Abstract
We describe a touchscreen method that satisfies a proposed ‘wish-list’ of desirables for a cognitive testing method for assessing rodent models of schizophrenia. A number of tests relevant to schizophrenia research are described which are currently being developed and validated using this method. These tests can be used to study reward learning, memory, perceptual discrimination, object-place associative learning, attention, impulsivity, compulsivity, extinction, simple Pavlovian conditioning, and other constructs. The tests can be deployed using a ‘flexible battery’ approach to establish a cognitive profile for a particular mouse or rat model. We have found these tests to be capable of detecting not just impairments in function, but enhancements as well, which is essential for testing putative cognitive therapies. New tests are being continuously developed, many of which may prove particularly valuable for schizophrenia research.
1. Introduction: Cognitive testing in preclinical schizophrenia research
“Antipsychotic drugs have emptied out our mental institutions, but have delivered their residents to impoverished lives outside. Research has linked elements of this poor psychosocial function to persistent cognitive impairments.”
It is now widely understood that the profound cognitive changes in schizophrenia, as much as the so-called ‘positive’ and ‘negative’ symptoms (see other articles in this issue), require understanding and treatment. To this end, major initiatives such as Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS), Cognitive Neuroscience Approaches to the Treatment of Impaired Cognition in Schizophrenia (CNTRICS), and elements of the European Innovative Medicines Initiative (IMI), have been set up to tackle this important facet of the disease.
It is also widely understood that animal models will play an indispensible role in this enterprise; indeed it has been said that it is hard to imagine any substantial progress in the pathophysiology or therapeutics of schizophrenia without good animal models (Nestler and Hyman, 2010). With respect to cognition, the ‘model’ in question is the appropriate combination of a e.g., genetic, pharmacological, lesion or environmental manipulation in an animal(usually a mouse or rat) with appropriate, accurate, reliable cognitive tests. It is a concern, then, that recently a number of articles have appeared with titles like “In search of a better mouse test”, emphasizing the need for improvement in this area(Nestler and Hyman, 2010; Tecott and Nestler, 2004; Wahlsten et al., 2003b). Clearly an important area of development must be the optimization of our cognitive testing methods in animal models.
With this in mind we can allow ourselves to ask what exactly it is we want from our rodent cognitive assays. We would like to suggest the following, probably relatively non-controversial ‘wish-list’ for the ideal cognitive testing method.
i. It should be automated
Notwithstanding some unique arguments to the contrary(Crabbe and Morris, 2004), most researchers agree that the same type of automation that has paid such dividends in areas like molecular biology is desirable for cognitive testing as well. Certainly the cognitive testing of clinical populations is becoming increasingly automated (e.g., the Cambridge Neuropsychological Test Automated Battery (CANTAB), CogState, Mindstreams), and so automation may help to achieve another desirable: effective translation (discussed in more detail below). But automation of cognitive testing in animal models confers advantages of its own; perhaps the most obvious to researchers ‘on the ground’ is a reduction in labour: while one experimenter is testing a single mouse in a maze, an experimenter armed with automated apparatus can test many mice simultaneously. Post-docs working in our lab, for example, routinely test 20 animals at a time, adding up to between 1 and 2 hundred a day. This realization negates the argument that some automated tests take more sessions to generate a result, as such tests would have to be 20 times slower before the equivalent non-automated testing methods start to make any time-economical sense. But it is the scientific arguments for automation that are perhaps the most compelling. With an automated test the computer can present many trials to an animal without having to interfere with the animal during testing. Such interference can have a huge effect, not just by introducing variability into the way the task is run, but also by introducing variability and potential confounds relating to the way the experimenter handles the animals, or even the sight or smell of the experimenter (Wahlsten et al., 2003a). Finally, because automated tasks are controlled by a computer, parameters such as delays and stimulus presentation are identical on every trial, for every animal, and the measures the computer gets back from the animal, for example latencies, can be accurate to the millisecond. All this adds up to a method that is much more likely to be reliable than non-automated methods.
ii. It should be non-aversive and low-stress
Although some animal models, including those of schizophrenia, utilize stress as a manipulation, unnecessary stress as a feature of a cognitive test is best avoided. Everyone knows that stress can interfere with our experimental manipulations, and can have strong modulatory effects on cognition(Joels and Baram, 2009). Unwanted stress can thereforebe a major impediment to the accurate assessment of cognition. Yet some of the most popular methods for cognitive testing involve cold water or electric shock. Such unwanted stressors are best avoided if possible, and so we would suggest that the ideal cognitive testing method should if possible be appetitive (reward-based) rather than aversive. Of course the use of appetitive reinforcers is not always problem-free either; some perturbations in experimental animals can affect animals’ motivation to perform vigorously to obtain reward. However appetitive reinforcers themselves generally have a less potentially confounding affect on the organism than stressors.
iii. It should assess multiple cognitive domains
Currently we often assess an animal’s memory in, say, a pool of water, its ability to extinguish a learned association using, say, footshock, and its attentional capacities using, say, an operant chamber. Then we try to compare across these tests. Obviously there are difficulties with this approach. Ideally we would be able to investigate all of these aspects of cognition under the same conditions -- in the same apparatus, using the same types of stimuli, and the same rewards and responses. We can then employ a ‘battery’ of tests to establish a cognitive profile of an animal, in conditions under which the tests can be compared in a less confounded manner.
iv. It should be translational
The goal of all of this is of course translation, from mouse to clinic. Currently the tests we use in animals are usually nothing like the tests we use in clinical populations; some of the tests mentioned above provide good examples of this disconnect. In order to translate effectively our findings from animals to humans, the tasks ideally would be as similar as possible. Such ‘face validity’ does not guarantee construct or predictive validity – further experimentation is needed to do that – but it makes these types of validity, and effective translation, much more likely than when the tests appear on the face of them to have little in common. With respect to schizophrenia, predictive validity presents a particular challenge as there are, as of yet, no approved treatments for the cognitive deficits in schizophrenia.
There are probably many as-yet unimagined methodologies that could satisfy this wish-list. In this review we would like to describe one method, which we have been working on for about 20 years now, which we think satisfies this list of desirables and could prove to be very valuable in the cognitive testing of rodent models of schizophrenia. We describe that method generally next, before describing some of the tests that are currently implemented and their relevance to schizophrenia research. We then describe the ‘flexible battery’ approach and the different ways in which such a battery might be deployed in practise.
2. The touchscreen testing method
With the wish-list in mind, we have developed an automated apparatus and method that uses a computer monitor to present stimuli, and an infra-red ‘touch-screen’ assembly with which an animal can register its response (Figure 1). Rewards can be food pellets or liquid reinforcers such as strawberry milkshake(which we have found to be particularly motivating, ameliorating some of the problems that can be associated with decreased motivation described above). We have developed a number of tests that can be run in this apparatus. These tests can be used to study reward learning, memory, perceptual discrimination, object-place associative learning, attention, impulsivity, compulsivity, extinction, simple Pavlovian conditioning, and other constructs. Next we describe some of these tests and their potential for use in schizophrenia research.
Figure 1.
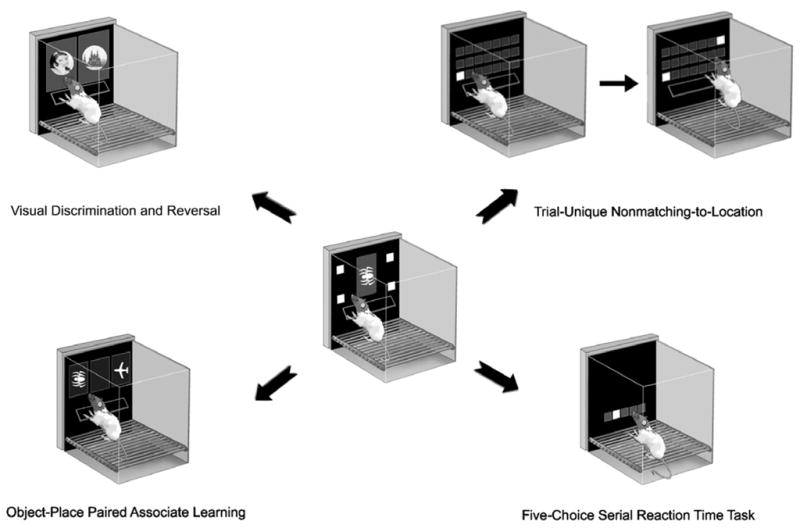
Centre: A rat using a touchscreen. Shapes and locations (delineated by white squares) can serve as stimuli; a nosepoke toward the screen serves as the response. The rat is shown using a ‘shelf’ directly under the stimulus display upon which it can rear and scan the stimuli before stretching across the shelf to make a response. The shelf serves to slow impulsive rats down, facilitating attention to the stimuli and minimizing impulsive responding, thus improving the rate of learning. Mice behave less impulsively when using the touchscreen, and so a shelf for mice is not required. Periphery: The basic method can be used to build a variety of tasks measuring learning, memory, attention, and other cognitive domains. Four examples are shown; the details of these tasks are provided in the text. Components of this figure are courtesy of John Talpos.
2.1 Visual Discrimination and Reversal: perceptual discrimination, association and inhibition
Visual discrimination and reversal learning is an intuitively straightforward touchscreen task (or rather, two tasks) which, nonetheless, permits the dissociation of multiple cognitive processes. The rodent is presented with two visual stimuli and learns that a response to one stimulus is rewarded (the S+) while a response to the other(the S-)is not (see Figure 1). The spatial location of the S+ and S- is irrelevant to task solution. Once the task has been acquired, the stimulus-reward contingencies can be reversed. A response to the S- is now rewarded, while the previously correct S+ goes unrewarded. Successful performance on this task thus involves a number of components. The subject must solve the perceptual discrimination and learn the initial stimulus-reward association, then, after the reversal, they must inhibit the prepotent response to the previously rewarded stimulus while learning the new stimulus-reward contingencies. These tasks are learned relatively quickly; Bussey et al. (2008) report that rats can achieve above 80% correct within several 60-trial sessions. The reversal takes longer, probably in the order of about tenor so such sessions. Although considerable progress has been made optimizing some parameters of the touchscreen method (e.g., Bussey et al., 2008), further improvements are being added all the time, and acquisition rates for these tasks are continuing to improve.
Reversal learning has been of particular interest with respect to schizophrenia. Reversal learning is known to depend upon the orbitofrontal region of prefrontal cortex (OFC) and interconnected subcortical areas, most notably the striatum(Bissonette et al., 2008; Chudasama and Robbins, 2003; McAlonan and Brown, 2003; Ragozzino, 2007). Dysfunction within this prefrontal-striatal circuitry is a key feature of schizophrenia (Robbins, 1990)and many studies have described impaired reversal learning in schizophrenia patients (Leeson et al., 2009a; Murray et al., 2008a; Pantelis et al., 1999; Waltz and Gold, 2007; but see also Joyce et al., 2002). Increasingly, such studies use computerised test batteries such as CANTAB (Robbins et al., 1994), in which the test for reversal learning is highly analogous to the rodent touchscreen version.
When rodents are tested on the touchscreen visual discrimination and reversal task, dissociations in performance may be observed between early post-reversal trials, in which the animal must inhibit responding to the previously correct stimulus in order to avoid perseverative errors, and later post-reversal trials, which reflect learning of the new association. Rats with lesions of the OFC, for example, displayed increased perseveration in earlier post-reversal trials, but did not differ from sham controls in the subsequent “new learning” phase (Chudasama and Robbins, 2003). Rats with lesions within the medial prefrontal cortex, by contrast, made significantly more errors than sham animals in the later post-reversal trials, but did not show increased perseveration (Bussey et al., 1997b; Chudasama and Robbins, 2003). A similar dissociation was seen in NR2A knockout mice, which showed impaired acquisition both of the initial discrimination and the new association post-reversal, but did not show altered perseveration (Brigman et al., 2008). Both types of error are likely to be relevant to schizophrenia. Perseveration is a common feature of the disorder, perhaps reflecting a reduced sensitivity to negative feedback (Leeson et al., 2009a). Striatal dysfunction has been implicated in the cognitive impairments in schizophrenia (Simpson et al., 2010) and dorsomedial striatal inactivation impairs the ability of animals to maintain a new response strategy during reversal learning (Ragozzino, 2007).
The touchscreen visual discrimination and reversal task is, in addition, amenable to pharmacological manipulation. For example, a D1-like receptor agonist produced an impairment in early reversal trials, without affecting acquisition of the initial visual discrimination (Izquierdo et al., 2006). While sub-chronic administration of the NMDA receptor antagonist phencyclidine (PCP) did not impair either acquisition or reversal in C57BL/6 mice (Brigman et al., 2009), increased perseveration can certainly be detected using this task (Chudasama and Robbins, 2003), and so ongoing research will attempt to identify a PCP protocol capable of reproducing this effect.
Acquisition of the discrimination is in itself a useful tool for studying visual processing in schizophrenia. Neurotransmitter systems such as the glutamatergic and cholinergic systems have been implicated in the disorder (Coyle, 2006; Raedler et al., 2007). Daily perirhinal cortex infusion of the GABA-A receptor agonist, muscimol, impaired acquisition of touchscreen visual discrimination in rats, as did perirhinal infusion of scopolamine or AP5 (Winters et al., 2010). Impaired acquisition was also seen in GLAST(EAAT-1, excitatory amino-acid transporter 1) knockout mice, further implicating the glutamatergic system (Karlsson et al., 2009). These studies featured the standard shape and photographic stimuli routinely used in touchscreen tasks. Discrimination and reversal can of course been carried out in standard ‘Skinner boxes’ using, e.g., lights as stimuli, and levers as responses. However in addition to the increased learning observed when stimulus-response contiguity is maximized, the touchscreen offers much richer possibilities for stimulus manipulation. In a recent study, for example, after rats acquired a shape discrimination we then ‘morphed’, or blended, the stimuli together to increase the perceptual similarity of the stimuli, bringing down baseline performance from ceiling in order better to detect improvements in performance following the administration of putative cognitive enhancers (McCarthy et al., 2011). Furthermore as with all touchscreen tasks the flexibility of stimuli allows for the presentation of stimuli identical to those presented to humans. For example a schizophrenia-relevant task we are currently building on top of the basic pair-wise discrimination template is the attentional set-shifting task, which we are presenting to rats and mice using the same types of shape and line stimuli used in the CANTAB version of this task (Brigman et al., 2005). The visual discrimination and reversal components would be folded in to the sequence of testing as they are in the CANTAB version. We are also developing a 3-stimulus version of the reversal task, which will allow us better to distinguish between perseveration and learned irrelevance (Arnsten et al., 1997; Jentsch et al., 2002; Garavan et al., 2000).
Impairments in visual processing are increasingly acknowledged in schizophrenia (Butler and Javitt, 2005)and have recently been highlighted by CNTRICS as a key area for task development(Green et al., 2009). This is in part because deficits in perceptual processing contribute to higher-level cognitive and social dysfunction, and thus to poorer functional outcome (Klosterkötter et al., 2001; Rassovsky et al., 2010). Visual processing deficits in schizophrenia are particularly prominent in the lower resolution magnocellular system (Butler and Javitt, 2005), although deficits in the faster, higher resolution, parvocellular system have also been reported (Slaghuis, 1998). Additional impairments are seen in gain control – the process by which neurons adapt their responses to optimise sensitivity to detect stimuli in a given environmental context (Dakin et al., 2005; Kim et al., 2006), and integration - the ability to extract a “global whole” from isolated local elements (Kurylo et al., 2007; Silverstein et al., 2006). The touchscreen method, with its rich possibilities for stimulus manipulation, may thus offer a new means of investigating some of these relatively overlooked, but robust and functionally relevant deficits. Although we have not yet attempted it, it is also of course possible to present moving stimuli to rodents, perhaps allowing the study of motion discrimination.
2.2 The TUNL Task: Working memory and pattern separation
Working memory dysfunction is a key aspect of cognitive impairment in schizophrenia. Identified by both the MATRICS (Nuechterlein et al., 2008a) and CNTRICS (Barch et al., 2009) initiatives as one of several dimensions of interest, working memory impairments have been reliably documented in schizophrenic patients, as well as back-translated to studies of primate and rodent models of schizophrenia. The work of Patricia Goldman-Rakic was instrumental in demonstrating that dopaminergic systems could underlie schizophrenia-like working memory impairments in rhesus monkeys, with similar results found in rodents (Castner et al., 2000; Floresco and Phillips, 2001; Phillips et al., 2004; Sawaguchi and Goldman-Rakic, 1991). Indeed, visuospatial working memory (SWM) has been identified as a putative cognitive endophenotype of schizophrenia (Park and Holzman, 1992; Park et al., 1995; Saperstein et al., 2006).
These findings regarding working memory have linked mainly to the role of prefrontal cortex in working memory (Courtney et al., 1998)and the hypofrontality observed in schizophrenia(Carter et al., 1998). However the hippocampus has also been implicated in schizophrenia (Harrison, 2004): volume is decreased in schizophrenic patients(Bogerts et al., 1990; Lawrie and Abukmeil, 1998; Nelson et al., 1998; Velakoulis et al., 1999), and hippocampal volume in patients can correlate with cognitive performance (Goldberg et al., 1994; Heckers et al., 1998). As the hippocampus is important for working memory in rodents (e.g., Kesner et al., 1993; McHugh et al., 2008; Wan et al., 1994), spatial working memory in particular has been seen as a fruitful approach to studying working memory in animals. The major automated working memory test in rodents has been the Delayed Non-Matching to Position (DNMTP) task, during a trial of which the animal is presented with one of two levers, and after a variable delay, must choose which of the two levers was not presented prior to the delay (i.e., the animal must ‘non-match’). DNMTP impairments result from manipulations to schizophrenia-relevant structures such as the hippocampus or prefrontal cortex (Aggleton et al., 1992; Dunnett et al., 1990), and more recently, impairments on DNMTP have been observed in mice with mutation of schizophrenia-associated genes such asDISC1(Li et al., 2007). The prefrontal cortex has been of particular interest, as lesions cause DNMTP impairment specific to working memory function (Granon et al., 1994). However, serious questions regarding the utility of DNMTP emerged when ‘mediating behaviours’ were observed in rats performing the task (Chudasama and Muir, 1997). These behaviours are possible because it is determined during the sample phase which location will be correct during the choice phase. Thus the rodent can use orientation toward the lever, or other responses, to bridge the delay and thus reduce or eliminate the need for memory per se. The need for a method of assessing working memory that minimises mediating behaviours led to the development of the Trial-Unique Delayed Non-Matching to Location (TUNL) task (Talpos et al., 2010).
An advantage of the touchscreen method for spatial tasks is that stimulus options are not limited to two locations determined by two levers as in DNMTP(Talpos et al., 2008). Thus although the TUNL task is similar to DNMTP in that two locations are used during a given trial, any of a number of alternative locations can serve as the correct stimulus on choice(Talpos et al., 2010; see Figure 1). Thus, unlike in DNMTP the animal cannot know, during the delay, which location will be correct on choice – and therefore cannot orient toward it. Indeed, systematic video analysis of potential mediating behaviours in TUNL showed little evidence for performance-enhancing mediating behaviours (Talpos et al., 2010).
The use of multiple locations confers an additional advantage; namely pairs of locations can be chosen which are either close together, far apart, or somewhere in between. This allows assessment of ‘pattern separation’ alongside the assessment of memory across a variable delay. ‘Pattern separation’ is the ability to form distinct representations from similar inputs that are closely presented in space (and/or time), and is thought to be important for keeping similar episodes distinct in memory. (This function of the hippocampus and its possible relevance to schizophrenia is discussed further in the next section.) Kesner and colleagues have shown that hippocampal lesions impair performance at small, but not large separations, indicating a deficit in pattern separation ability(Kesner et al., 2004). Talpos et al (2010) examined the effects of excitotoxic lesions of the hippocampus on performance of TUNL under conditions of different separations and delays. Hippocampal lesions were shown to significantly impair performance when the delay period was increased, or the separation decreased. Intriguingly, recent preliminary results indicate that prefrontal cortex lesions in the rat impair memory across a delay in TUNL, but not pattern separation (K.A.L. McAllister et al., unpublished results). If this result holds up, TUNL would represent a task for which the hippocampus and PFC are dissociable, but at the same time interact in the service of working memory. This is particularly relevant to schizophrenia, as it has been widely acknowledged that structural and functional changes in both the hippocampus and prefrontal cortex play an important role in the pathophysiology of schizophrenia (see above). Furthermore, schizophrenic brains show altered connectivity between hippocampus and prefrontal cortex(Meyer-Lindenberg et al., 2005), and impaired hippocampal-prefrontal synchrony during performance on a working memory task has been observed in a mouse with a microdeletion on human chromosome 22 (22q11.2), a genetic risk factor for schizophrenia(Sigurdsson et al., 2010). This mirrors human data where abnormal coupling is seen between the prefrontal cortex and hippocampus in patients with schizophrenia and healthy carriers of rs1344706 risk genotypes (Esslinger et al., 2009).
A disadvantage of TUNL is that it takes several weeks to train; however we are currently working on more rapidly-acquired versions, as well as a version for the mouse.
2.3 The Location Discrimination (LD) task: Focus on dentate gyrus and neurogenesis-dependent pattern separation
As described above, schizophrenic brains can exhibit gross changes in hippocampal morphology. However more detailed analyses have led many researchers to emphasise more subtle changes within the subregions of the hippocampus, and in particular in the dentate gyrus (DG), including changes in adult neurogenesis in this region (DeCarolis and Eisch, 2010; Eisch et al., 2008; Reif et al., 2006; Reif et al., 2007; Toro and Deakin, 2007). The finding of aberrant neurogenesis is consistent with the idea that the aetiology of schizophrenia may, at least in part, involve abnormal neurodevelopment. Notably, altered neurogenesis is also observed in mouse models of schizophrenia susceptibility genes, such as mice that have a mutant form of DISC1 (Duan et al., 2007) or constitutively lack the bHLH transcription factor NPAS3 (Pieper et al., 2005). Furthermore, pharmacological models of schizophrenia, such as treatment with the NMDA receptor antagonists PCP and ketamine have been shown to decrease hippocampal cell proliferation and survival (Keilhoff et al., 2004; Liu et al., 2006; Maeda et al., 2007). Disturbances in components of the Wnt signalling pathway, which mediates neurogenesis, have additionally been reported in schizophrenia (Beasley et al., 2001; Cotter et al., 1998; Kozlovsky et al., 2005). Additionally, within the DG, a reduction in the number of mossy fibers synapses onto CA3 neurons has been documented in patients (Kolomeets et al., 2005; Kolomeets et al., 2007) in addition to decreased levels of NR1 mRNA, the obligate subunit of the NMDA receptor, which has been shown to be selectively reduced in the DG (Gao et al., 2000; Law and Deakin, 2001).
As described in the previous section, the DG in particular has been implicated in pattern separation. This idea is based on a number of sources of evidence, including the architecture of the DG and the nature of its projections to the CA3 (reviewed by Aimone et al., 2010; Deng et al., 2010), computational modeling (O’Reilly and McClelland, 1994; Rolls and Kesner, 2006), lesion studies in rodents (Gilbert et al., 2001; Hunsaker and Kesner, 2008), electrophysiological data (Leutgeb et al., 2007) and reports that NMDA receptors within the DG, specifically the NR1 subunit, are important for pattern separation (McHugh et al., 2007). Additionally, human fMRI studies also indicate that activity in the DG/CA3 regions is correlated with pattern separation demands (Bakker et al., 2008). Using a spatial pattern separation task that has been developed in the touchscreen (McTighe et al., 2009), a functional role for hippocampal neurogenesis in pattern separation has recently been described (Clelland et al., 2009). Although the TUNL task described above has the advantage that it measures memory across a delay in addition to pattern separation, the LD task was designed to be a more rapidly-acquired task that focuses selectively on pattern separation. In the LD task, animals are trained to discriminate the correct one of two spatial locations delineated by illuminated squares. By varying the distance between the choice locations, the requirement for pattern separation can be manipulated. Training on this task takes only a few weeks. Mice with neurogenesis ablation perform similarly to control mice when discriminating two stimuli that were located spatially distant to one another, but were impaired when the stimuli were located closer in proximity, suggesting that neurogenesis is required for spatial pattern separation(Clelland et al., 2009). In line with these findings, a subsequent study revealed that mice with increased neurogenesis induced by wheel-running displayed enhanced pattern separation ability compared to non-exercising mice (Creer et al., 2010).
Thus the LD task is highly sensitive to hippocampal dysfunction, and in particular DG neurogenesis impairment. As the DG and neurogenesis have been implicated in schizophrenia, this task may be a rapid and useful assay of this aspect of dysfunction in schizophrenia. Of course the foregoing discussion raises the question of whether there is a link between schizophrenia and pattern separation in humans, which could of course contribute to the working memory impairments seen in schizophrenia (outlined above). Although no study has yet explicitly examined pattern separation in schizophrenic patients, growing evidence suggests this may be a relevant cognitive function of interest for future studies(Tamminga et al., 2010).
2.4 Object-place paired-associate learning
Schizophrenic patients are impaired on certain forms of associative learning i.e. the correct recall of contextual information regarding certain events, which can lead to errors in source monitoring (Brebion et al., 1997; Diaz-Asper et al., 2008; Johnson et al., 1993; Keefe et al., 1999; Vinogradov et al., 1997; Waters et al., 2004). Impairments in object-place associative memory in schizophrenic patients have been evaluated using the object-place paired-associate-learning (PAL) test from CANTAB. Studies using the CANTAB-PAL have revealed an impairment in patients with established schizophrenia (Aubin et al., 2009; Chouinard et al., 2007; Donohoe et al., 2008; Wood et al., 2002). Furthermore, PAL was found to be impaired in the prodromal phase of the disease and in individuals with an increased risk of developing schizophrenia (Barnett et al., 2005; Bartok et al., 2005), although this finding is not supported by all studies (Wood et al., 2002). Notably, PAL performance was found to be correlated with symptom severity and normal daily functioning (Aubin et al., 2009; Barnett et al., 2005; Prouteau et al., 2004, 2005; Ritsner and Blumenkrantz, 2007) and test results allow differentiation between schizophrenia and schizoaffective disorder (Stip et al., 2005).
Encoding and retrieval of paired associates in humans is thought to rely on concurrent prefrontal and hippocampal activation (Eichenbaum, 1999, 2000; Milner et al., 1997; Owen et al., 1995; Simons and Spiers, 2003). Similarly in rodents both the hippocampus and prefrontal cortex are thought to comprise a circuit underlying object-place learning (unpublished findings reported in (Warburton and Brown, 2010)), and as discussed above, schizophrenia has been associated with deficient prefrontal-hippocampal interaction. Thus object-place paired associate learning seems to rely on structures implicated in schizophrenia, perhaps explaining why paired-associate learning, and associative learning in general, can be compromised in schizophrenia.
With this background in mind, we sought to develop a task for the touchscreen in which rodents learned associations between objects (shapes) and locations on the computer screen (Talpos et al., 2009). In this task, animals learn to associate three different visual patterns (A, B and C) with three unique spatial locations (1, 2 and 3) on a screen. Pattern A is only correct if it appears in location 1; B is correct in location 2 and C is correct in 3. On each trial animals are presented with two choices e.g. A1 and B3 and are subsequently required to touch the screen in the correct location (A1) in order to receive a food reward. Thus the touchscreen paired-associate learning task involves the association of a certain object (visual pattern) with a location on the screen. Post-acquisition performance of this task has been shown to require an intact dorsal hippocampus, and more specifically activation of NMDA and AMPA receptors in the hippocampus (Talpos et al., 2009). Experiments in mice indicate dependence on the cholinergic system (Bartko et al., 2010). Mice carrying deletions of the schizophrenia-associated gene Dlg2(Consortium, 2008; Walsh et al., 2008) show severe impairments in this task (Nithianantharajah et al., 2009). Ongoing research projects include investigating the role of other receptor systems and structures such as the prefrontal cortexin this task, and developing more rapidly acquired versions of the task (currently rats require a mean of approximately 20- 30 90-trial sessions to reach a criterion of 80% correct).
2.5 The 5-choice task: Attention, impulsivity and compulsivity
Attentional changes in schizophrenia are well known (Fioravanti et al., 2005; Luck and Gold, 2008; Nuechterlein et al., 2006; Young et al., 2009) and are often assessed using the continuous performance test (CPT). The CPT is a paradigm comprising several procedural variants, many of which have proven reliable indicators for the presence of, and susceptability to, schizophrenia. In its basic form, the CPT involves rapid, externally-paced (i.e., not under subject control) presentation of a large number of consecutive stimuli, to which subjects are instructed to respond only upon detection of a designated target stimulus or sequence. The CPT is generally considered primarily as a measure of vigilance or sustained attention, with performance variables (e.g., accuracy or response latency) typically worsening as the session proceeds. However, performance is dependent on task parameters and the task may be modified to be more or less sensitive to elements of sustained attention, selective attention, impulsivity or executive control. Versions of the CPT have recently been selected by both the MATRICS and CNTRICS groups for inclusion in their batteries for assessing cognitive dysfunction in schizophrenia patients (Barch et al., 2009; Nuechterlein et al., 2008b).
Although the CPT appears to be sensitive to certain attentional and cognitive processes disturbed in schizophrenia, uncovering their neural and neurochemical basis, as well as the mechanisms of pharmacotherapeutic agents would greatly benefit from a translational preclinical model of cognitive function. The five-choice serial reaction time task (5-CSRTT) for the rodent has been developed and proposed as an analogue of the CPT in humans (see Chudasama and Robbins, 2004, 2006; Robbins, 2002 for recent extensive reviews). Similar to the CPT, the 5-CSRTT involves presentation of a large number of discrete consecutive trials. On each trial, subjects are required to scan a horizontal array of five locations (i.e., divided attention) for the presence of a brief light stimulus during a short (e.g., 5 s) inter-trial interval (ITI) (See Figure 1). Behavioural measures include accuracy, omissions (when the animal does not nose poke any of the front panel locations within the ‘limited hold’ period following stimulus offset), premature responses (responses during the ITI, prior to the presentation of the visual stimulus, an index of impulsivity), perseverative responses (repeated responses, a measure of compulsivity), as well as response and reward collection latencies.
Dysfunction in regions of the prefrontal cortex is one of the most studied and consistent findings in schizophrenia research (see above) and has been associated with disruptions in performance on versions of the CPT (Fallgatter, 2001; Yoon et al., 2008). Moreover, dysregulation of various neurotransmitters in these prefrontal regions, in particular dopamine and glutamate, have been extensively documented in schizophrenia and have been linked to performance changes in cognitive tasks such as the CPT (Braver et al., 1999; Coyle, 2006; Paz et al., 2008; Purdon et al., 2008; Stone et al., 2007; Tamminga, 1999). Similar neural systems underpin performance on the 5-CSRTT. In the rat, large excitotoxic lesions of the mPFC cause substantial but fairly selective reductions in response accuracy and increases in response latency compared with sham-lesioned controls (Muir et al., 1996; Passetti et al., 2003). More discrete excitotoxic lesions show that the effect on discrimination accuracy, particularly following variable ITI and noise burst challenges, may be restricted to damage of the anterodorsal mPFC (Chudasama et al., 2003; Passetti et al., 2003). Similar decrements in response accuracy and increases in response latencies are observed following lesions of the dorsomedial striatum (Rogers et al., 2001). This functional circuitry is further supported by the observation that asymmetrical, disconnection preparations (in which a lesion to the mPFC in one hemisphere is combined with a lesion to the medial striatum in the other hemisphere), produce effects similar to that of the bilateral lesions (Christakou et al., 2001). In-vivo microdialysis studies have revealed that cortical, and particularly prelimbic, DA is highly engaged during 5-CSRTT performance (Dalley et al., 2002b). Site-specific infusion of dopamine D1 receptor agonists directly into the mPFC enhances accuracy in animals having low baseline performance, while, conversely, dopamine D1 receptor antagonists impair accuracy in animals with relatively high baseline performance (Granon et al., 2000). In contrast, mPFC infusion of the D2 receptor antagonist, sulpiride, showed no significant effects in normal animals, although intra-accumbens infusion of sulpiride could remediate the impairments in attentional accuracy produced by mPFC lesions (Pezze et al., 2009). In addition, site-specific bilateral injection of competitive NMDA antagonist, CPP, into the mPFC of the rat produced reductions in choice accuracy, as well as increases in omissions and correct response latency (Baviera et al., 2008; Murphy et al., 2005).
Putative animal models of schizophrenia affect performance on the 5-CSRTT. Rats given repeated treatment with amphetamine showed impairments in choice accuracy and increased trial omissions under reduced stimulus duration conditions (Fletcher et al., 2007). These effects were reversed by infusion of the D1 receptor agonist, SKF38393, directly into the mPFC. Several studies have demonstrated that acute systemic administration of NMDA receptor antagonists MK-801 or PCP can cause reductions in choice accuracy and response latencies in rats and mice on the 5-CSRTT(see Amitai and Markou, 2010 for review; Amitai et al., 2007; Paine and Carlezon Jr., 2009; Paine et al., 2007). Moreover, repeated dosing of PCP in rats produces significant reductions in discrimination accuracy, as well as increases in correct response latency and premature responses (Amitai and Markou, 2009; Amitai et al., 2007). Sub-chronic or chronic intermittent treatment PCP may result in somewhat milder effects, with moderate slowing of responding (Thomson et al., 2010) and subtle impairments in accuracy observed only after the ITI is reduced (preliminary unpublished results described in Neill et al., 2010). Neonatal ventral hippocampal lesions (nVHL) were observed to produce mild impairments in choice accuracy and more pronounced increases in correct response latencies, which could be exacerbated by introducing distracting white noise prior to stimulus presentation, but not by varying the stimulus duration or ITI(Le Pen et al., 2003). In addition, nVHL rats showed increased sensitivity to the performance disruptive effects of acute PCP, but not acute amphetamine, treatment(Le Pen et al., 2003). In genetic mouse models, heterozygous reeler mice did not exhibit any behavioural deficits in acquisition or performance on a modified 3-choice procedure (Krueger et al., 2006). However, a recent study using an imprinting centre deletion mouse model for PWS showed these mice to have decreased choice accuracy, increased omissions, and longer correct reaction times compared to controls, which were exacerbated under reduced stimulus duration conditions (Relkovic et al., 2010).
The 5-CSRTT also appears sensitive to the remediating effects of first and second generation antipsychotic medications. Under baseline 5-CSRTT conditions using ‘normal’ rats, the typical antipsychotics, haloperidol or sulpiride, and the atypical antipsychotics clozapine, risperidone, quetiapine, or olanzapine, do not affect performance except for the behavioural disruptions of decreased correct responses (all except haloperidol), and increased omissions (sulpiride, clozapine, risperidone and olanzapine) at higher doses (Amitai et al., 2007; Harrison et al., 1997; Paine and Carlezon Jr., 2009). However, in mPFC-lesioned animals, systemic administration of sulpiride can ameliorate the impairment in attentional accuracy (Passetti et al., 2003), an improvement that is also observed after site-specific infusions of sulpiride into the nucleus accumbens (Pezze et al., 2009). Acute administration of low doses of clozapine, but not haloperidol, can remediate the accuracy deficits and increased omissions induced by acute MK-801 administration (Paine and Carlezon Jr., 2009). Clozapine, but not haloperidol, can improve the accuracy deficits induced by mPFC infusion of CPP (both clozapine and haloperidol reduced the mPFC CPP-induced elevations in premature responses, but neither were effective in altering the increased omissions and correct response latencies)(Baviera et al., 2008). In another pair of studies, low doses of sertindole and clozapine prevented mPFC infusion of CPP-induced accuracy deficits, anticipatory over-responding and the rise in glutamate release induced by CPP (Carli et al., 2010a), and oral aripiprazole and olanzapine, but not haloperidol, abolished a CPP-induced accuracy deficit and were associated with a decrease in glutamate release (Carli et al., 2010b). Further, chronic treatment with clozapine (Amitai et al., 2007), but not quetiapine (Amitai and Markou, 2009)can attenuate the reductions in accuracy and increases in premature responding following repeated PCP exposure.
The 5-CSRTT thus appears to be a highly promising translational behavioural paradigm that can sensitively assess aspects of attentional/cognitive dysfunction that is observed clinically in schizophrenia. Although the task has typically been carried out in the “nine-hole box” apparatus, the task runs well in touchscreens (Sahgal and Steckler, 1994; Steckler and Sahgal, 1995). Indeed we have found that mice progress from the end of pre-training to a baseline stimulus duration of 2 seconds within 3 weeks. We have obtained stimulus duration-dependent effects on accuracy in muscarinic knock-out mice and mouse models of Alzheimer’s disease (Romberg et al., 2011), and have shown facilitations of attention by the anticholinesterase donepezil in both rats and mice (McTighe et al, unpublished results; Romberg et al., 2011). The reversal of impairments in vigilance in Alzheimer’s mice with donepezil parallels strikingly the same effects in patients on an analogous task, indicating predictive validity at least with respect to this task and Alzheimer’s disease. With respect to schizophrenia, given that muscarinic agonists have been suggested as a treatment (Sellin et al., 2008), is the finding that mice with deletion of the M1 receptor show increased striatal dopamine release (Gerber et al., 2001), and abnormal responding consistent with striatal dopaminergic manipulations in rats (Barko et al, in revision). Thus the touchscreen may prove to be a particularly useful way to run the 5-CSRTT in mice and rats, with the advantage of improved comparability with results from other tests of learning, memory etc carried out in the same apparatus. In addition, the flexibility of stimulus material in the touchscreen could allow the marriage of spatial locations as in the 5-CSRTT and non-spatial (e.g., shape) stimuli to create tests of object-based attentional tasks using, e.g., rapid visual presentation of non-spatial stimuli, or the use of non-spatial stimuli as distractors. Such modifications are currently under development.
3.6 Extinction: Inhibition of responding in the face of non-reward
“My first extinction curve showed up by accident. A rat was pressing the lever in an experiment on satiation when the pellet dispenser jammed. I was not there at the time, and when I returned I found a beautiful curve. The rat had gone on pressing although no pellets were received…. I was terribly excited. It was a Friday afternoon and there was no one in the laboratory who I could tell. All that weekend I crossed streets with particular care and avoided all unnecessary risks to protect my discovery from loss through my accidental death” (Skinner, 1979).
Fortunately for posterity, Skinner made it through the weekend and went on to describe his observation of an example of operant extinction in his rats. As Skinner noted, Pavlov had reported extinction of classical conditioned responses some fifty years earlier (Pavlov, 1927). Both forms of extinction can be operationally defined as a form of learning in which a response is reduced after repeated experience of a stimulus that elicits the response, in the absence of the previously paired reinforcer.
Extinction has been surprisingly understudied in schizophrenic patients. However, recent evidence indicates that extinction of fear responses, which has been intensively studied in the context of anxiety disorders (Holmes and Quirk, 2010), is also impaired in schizophrenia. Holt and colleagues (2008) trained subjects to associate a visual stimulus with electric shock in one room and then extinguished the fear (skin conductance) response evoked by repeatedly presenting the stimulus without shock in a different test room. Medicated schizophrenic patients were more likely than normal controls to retain fear responses the following day when presented with the stimulus in the extinction test room, indicating a deficit in extinction recall and/or a failure to link the extinction room with a safety signal. Given the dependence of extinction on prefrontal and hippocampal function (Corcoran and Quirk, 2007; Holmes and Wellman, 2009), the deficits might be related to abnormalities in these regions, which are thoughtto be dysfunctional in schizophrenic patients (Nelson et al., 1998; Taylor et al., 2007).
We have used a touchscreen extinction task to test mice with mutations in various molecules of the glutamatergic system, a neurotransmitter system which has been repeatedly implicated in schizophrenia (Coyle, 2006). We employed a procedure in which mice are first trained to reliably make a touchscreen response at a simple visible stimulus (white square) for food reward. Extinction is then measured as the rate at which the mouse demonstrates low levels (>20%) of responding over (30-trial) daily sessions. This method has been extensively characterized across different inbred mouse strains (Hefner et al., 2008; Lederle et al., 2010). Mutant mice lacking the NR2A subunit of the NMDA receptor have a significant impairment in pairwise visual discrimination, but extinguish a stimulus-response as well as do littermate controls (Brigman et al., 2008). A similar phenotypic profile of impaired discrimination but intact extinction is found in mutants with deletion of the glutamate transporter(Karlsson et al., 2009), GLAST (EAAT-1, excitatory amino-acid transporter 1), a mutant which exhibits a constellation of antipsychotic-reversible ‘schizophrenia-related’ phenotypes (Karlsson et al., 2008). By contrast, in another mutant with ‘schizophrenia-related’ behavioral abnormalities (Fitzgerald et al., 2010; Wiedholz et al., 2008), the AMPA receptor GluA1 subunit knockout mouse, discrimination and reversal are unimpaired while extinction is severely impaired(Barkus et al., submitted).
A general conclusion is that these tasks provide assays of different forms of ‘cognitive flexibility’ and ‘perseveration’ that are dissociable at the molecular level. This underscores the utility of adopting a battery approach to assessing cognitive function in future studies (see discussion below). Precisely why it is that targeting these different nodes within the glutamate system produces different patterns of impairment on these tasks is currently unclear. Indeed, this will be a key question for future studies aimed at dissecting the role of this and interacting systems in mediating schizophrenia-related cognitive impairment.
3.7 Autoshaping: Pavlovian approach and the mesolimbic dopamine system
The role of striatal dopamine in schizophrenia has long been known(Kleinman et al., 1988), and its role in cognition in particular has been forcefully argued(Simpson et al., 2010). Of particular interest is the ventral striatum (Robbins, 1990). One recent idea is that dopaminergic neurons that extend from the midbrain to the ventral striatum code “reward prediction error”, the teaching signal which underlies the acquisition of stimulus-outcome associations (Schultz and Dickinson, 2000). A recent fascinating finding is that patients with psychosis exhibit abnormal physiological responses associated with prediction error in the striatum (as well as other structures) (Murray et al., 2008b).
The striatal dopamine system, and midbrain-ventral striatal “prediction error” system in particular, can be targeted using a simple task we call “Autoshaping”(Bussey et al., 1997a). This is a very rapidly administered test of simple classical conditioning that capitalizes on and measures “Pavlovian approach” to stimuli predictive of reward. On a given trial of this task a stimulus (vertical white bar) appears on the left or the right of the screen for several seconds. Either the left or the right stimulus is deemed the CS+; the other is the CS-. Immediately on offset of the stimulus CS+, a food reward is delivered. After the CS- no reward is delivered. Over trials, the mouse or rat learns that the CS+ predicts reward and makes increasingly more approaches and touches to it. Approaches and touches to the CS- decrease or stay the same. The difference between the number or proportion of approaches or touches to the CS+ and CS-, and well as the difference between the latency to approach or touch, can be taken as an indication of learning. The task is very rapidly acquired; significant discrimination by rats can be observed even within a single session. Lesions of the ventral striatum, dopamine receptor antagonism, and dopamine depletion within this region, impair learning (Dalley et al., 2002a; Dalley et al., 2005; Parkinson et al., 2002). This task dissociates striatal dopamine from other systems, for example, the task is not affected by lesions of the prefrontal cortex (Bussey et al., 1997a) or the hippocampus and related structures (Bussey et al., 2000; Ito et al., 2005). The Autoshaping test has already been used in many studies involving lesion and drug manipulations (Cardinal et al., 2003; Di Ciano et al., 2001; Parkinson et al., 2002; Parkinson et al., 1999; Parkinson et al., 2000). For the purposes of schizophrenia research, because it is a classical conditioning procedure it could perhaps be modified to administer other schizophrenia-relevant tests, for example latent inhibition (Solomon et al., 1981; Weiner and Feldon, 1987; Weiner et al., 1984).
4. A ‘flexible’ battery approach
“All putative animal models [of neuropsychiatric disorders] should be evaluated with the broadest range possible of behavioral assays” – Nestler & Hyman 2010
Although each of the above tasks can be used alone, we would advocate the use of a ‘battery’ approach to establish a cognitive profile of an animal. In this way a given task in the battery not only yields useful results in its own right, it can serve as a control for other tasks in the battery. Thus, if an animal fails object-place paired associate learning but performs well on visual discrimination and reversal, it is unlikely that the paired associate learning impairment is due to a difficulty with perceptually discriminating objects. Similarly if an animal is impaired on TUNL but not 5-CSRTT, an attentional explanation for the TUNL impairment would seem unlikely. These comparisons can be made with confidence, because all of the tasks use the same types of stimuli, responses and reward, and of course are all carried out in the same testing apparatus.
Yet to suggest a battery approach is not to suggest slavish adherence to animmutable sequence of tests; for example each of these tasks can be followed by behavioural probes to follow up interesting findings. For example, shape or photographic stimuli can be ‘morphed’ to increase the premium on perceptual discrimination (McCarthy et al, 2011). Parameters in TUNL can be further varied, for example inter-trial intervals might be varied to increase or decrease interference. The 5-CSRTT can be followed by sessions of rapid presentation of stimuli during long sessions in order to examine sustained vigilance. And so on.
How, practically, does one implement the battery? There are different models, depending on one’s needs. The extremes are 1. Run each task in a new cohort of naïve animals, or 2. Run many tasks in each animal (we are currently working out what tasks ‘go together’ best, and are settling on a sequence in which the tasks appear not to interfere with one another). Option 1 might be the better choice for, say, a progressive disease model or a drug study; option 2 has its own advantages, for example fewer animals are used, which is desirable in cases where large numbers of animals are difficult to obtain. Which approach is taken depends on several factors that may differ from experiment to experiment.
Finally of course brand new tasks for the battery are being developed all the time, some of which could prove particularly useful for schizophrenia research; we have already alluded above to attentional set-shifting tests similar to those administered using the CANTAB method (Leeson et al., 2009b); in addition we are having success developing a touchscreen version of the object recognition memory test used so widely to study animal models of schizophrenia(e.g., Duffy et al.; Grayson et al., 2007). Furthermore, some researchers are currently expanding the method to incorporate other techniques such as in vivo electrophysiology and dialysis.
Are there any disadvantages to this method? An obvious one is that, being a relatively new method, there does not yet exist the extensive database behind it that some methods – for example, the Morris water maze – enjoy. But the touchscreen database is expanding: at this time we know of roughly 30 labs using touchscreen technology with rodents, and so the validation and development of this method is continuing apace. Another has been alluded to above: although the touchscreen method avoids the aversive stimuli and stressors endemic to some other methods, any method which uses gustatory rewards is vulnerable to the potential effects of some perturbations on motivation to perform for reward. Indeed we have experienced such problems with certain mouse models, and to some extent were able to mitigate them by, for example, using larger volumes of highly palatable reinforcers (e.g., 20ul strawberry milkshake), and requiring fewer trials per session (from the control groups as well, of course). Finally, although all things considered we strongly advocate a battery approach in which all tests are carried out in the same apparatus, such an approach might make within-subjects task designs more vulnerable to negative or positive transfer between tasks. Of course this is a potential problem when any within-subjects battery is used; however the problem could be exacerbated when contexts are so similar between tasks. When designing within-subjects testing sequences, we try to combine tests which are least likely to interfere with one another – although such interference can only be definitively ruled out by retesting naive animals on the task in question.
There is still much work to be done. Much more validation is required to establish the neural circuits and transmitters involved in the tasks. We are currently piloting the mouse and rat tasks in human subjects, to allow even tighter translation and the ability to test whether the same circuits, neurotransmitters etc, and psychological processes (e.g., habit versus goal-directed performance) are involved in these tasks in rodents and humans. Another issue we will need to address is test-retest reliability, and what are the pharmacological sensitivities of each of the tasks. What is already clear from the foregoing discussion is that the touchscreen method offers much more than a ‘glorified Skinner box’. The apparatus can theoretically be used to run any task that can be run in a box with lights and levers, but the myriad of stimuli that are possible, and the multiple locations in which they can be presented, offer virtually unlimited possibilities for task development.
5. Summary
The touchscreen testing method satisfies what we think is a fairly non-controversial ‘wish-list’ of desirables for a cognitive testing method for assessing rodent models of schizophrenia. A number of tests relevant to schizophrenia research are currently being validated. These tasks can be deployed using a ‘flexible battery’ approach to establish a cognitive profile for a particular mouse model. We have found these tasks to be capable of detecting not just impairments in function, but enhancements as well, which is essential for testing putative cognitive therapies. New tasks are continuously coming ‘on-line’, many of which may prove particularly valuable for schizophrenia research.
Highlights.
We describe a touchscreen method for assaying cognition in rodent models.
A number of tests relevant to schizophrenia research are described.
The tests can be used to study multiple aspects of cognition.
The tests can be combined in a flexible battery to establish a cognitive profile.
The tests are capable of detecting both impairments and enhancements in function.
Acknowledgments
All authors contributed equally to the preparation of this manuscript; names are listed alphabetically. The authors would like to thank Trevor Robbins for comments on an early version of the manuscript and John Talpos for kindly providing some components of Figure 1. This work was supported by funding from the Commonwealth Trust, Janssen Pharmaceutica, the National Institute on Alcohol Abuse and Alcoholism Intramural Research Program, and the Innovative Medicines Initiative Joint Undertaking (IMI) under grant agreement n° 115008. IMI is a public-private partnership between the European Union and the European Federation of Pharmaceutical Industries and Associations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Keith AB, Rawlins JN, Hunt PR, Sahgal A. Removal of the hippocampus and transection of the fornix produce comparable deficits on delayed non-matching to position by rats. Behav Brain Res. 1992;52:61–71. doi: 10.1016/s0166-4328(05)80325-0. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci. 2010;14:325–337. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Markou A. Increased Impulsivity and Disrupted Attention Induced by Repeated Phencyclidine are not Attenuated by Chronic Quetiapine Treatment. Pharmacology, Biochemistry & Behavior. 2009;93:248–257. doi: 10.1016/j.pbb.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Markou A. Disruption of performance in the five-choice serial reaction time task induced by administration of N-methyl-D-aspartate receptor antagonists: relevance to cognitive dysfunction in schizophrenia. Biological psychiatry. 2010;68:5–16. doi: 10.1016/j.biopsych.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology. 2007;193:521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Lin CH, Van Dyck CH, Stanhope KJ. The effects of 5-HT3 receptor antagonists on cognitive performance in aged monkeys. Neurobiol Aging. 1997;18:21–28. doi: 10.1016/s0197-4580(96)00162-5. [DOI] [PubMed] [Google Scholar]
- Aubin G, Stip E, Gelinas I, Rainville C, Chapparo C. Daily activities, cognition and community functioning in persons with schizophrenia. Schizophr Res. 2009;107:313–318. doi: 10.1016/j.schres.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Barch DM, Berman MG, Engle R, Jones JH, Jonides J, Macdonald A, Nee DE, Redick TS, Sponheim SR. CNTRICS final task selection: working memory. Schizophrenia Bulletin. 2009;35:136–152. doi: 10.1093/schbul/sbn153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus C, Feyder M, Graybeal C, Wright T, Wiedholz L, Izquierdo A, Saksida LM, Bussey TJ, Bannerman D, Sprengel R, Holmes A. GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative symptoms of schizophrenia and schizoaffective disorder. TBD. doi: 10.1016/j.neuropharm.2011.06.005. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Sahakian BJ, Werners U, Hill KE, Brazil R, Gallagher O, Bullmore ET, Jones PB. Visuospatial learning and executive function are independently impaired in first-episode psychosis. Psychol Med. 2005;35:1031–1041. doi: 10.1017/s0033291704004301. [DOI] [PubMed] [Google Scholar]
- Bartko SJ, Vendrell I, Saksida LM, Bussey TJ. A computer-automated touchscreen paired-associates learning (PAL) task for mice: impairments following administration of scopolamine or dicyclomine, and improvements following donepezil. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-2050-1. in press. [DOI] [PubMed] [Google Scholar]
- Bartok E, Berecz R, Glaub T, Degrell I. Cognitive functions in prepsychotic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:621–625. doi: 10.1016/j.pnpbp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Baviera M, Invernizzi RW, Carli M. Haloperidol and clozapine have dissociable effects in a model of attentional performance deficits induced by blockade of NMDA receptors in the mPFC. Psychopharmacology. 2008;196:269–280. doi: 10.1007/s00213-007-0959-9. [DOI] [PubMed] [Google Scholar]
- Beasley C, Cotter D, Khan N, Pollard C, Sheppard P, Varndell I, Lovestone S, Anderton B, Everall I. Glycogen synthase kinase-3beta immunoreactivity is reduced in the prefrontal cortex in schizophrenia. Neurosci Lett. 2001;302:117–120. doi: 10.1016/s0304-3940(01)01688-3. [DOI] [PubMed] [Google Scholar]
- Bissonette G, Martins G, Franz T, Harper E, Schoenbaum G, Powell E. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28:11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogerts B, Ashtari M, Degreef G, Alvir JM, Bilder RM, Lieberman JA. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res. 1990;35:1–13. doi: 10.1016/0925-4927(90)90004-p. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biological psychiatry. 1999;46:312–328. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Brebion G, Smith MJ, Gorman JM, Amador X. Discrimination accuracy and decision biases in different types of reality monitoring in schizophrenia. J Nerv Ment Dis. 1997;185:247–253. doi: 10.1097/00005053-199704000-00005. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Bussey TJ, Saksida LM, Rothblat LR. Discrimination of multidimensional visual stimuli by mice: Intra- and extradimensional shifts. Behavioral Neuroscience. 2005;119:839–842. doi: 10.1037/0735-7044.119.3.839. [DOI] [PubMed] [Google Scholar]
- Brigman J, Ihne J, Saksida L, Bussey T, Holmes A. Effects of Subchronic Phencyclidine (PCP) Treatment on Social Behaviors, and Operant Discrimination and Reversal Learning in C57BL/6J Mice. Front Behav Neurosci. 2009;3:2. doi: 10.3389/neuro.08.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem. 2008;15:50–54. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Duck J, Muir JL, Aggleton JP. Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behavioural Brain Research. 2000;111:187–202. doi: 10.1016/s0166-4328(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Everitt BJ, Robbins TW. Dissociable effects of cingulate and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: implications for the neurobiology of emotion. Behavioral Neuroscience. 1997a;111:908–919. doi: 10.1037//0735-7044.111.5.908. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci. 1997b;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Padain TL, Skillings EA, Winters BD, Morton AJ, Saksida LM. The touchscreen cognitive testing method for rodents: How to get the best out of your rat. Learning and Memory. 2008;15:516–523. doi: 10.1101/lm.987808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler P, Javitt D. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18:151–157. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Marbini HD, Toner AJ, Bussey TJ, Robbins TW, Everitt BJ. Role of the anterior cingulate cortex in the control over behavior by Pavlovian conditioned stimuli in rats. Behav Neurosci. 2003;117:566–587. doi: 10.1037/0735-7044.117.3.566. [DOI] [PubMed] [Google Scholar]
- Carli M, Calcagno E, Mainini E, Arnt J, Invernizzi RW. Sertindole restores attentional performance and suppresses glutamate release induced by the NMDA receptor antagonist CPP. Psychopharmacology (Berl) 2010a doi: 10.1007/s00213-010-2066-6. [DOI] [PubMed] [Google Scholar]
- Carli M, Calcagno E, Mainolfi P, Mainini E, Invernizzi RW. Effects of aripiprazole, olanzapine, and haloperidol in a model of cognitive deficit of schizophrenia in rats: relationship with glutamate release in the medial prefrontal cortex. Psychopharmacology (Berl) 2010b doi: 10.1007/s00213-010-2065-7. [DOI] [PubMed] [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287:2020–2022. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- Chouinard S, Stip E, Poulin J, Melun JP, Godbout R, Guillem F, Cohen H. Rivastigmine treatment as an add-on to antipsychotics in patients with schizophrenia and cognitive deficits. Curr Med Res Opin. 2007;23:575–583. doi: 10.1185/030079906X167372. [DOI] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, Everitt BJ. Functional Disconnection of a Prefrontal Cortical-Dorsal Striatal System Disrupts Choice Reaction Time Performance: Implications for Attentional Function. Behavioral Neuroscience. 2001;115:812–825. doi: 10.1037//0735-7044.115.4.812. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Muir JL. A behavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology (Berl) 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behavioural Brain Research. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins T. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Psychopharmacological approaches to modulating attention in the five-choice serial reaction time task: implications for schizophrenia. Psychopharmacology. 2004;174:86–98. doi: 10.1007/s00213-004-1805-y. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biological psychology. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium IS. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Recalling safety: cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr. 2007;12:200–206. doi: 10.1017/s1092852900020915. [DOI] [PubMed] [Google Scholar]
- Cotter D, Kerwin R, al-Sarraji S, Brion JP, Chadwich A, Lovestone S, Anderton B, Everall I. Abnormalities of Wnt signalling in schizophrenia--evidence for neurodevelopmental abnormality. Neuroreport. 1998;9:1379–1383. doi: 10.1097/00001756-199805110-00024. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Haxby JV, Ungerleider LG. The role of prefrontal cortex in working memory: examining the contents of consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1819–1828. doi: 10.1098/rstb.1998.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Morris RG. Festina lente: late-night thoughts on high-throughput screening of mouse behavior. Nat Neurosci. 2004;7:1175–1179. doi: 10.1038/nn1343. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin S, Carlin P, Hemsley D. Weak suppression of visual context in chronic schizophrenia. Curr Biol. 2005;15:R822–824. doi: 10.1016/j.cub.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Chudasama Y, Theobald DE, Pettifer CL, Fletcher CM, Robbins TW. Nucleus accumbens dopamine and discriminated approach learning: interactive effects of 6-hydroxydopamine lesions and systemic apomorphine administration. Psychopharmacology (Berl) 2002a;161:425–433. doi: 10.1007/s00213-002-1078-2. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DE, Armstrong HC, Corlett PR, Chudasama Y, Robbins TW. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proc Natl Acad Sci U S A. 2005;102:6189–6194. doi: 10.1073/pnas.0502080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology. 2002b;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- DeCarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology. 2010;58:884–893. doi: 10.1016/j.neuropharm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21:9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Asper C, Malley J, Genderson M, Apud J, Elvevag B. Context binding in schizophrenia: effects of clinical symptomatology and item content. Psychiatry Res. 2008;159:259–270. doi: 10.1016/j.psychres.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Donohoe G, Spoletini I, McGlade N, Behan C, Hayden J, O’Donoghue T, Peel R, Haq F, Walker C, O’Callaghan E, Spalletta G, Gill M, Corvin A. Are relational style and neuropsychological performance predictors of social attributions in chronic schizophrenia? Psychiatry Res. 2008;161:19–27. doi: 10.1016/j.psychres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In- Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy L, Cappas E, Lai D, Boucher AA, Karl T. Cognition in transmembrane domain neuregulin 1 mutant mice. Neuroscience. 2010;170:800–807. doi: 10.1016/j.neuroscience.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Wareham AT, Torres EM. Cholinergic blockade in prefrontal cortex and hippocampus disrupts short-term memory in rats. Neuroreport. 1990;1:61–64. doi: 10.1097/00001756-199009000-00017. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behav Brain Res. 1999;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, Haddad L, Mier D, Opitz von Boberfeld C, Raab K, Witt SH, Rietschel M, Cichon S, Meyer-Lindenberg A. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- Fallgatter A. Electrophysiology of the prefrontal cortex in healthy controls and schizophrenic patients: a review. Journal of neural transmission. 2001;108:679–694. doi: 10.1007/s007020170045. [DOI] [PubMed] [Google Scholar]
- Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychology review. 2005;15:73–95. doi: 10.1007/s11065-005-6254-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PF, Barkus C, Feyder M, Wiedholz L, Chen Y-C, Karlsson RM, Machado-Vieira R, Graybeal C, Sharp T, Zarate CA, Harvey-White J, Du J, Sprengel R, Gass P, Bannerman DM, Holmes A. Does gene deletion of AMPA GluA1 phenocopy features of schizoaffective disorder? Neurobiol Dis. 2010 doi: 10.1016/j.nbd.2010.08.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav Neurosci. 2001;115:934–939. [PubMed] [Google Scholar]
- Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- Garavan H, Morgan RE, Mactutus CF, Levitsky DA, Booze RM, Strupp BJ. Prenatal cocaine exposure impairs selective attention: evidence from serial reversal and extradimensional shift tasks. Behav Neurosci. 2000;114(4):725–38. [PubMed] [Google Scholar]
- Gerber DJ, Sotnikova TD, Gainetdinov RR, Huang SY, Caron MG, Tonegawa S. Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:15312–15317. doi: 10.1073/pnas.261583798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer M, Tamminga C. Measurement and treatment research to improve cognition in schizophrenia: neuropharmacological aspects. Psychopharmacology. 2004;174:1–2. [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Torrey EF, Berman KF, Weinberger DR. Relations between neuropsychological performance and brain morphological and physiological measures in monozygotic twins discordant for schizophrenia. Psychiatry Res. 1994;55:51–61. doi: 10.1016/0925-4927(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. The Journal of neuroscience. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granon S, Vidal C, Thinus-Blanc C, Changeux JP, Poucet B. Working memory, response selection, and effortful processing in rats with medial prefrontal lesions. Behav Neurosci. 1994;108:883–891. doi: 10.1037//0735-7044.108.5.883. [DOI] [PubMed] [Google Scholar]
- Grayson B, Idris NF, Neill JC. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behav Brain Res. 2007;184:31–38. doi: 10.1016/j.bbr.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Green M, Butler P, Chen Y, Geyer M, Silverstein S, Wynn J, Yoon J, Zemon V. Perception measurement in clinical trials of schizophrenia: promising paradigms from CNTRICS. Schizophr Bull. 2009;35:163–181. doi: 10.1093/schbul/sbn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology. 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Quirk GJ. Pharmacological facilitation of fear extinction and the search for adjunct treatments for anxiety disorders--the case of yohimbine. Trends Pharmacol Sci. 2010;31:2–7. doi: 10.1016/j.tips.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, Cassidy BS, Walsh JP, Goff DC. Extinction Memory Is Impaired in Schizophrenia. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Evaluating the differential roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 during a temporal ordering for spatial locations task. Hippocampus. 2008;18:955–964. doi: 10.1002/hipo.20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Everitt BJ, Robbins TW. The hippocampus and appetitive Pavlovian conditioning: effects of excitotoxic hippocampal lesions on conditioned locomotor activity and autoshaping. Hippocampus. 2005;15:713–721. doi: 10.1002/hipo.20094. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wiedholz L, Millstein R, Yang R, Bussey T, Saksida L, Holmes A. Genetic and dopaminergic modulation of reversal learning in a touchscreen-based operant procedure for mice. Behav Brain Res. 2006;171:181–188. doi: 10.1016/j.bbr.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002 Feb;26(2):183–90. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychol Bull. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Joyce E, Hutton S, Mutsatsa S, Gibbins H, Webb E, Paul S, Robbins T, Barnes T. Executive dysfunction in first-episode schizophrenia and relationship to duration of untreated psychosis: the West London Study. Br J Psychiatry Suppl. 2002;43:s38–44. doi: 10.1192/bjp.181.43.s38. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Tanaka K, Heilig M, Holmes A. Loss of glial glutamate and aspartate transporter (excitatory amino acid transporter 1) causes locomotor hyperactivity and exaggerated responses to psychotomimetics: rescue by haloperidol and metabotropic glutamate 2/3 agonist. Biol Psychiatry. 2008;64:810–814. doi: 10.1016/j.biopsych.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2009;34:1578–1589. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Arnold MC, Bayen UJ, Harvey PD. Source monitoring deficits in patients with schizophrenia; a multinomial modelling analysis. Psychol Med. 1999;29:903–914. doi: 10.1017/s0033291799008673. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Bernstein HG, Becker A, Grecksch G, Wolf G. Increased neurogenesis in a rat ketamine model of schizophrenia. Biol Psychiatry. 2004;56:317–322. doi: 10.1016/j.biopsych.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Bolland BL, Dakis M. Memory for spatial locations, motor responses, and objects: triple dissociation among the hippocampus, caudate nucleus, and extrastriate visual cortex. Exp Brain Res. 1993;93:462–470. doi: 10.1007/BF00229361. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Kim D, Wylie G, Pasternak R, Butler P, Javitt D. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophr Res. 2006;82:1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman JE, Casanova MF, Jaskiw GE. The neuropathology of schizophrenia. Schizophr Bull. 1988;14:209–216. doi: 10.1093/schbul/14.2.209. [DOI] [PubMed] [Google Scholar]
- Klosterkötter J, Hellmich M, Steinmeyer E, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Kolomeets NS, Orlovskaya DD, Rachmanova VI, Uranova NA. Ultrastructural alterations in hippocampal mossy fiber synapses in schizophrenia: a postmortem morphometric study. Synapse. 2005;57:47–55. doi: 10.1002/syn.20153. [DOI] [PubMed] [Google Scholar]
- Kolomeets NS, Orlovskaya DD, Uranova NA. Decreased numerical density of CA3 hippocampal mossy fiber synapses in schizophrenia. Synapse. 2007;61:615–621. doi: 10.1002/syn.20405. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Nadri C, Agam G. Low GSK-3beta in schizophrenia as a consequence of neurodevelopmental insult. Eur Neuropsychopharmacol. 2005;15:1–11. doi: 10.1016/j.euroneuro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Krueger DD, Howell JL, Hebert BF, Olausson P, Taylor JR, Nail AC. Assessment of cognitive function in the heterozygous reeler mouse. Psychopharmacology. 2006;189:95–104. doi: 10.1007/s00213-006-0530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurylo D, Pasternak R, Silipo G, Javitt D, Butler P. Perceptual organization by proximity and similarity in schizophrenia. Schizophr Res. 2007;95:205–214. doi: 10.1016/j.schres.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Deakin JF. Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport. 2001;12:2971–2974. doi: 10.1097/00001756-200109170-00043. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry. 1998;172:110–120. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Grottick AJ, Higgins GA, Moreau JL. Phencyclidine exacerbates attentional deficits in a neurodevelopmental rat model of schizophrenia. Neuropsychopharmacology. 2003;28:1799–1809. doi: 10.1038/sj.npp.1300208. [DOI] [PubMed] [Google Scholar]
- Lederle L, Weber S, Wright T, Feyder M, Brigman J, Crombag H, Saksida LM, Bussey TJ, Holmes A. Reward-related behavioral paradigms for addiction research in the mouse: performance of common inbred strains. PLoS ONE. 2010 doi: 10.1371/journal.pone.0015536. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson V, Robbins T, Matheson E, Hutton S, Ron M, Barnes T, Joyce E. Discrimination learning, reversal, and set-shifting in first episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009a;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TR, Joyce EM. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009b;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Li W, Zhou Y, Jentsch JD, Brown RA, Tian X, Ehninger D, Hennah W, Peltonen L, Lonnqvist J, Huttunen MO, Kaprio J, Trachtenberg JT, Silva AJ, Cannon TD. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc Natl Acad Sci U S A. 2007;104:18280–18285. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Suzuki T, Seki T, Namba T, Tanimura A, Arai H. Effects of repeated phencyclidine administration on adult hippocampal neurogenesis in the rat. Synapse. 2006;60:56–68. doi: 10.1002/syn.20275. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. The Construct of Attention in Schizophrenia. Biological psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Sugino H, Hirose T, Kitagawa H, Nagai T, Mizoguchi H, Takuma K, Yamada K. Clozapine prevents a decrease in neurogenesis in mice repeatedly treated with phencyclidine. J Pharmacol Sci. 2007;103:299–308. doi: 10.1254/jphs.fp0061424. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McCarthy AD, Owens IJ, Bansal AT, McTighe SM, Bussey TJ, Saksida LM. ıFK962 and Donepezil act synergistically to improve cognition in rats: potential as an add-on therapy for Alzheimer’s Disease. Pharmacology, Biochemistry and Behavior. 2011;98:76–80. doi: 10.1016/j.pbb.2010.11.019. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Niewoehner B, Rawlins JN, Bannerman DM. Dorsal hippocampal N-methyl-D-aspartate receptors underlie spatial working memory performance during non-matching to place testing on the T-maze. Behav Brain Res. 2008;186:41–47. doi: 10.1016/j.bbr.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- McTighe SM, Mar AC, Romberg C, Bussey TJ, Saksida LM. A new touchscreen test of pattern separation: effect of hippocampal lesions. Neuroreport. 2009;20:881–885. doi: 10.1097/WNR.0b013e32832c5eb2. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- Milner B, Johnsrude I, Crane J. Right medial temporal-lobe contribution to object-location memory. Philos Trans R Soc Lond B Biol Sci. 1997;352:1469–1474. doi: 10.1098/rstb.1997.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cerebral cortex. 1996;6:470–481. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Dalley JW, Robbins TW. Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology. 2005;179:99–107. doi: 10.1007/s00213-004-2068-3. [DOI] [PubMed] [Google Scholar]
- Murray G, Cheng F, Clark L, Barnett J, Blackwell A, Fletcher P, Robbins T, Bullmore E, Jones P. Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull. 2008a;34:848–855. doi: 10.1093/schbul/sbn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray GK, Corlett PR, Clark L, Pessiglione M, Blackwell AD, Honey G, Jones PB, Bullmore ET, Robbins TW, Fletcher PC. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008b;13:239, 267–276. doi: 10.1038/sj.mp.4002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacology & therapeutics. 2010;128:419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Komiyama NH, Elsegood KA, Fricker DG, Saksida LM, Bussey TJ, Grant SGN. Dissociation of cognitive functions in mice with mutations in NMDA receptor binding scaffold proteins using a touchscreen-automated cognitive test battery: Implications for schizophrenia. Society for Neuroscience; Chicago, IL: 2009. [Google Scholar]
- Nuechterlein K, Pashler H, Subotnik K. Translating basic attentional paradigms to schizophrenia research: reconsidering the nature of the deficits. Developmental Psychopathology. 2006;18:831–851. doi: 10.1017/s095457940606041x. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008a;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Iii FJF, Gold JM, Goldberg T, Heaton RK, Keefe RSE, Kraemer H, Mesholamgately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. American journal of psychiatry. 2008b;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW. Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1995;33:1–24. doi: 10.1016/0028-3932(94)00098-a. [DOI] [PubMed] [Google Scholar]
- Paine TA, Carlezon WA., Jr Effects of antipsychotic drugs on MK-801-induced attentional and motivational deficits in rats. Neuropharmacology. 2009;56:788–797. doi: 10.1016/j.neuropharm.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA. Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biological psychiatry. 2007;62:687–693. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barber F, Barnes T, Nelson H, Owen A, Robbins T. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res. 1999;37:251–270. doi: 10.1016/s0920-9964(98)00156-x. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics Show Spatial Working Memory Deficits. Archives of General Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS, Goldmanrakic PS. Spatial Working-Memory Deficits in the Relatives of Schizophrenic-Patients. Archives of General Psychiatry. 1995;52:821–828. doi: 10.1001/archpsyc.1995.03950220031007. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur J Neurosci. 2000;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Passetti F, Levita L, Robbins TW. Sulpiride alleviates the attentional impairments of rats with medial prefrontal cortex lesions. Behavioural Brain Research. 2003;138:59–69. doi: 10.1016/s0166-4328(02)00229-2. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. Oxford University Press; London: 1927. [Google Scholar]
- Paz RD, Tardito S, Atzori M, Tseng KY. Glutamatergic Dysfunction in Schizophrenia: from basic neuroscience to clinical psychopharmacology. European neuropsychopharmacology. 2008;18:773–786. doi: 10.1016/j.euroneuro.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze MA, Dalley JW, Robbins TW. Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D(2/3) receptor antagonist sulpiride. Psychopharmacology. 2009;202:307–313. doi: 10.1007/s00213-008-1384-4. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Floresco SB. Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J Neurosci. 2004;24:547–553. doi: 10.1523/JNEUROSCI.4653-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper AA, Wu X, Han TW, Estill SJ, Dang Q, Wu LC, Reece-Fincanon S, Dudley CA, Richardson JA, Brat DJ, McKnight SL. The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc Natl Acad Sci U S A. 2005;102:14052–14057. doi: 10.1073/pnas.0506713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouteau A, Verdoux H, Briand C, Lesage A, Lalonde P, Nicole L, Reinharz D, Stip E. Self-assessed cognitive dysfunction and objective performance in outpatients with schizophrenia participating in a rehabilitation program. Schizophr Res. 2004;69:85–91. doi: 10.1016/j.schres.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Prouteau A, Verdoux H, Briand C, Lesage A, Lalonde P, Nicole L, Reinharz D, Stip E. Cognitive predictors of psychosocial functioning outcome in schizophrenia: a follow-up study of subjects participating in a rehabilitation program. Schizophr Res. 2005;77:343–353. doi: 10.1016/j.schres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Purdon SE, Valiakalayil A, Hanstock CC, Seres P, Tibbo P. Elevated 3T proton MRS glutamate levels associated with poor Continuous Performance Test (CPT-0X) scores and genetic risk for schizophrenia. Schizophrenia research. 2008;99:218–224. doi: 10.1016/j.schres.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Raedler T, Bymaster F, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry. 2007;12:232–246. doi: 10.1038/sj.mp.4001924. [DOI] [PubMed] [Google Scholar]
- Ragozzino M. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Rassovsky Y, Horan W, Lee J, Sergi M, Green M. Pathways between early visual processing and functional outcome in schizophrenia. Psychol Med. 2010:1–11. doi: 10.1017/S0033291710001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Reif A, Schmitt A, Fritzen S, Lesch KP. Neurogenesis and schizophrenia: dividing neurons in a divided mind? Eur Arch Psychiatry Clin Neurosci. 2007;257:290–299. doi: 10.1007/s00406-007-0733-3. [DOI] [PubMed] [Google Scholar]
- Relkovic D, Doe CM, Humby T, Johnstone KA, Resnick JL, Holland AJ, Hagan JJ, Wilkinson LS, Isles AR. Behavioural and cognitive abnormalities in an imprinting centre deletion mouse model for Prader-Willi syndrome. European journal of neuroscience. 2010;31:156–164. doi: 10.1111/j.1460-9568.2009.07048.x. [DOI] [PubMed] [Google Scholar]
- Ritsner MS, Blumenkrantz H. Predicting domain-specific insight of schizophrenia patients from symptomatology, multiple neurocognitive functions, and personality related traits. Psychiatry Res. 2007;149:59–69. doi: 10.1016/j.psychres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Robbins T. The case of frontostriatal dysfunction in schizophrenia. Schizophr Bull. 1990;16:391–402. doi: 10.1093/schbul/16.3.391. [DOI] [PubMed] [Google Scholar]
- Robbins T, James M, Owen A, Sahakian B, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Baunez C, Everitt BJ, Robbins TW. Lesions of the Medial and Lateral Striatum in the Rat Produce Differential Deficits in Attentional Performance. Behavioral Neuroscience. 2001;115:799–811. doi: 10.1037//0735-7044.115.4.799. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Romberg C, Bussey TJ, Saksida LM. Attentional impairments in the triple transgenic mouse model of Alzheimer’s disease: Rescue with donepezil (Aricept) Journal of Neuroscience. 2011 doi: 10.1523/JNEUROSCI.5242-10.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahgal A, Steckler T. TouchWindows and operant behaviour in rats. Journal of neuroscience methods. 1994;55:59–64. doi: 10.1016/0165-0270(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Saperstein AM, Fuller RL, Avila MT, Adami H, McMahon RP, Thaker GK, Gold JM. Spatial working memory as a cognitive endophenotype of schizophrenia: Assessing risk for pathophysiological dysfunction. Schizophrenia Bulletin. 2006;32:498–506. doi: 10.1093/schbul/sbj072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Sellin AK, Shad M, Tamminga C. Muscarinic agonists for the treatment of cognition in schizophrenia. CNS spectrums. 2008;13:985–996. doi: 10.1017/s1092852900014048. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S, Hatashita-Wong M, Schenkel L, Wilkniss S, Kovács I, Fehér A, Smith T, Goicochea C, Uhlhaas P, Carpiniello K, Savitz A. Reduced top-down influences in contour detection in schizophrenia. Cogn Neuropsychiatry. 2006;11:112–132. doi: 10.1080/13546800444000209. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Simpson E, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF. The Shaping of a Behaviorist. New York University Press; New York: 1979. [Google Scholar]
- Slaghuis W. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J Abnorm Psychol. 1998;107:49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Crider A, Winkelman JW, Turi A, Kamer RM, Kaplan LJ. Disrupted latent inhibition in the rat with chronic amphetamine or haloperidol-induced supersensitivity: relationship to schizophrenic attention disorder. Biol Psychiatry. 1981;16:519–537. [PubMed] [Google Scholar]
- Steckler T, Sahgal A. Psychopharmacological studies in rats responding at touch-sensitive devices. Psychopharmacology. 1995;118:226–229. doi: 10.1007/BF02245846. [DOI] [PubMed] [Google Scholar]
- Stip E, Sepehry AA, Prouteau A, Briand C, Nicole L, Lalonde P, Lesage A. Cognitive discernible factors between schizophrenia and schizoaffective disorder. Brain Cogn. 2005;59:292–295. doi: 10.1016/j.bandc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia--a synthesis and selective review. J Psychopharmacol. 2007;21:440–452. doi: 10.1177/0269881106073126. [DOI] [PubMed] [Google Scholar]
- Talpos JC, Dias R, Bussey TJ, Saksida LM. Hippocampal lesions in rats impair learning and memory for locations on a touchsensitive computer screen: the “ASAT” task. Behav Brain Res. 2008;192:216–225. doi: 10.1016/j.bbr.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Talpos JC, McTighe SM, Dias R, Saksida LM, Bussey TJ. Trial-unique, delayed nonmatching-to-location (TUNL): A novel, highly hippocampus-dependent automated touchscreen test of location memory and pattern separation. Neurobiol Learn Mem. 2010 doi: 10.1016/j.nlm.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpos JC, Winters BD, Dias R, Saksida LM, Bussey TJ. A novel touchscreen-automated paired-associate learning (PAL) task sensitive to pharmacological manipulation of the hippocampus: a translational rodent model of cognitive impairments in neurodegenerative disease. Psychopharmacology (Berl) 2009;205:157–168. doi: 10.1007/s00213-009-1526-3. [DOI] [PubMed] [Google Scholar]
- Tamminga C. Glutamatergic aspects of schizophrenia. British journal of psychiatry supplement. 1999;37:12–15. [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The Hippocampal Formation in Schizophrenia. Am J Psychiatry. 2010 doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Welsh RC, Chen AC, Velander AJ, Liberzon I. Medial frontal hyperactivity in reality distortion. Biol Psychiatry. 2007;61:1171–1178. doi: 10.1016/j.biopsych.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Nestler EJ. Neurobehavioral assessment in the information age. Nat Neurosci. 2004;7:462–466. doi: 10.1038/nn1225. [DOI] [PubMed] [Google Scholar]
- Thomson DM, McVie A, Morris BJ, Pratt JA. Dissociation of acute and chronic intermittent phencyclidine-induced performance deficits in the 5-choice serial reaction time task: influence of clozapine. Psychopharmacology. 2011;213:681–95. doi: 10.1007/s00213-010-2020-7. [DOI] [PubMed] [Google Scholar]
- Toro CT, Deakin JF. Adult neurogenesis and schizophrenia: a window on abnormal early brain development? Schizophr Res. 2007;90:1–14. doi: 10.1016/j.schres.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Pantelis C, McGorry PD, Dudgeon P, Brewer W, Cook M, Desmond P, Bridle N, Tierney P, Murrie V, Singh B, Copolov D. Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:133–141. doi: 10.1001/archpsyc.56.2.133. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Willis-Shore J, Poole JH, Marten E, Ober BA, Shenaut GK. Clinical and neurocognitive aspects of source monitoring errors in schizophrenia. Am J Psychiatry. 1997;154:1530–1537. doi: 10.1176/ajp.154.11.1530. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003a;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Rustay NR, Metten P, Crabbe JC. In search of a better mouse test. Trends Neurosci. 2003b;26:132–136. doi: 10.1016/S0166-2236(03)00033-X. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Waltz J, Gold J. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan RQ, Pang K, Olton DS. Hippocampal and amygdaloid involvement in nonspatial and spatial working memory in rats: effects of delay and interference. Behav Neurosci. 1994;108:866–882. doi: 10.1037//0735-7044.108.5.866. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Brown MW. Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia. 2010;48:2262–2272. doi: 10.1016/j.neuropsychologia.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Waters FA, Maybery MT, Badcock JC, Michie PT. Context memory and binding in schizophrenia. Schizophr Res. 2004;68:119–125. doi: 10.1016/S0920-9964(03)00221-4. [DOI] [PubMed] [Google Scholar]
- Weiner I, Feldon J. Facilitation of latent inhibition by haloperidol in rats. Psychopharmacology (Berl) 1987;91:248–253. doi: 10.1007/BF00217073. [DOI] [PubMed] [Google Scholar]
- Weiner I, Lubow RE, Feldon J. Abolition of the expression but not the acquisition of latent inhibition by chronic amphetamine in rats. Psychopharmacology (Berl) 1984;83:194–199. doi: 10.1007/BF00429734. [DOI] [PubMed] [Google Scholar]
- Wiedholz LM, Owens WA, Horton RE, Feyder M, Karlsson RM, Hefner K, Sprengel R, Celikel T, Daws LC, Holmes A. Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol Psychiatry. 2008;13:631–640. doi: 10.1038/sj.mp.4002056. [DOI] [PubMed] [Google Scholar]
- Winters B, Bartko S, Saksida L, Bussey T. Muscimol, AP5, or scopolamine infused into perirhinal cortex impairs two-choice visual discrimination learning in rats. Neurobiol Learn Mem. 2010;93:221–228. doi: 10.1016/j.nlm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Proffitt T, Mahony K, Smith DJ, Buchanan JA, Brewer W, Stuart GW, Velakoulis D, McGorry PD, Pantelis C. Visuospatial memory and learning in first-episode schizophreniform psychosis and established schizophrenia: a functional correlate of hippocampal pathology? Psychol Med. 2002;32:429–438. doi: 10.1017/s0033291702005275. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Ursu S, Ryan Walter BS, Walters R, Wendelken C, Ragland JD, Carter CS. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. American journal of psychiatry. 2008;165:1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacology & therapeutics. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


