Google Scholar]
- 5.Gabay C, Kushner I. Acute phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 6.Szalai AJ. The antimicrobial activity of C-reactive protein. Microbes Infect. 2002;4:201–205. doi: 10.1016/s1286-4579(01)01528-3. [DOI] [PubMed] [Google Scholar]
- 7.Bisoendial RJ, Boekholdt SM, Vergeer M, Stroes ES, Kastelein JJ. C-reactive protein is a mediator of cardiovascular disease. Eur Heart J. 2010;31:2087–2091. doi: 10.1093/eurheartj/ehq238. [DOI] [PubMed] [Google Scholar]
- 8.Mallya RK, de Beer FC, Berry H, Hamilton ED, Mace BE, Pepys MB. Correlation of clinical parameters of disease activity in rheumatoid arthritis with serum concentration of C-reactive protein and erythrocyte sedimentation rate. J Rheumatol. 1982;9:224–228. [PubMed] [Google Scholar]
- 9.Volakanis JE. Complement activation by C-reactive protein complexes. Ann N Y Acad Sci. 1982;389:235–250. doi: 10.1111/j.1749-6632.1982.tb22140.x. [DOI] [PubMed] [Google Scholar]
- 10.Molenaar ET, Voskuyl AE, Familian A, van Mierlo GJ, Dijkmans BA, Hack CE. Complement activation in patients with rheumatoid arthritis mediated in part by C-reactive protein. Arthritis. Rheum. 2001;44:997–1002. doi: 10.1002/1529-0131(200105)44:5<997::AID-ANR178>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Mold C, Gresham HD, Du Clos TW. Serum amyloid P component and C-reactive protein mediate phagocytosis through murine FcγRs. J Immunol. 2001;166:1200–1205. doi: 10.4049/jimmunol.166.2.1200. [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Marnell LL, Marjon KD, Mold C, Du Clos TW, Sun PD. Structural recognition and functional activation of FcγR by innate pentraxins. Nature. 2008;456:989–992. doi: 10.1038/nature07468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993;91:1351–1357. doi: 10.1172/JCI116336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walters MT, Stevenson FK, Goswami R, Smith JL, Cawley MI. Comparison of serum and synovial fluid concentrations of beta 2-microglobulin and C-reactive protein in relation to clinical disease activity and synovial inflammation in rheumatoid arthritis. Ann. Rheum. Dis. 1989;48:905–911. doi: 10.1136/ard.48.11.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamani B, Jamali R, Ehteram H. Synovial fluid adenosine deaminase and high-sensitivity C-reactive protein activity in differentiating monoarthritis. Rheumatol Int. 2010 doi: 10.1007/s00296-010-1602-3. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Matsui T, Kuga Y, Kaneko A, Nishino J, Eto Y, Chiba N, et al. Disease Activity Score 28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients in Japan. Ann. Rheum. Dis. 2007;66:1221–1226. doi: 10.1136/ard.2006.063834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kushner I, Somerville-Volanakis J. Studies of synovial and serum C-reactive protein in experimental arthritis in rabbits. Proc Soc Exp Biol Med. 1973;142:112–114. doi: 10.3181/00379727-142-36969. [DOI] [PubMed] [Google Scholar]
- 18.Phillips NC. Exacerbation of experimental poly-D-lysine arthritis by C-reactive protein. Agents Actions. 1982;12:344–347. doi: 10.1007/BF01965401. [DOI] [PubMed] [Google Scholar]
- 19.Jiang S, Xia D, Samols D. Expression of rabbit C-reactive protein in transgenic mice inhibits development of antigen-induced arthritis. Scand. J. Rhuematol. 2006;35:351–355. doi: 10.1080/03009740600757963. [DOI] [PubMed] [Google Scholar]
- 20.Ciliberto G, Arcone R, Wagner EF, Ruther U. Inducible and tissue-specific expression of human C-reactive protein in transgenic mice. The EMBO Journal. 1987;6:4017–4022. doi: 10.1002/j.1460-2075.1987.tb02745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szalai AJ, McCrory MA. Varied biologic functions of C-reactive protein: lessons learned from transgenic mice. Immunologic Research. 2002;26:279–287. doi: 10.1385/IR:26:1-3:279. [DOI] [PubMed] [Google Scholar]
- 22.Szalai AJ, Nataf S, Hu XZ, Barnum SR. Experimental allergic encephalomyelitis is inhibited in transgenic mice expressing human C-reactive protein. J Immunol. 2002;1;168:5792–5797. doi: 10.4049/jimmunol.168.11.5792. [DOI] [PubMed] [Google Scholar]
- 23.Whitehead AS, Zahedi K, Rits M, Mortensen RF, Lelias JM. Mouse C-reactive protein. Generation of cDNA clones, structural analysis, and induction of mRNA during inflammation. Biochem J. 1990;266:283–290. doi: 10.1042/bj2660283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inglis JJ, Simelyte E, McCann FE, Criado G, Williams RO. Protocol for the induction of arthritis in C57BL/6 mice. Nat. Protoc. 2008;3:612–618. doi: 10.1038/nprot.2008.19. [DOI] [PubMed] [Google Scholar]
- 25.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat. Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 26.Barck KH, Lee WP, Diehl LJ, Ross J, Gribling P, Zhang Y, et al. Quantification of cortical bone loss and repair for therapeutic evaluation in collagen-induced arthritis, by micro-computed tomography and automated image analysis. Arthritis. Rheum. 2004;50:3377–3386. doi: 10.1002/art.20557. [DOI] [PubMed] [Google Scholar]
- 27.Apostolopoulos J, Hickey MJ, Sharma L, Davenport P, Moussa L, James WG, et al. The cytoplasmic domain of tissue factor in macrophages augments cutaneous delayed-type hypersensitivity. J. Leukoc. Biol. 2008;83:902–911. doi: 10.1189/jlb.0607353. [DOI] [PubMed] [Google Scholar]
- 28.Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. 1999;7:169–177. doi: 10.1016/S0969-2126(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 29.Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117:104–111. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Mold C, Gewurz H, Du Clos TW. Regulation of complement activation by C-reactive protein. Immunopharmacology. 1999;42:23–30. doi: 10.1016/s0162-3109(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 31.Szalai AJ, Nataf S, Hu XZ, Barnum SR. Experimental allergic encephalomyelitis is inhibited in transgenic mice expressing human C-reactive protein. J Immunol. 2002;168:5792–5797. doi: 10.4049/jimmunol.168.11.5792. [DOI] [PubMed] [Google Scholar]
- 32.Szalai AJ, Weaver CT, McCrory MA, van Ginkel FW, Reiman RM, Kearney JF, Marion TN, Volanakis JE. Delayed lupus onset in (NZB × NZW)F1 mice expressing a human C-reactive protein transgene. Arthritis Rheum. 2003;48:1602–1611. doi: 10.1002/art.11026. [DOI] [PubMed] [Google Scholar]
- 33.Hu XZ, Wright TT, Jones NR, Ramos TN, Skibinski GA, McCrory MA, et al. Inhibition of Experimental Autoimmune Encephalomyelitis in Human C-Reactive Protein Transgenic Mice Is FcγRIIB Dependent. J. Autoimmune. Dis. 2011 doi: 10.4061/2011/484936. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuasa T, Kubo S, Yoshino T, Ujike A, Matsumura K, Ono M, et al. Deletion of Fcγ Receptor IIB renders H-2b mice susceptible to collagen-induced arthritis. J. Exp. Med. 1999;189:187–194. doi: 10.1084/jem.189.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teupser D, Weber O, Rao TN, Sass K, Thiery J, Fehling HJ. No Reduction of Atherosclerosis in C-reactive Protein (CRP)-deficient Mice. J Biol Chem. 2011;286:6272–6279. doi: 10.1074/jbc.M110.161414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.