Abstract
Bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), recently received FDA approval for recurrent glioblastoma. Additionally, several VEGF receptor (VEGFR) tyrosine kinase inhibitors (TKIs) have entered trials for recurrent glioma. Phase II studies of bevacizumab for recurrent GBM have reported incidents of ischemic stroke (IS) and intracranial hemorrhage (ICH); however, their clinical features and outcomes were not described in detail. We conducted a retrospective study of recurrent malignant glioma patients with radiographically-confirmed IS or ICH while on anti-angiogenic therapy. The study population included patients treated between 2005 and 2010 at the National Cancer Institute on four different phase I and II trials of antiangiogenic agents for recurrent malignant glioma, as well as patients receiving bevacizumab off clinical trial during this same period. Eight patients developed IS (50% lacunar) and 14 experienced ICH (79% intratumoral) while on antiangiogenic therapy for malignant glioma recurrence. The median age was 53 years, 17 patients (77%) were men, and 59% had glioblastoma. The frequencies of IS and ICH were 1.9% and 1.9% in bevacizumab trials. None of the patients on VEGFR TKI trials developed IS, while 3.8% experienced ICH. Patients with IS were treated with anti-angiogenic agents longer than those with ICH (median, 16.2 vs. 2.6 months, P = 0.001). Median survival was 7.8 months after IS and 2.6 months after ICH. The most common IS subtype was lacunar, while most ICHs were asymptomatic and intratumoral. Overall, IS seems to be a complication of prolonged antiangiogenic therapy, while intratumoral bleeds often occur in the setting of tumor progression.
Keywords: Glioma, Glioblastoma, Antiangiogenic agents, Bevacizumab, stroke, CNS hemorrhage
Introduction
Malignant gliomas overexpress vascular endothelial growth factor (VEGF), a key promoter of angiogenesis [1]. Bevacizumab, an antiangiogenic antibody against VEGF, recently received accelerated FDA approval for recurrent glioblastoma (GBM) [2, 3]. This agent prolongs progression-free survival, promotes unprecedented radiographic response rates of more than 25%, and reduces the corticosteroid doses necessary to manage peritumoral edema, though an overall survival benefit has not been definitively demonstrated [2-4]. Additionally, several VEGF receptor (VEGFR) tyrosine kinase inhibitors (TKIs) are currently in clinical trials for malignant glioma.
Randomized trials of bevacizumab [5] and VEGFR TKIs [6] for non-CNS malignancies have demonstrated an increased risk of hemorrhage and ischemic stroke (IS). Similarly, smaller phase II studies of bevacizumab for recurrent GBM have reported incidents of IS and intracranial hemorrhage (ICH). However, their clinical features and outcomes were not described in detail, as these reports used the Common Toxicity Criteria for oncological trials [2, 3]. Glioma patients already have an inherently elevated risk of IS and ICH [7, 8]. In light of increasing use of VEGF-directed therapies, we evaluated the incidence, clinical features, and outcomes of IS and ICH in glioma patients receiving antiangiogenic therapy (AT).
Methods
We conducted a retrospective study of recurrent malignant glioma patients with radiographically-confirmed IS or ICH while on AT. We reviewed adverse events from 4 phase I/II trials of AT for recurrent malignant glioma conducted at the National Cancer Institute (NCI) between 2005 and 2010 (Tables 1, 2). Additionally, we included patients receiving bevacizumab off trial who were evaluated at the NCI during this same period. We collected relevant data for each patient, including basic demographics; risk factors for IS or ICH; history of treatment with surgery, radiation, chemotherapy, and antiangiogenic agents; location of tumor versus location of IS or ICH; clinical presentation; management following adverse event; subsequent anti-tumor therapies; and survival duration.
Table 1.
Incidence of ischemic strokes and intracranial hemorrhages in clinical trials of vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKIs) for recurrent malignant gliomas conducted by the Neuro-Oncology Branch at the NIH
| Clinical trials.gov identifier | Drugs | No. of patients | No. of ischemic strokes | No. of intracranial hemorrhages |
|---|---|---|---|---|
| NCT00293566 | Vandetanib | 83 | 0 | 3 |
| NCT00923117 | Sunitinib | 75 | 0 | 3 |
| Total | 158 | 0 (0%) | 6 (3.8%) |
Table 2.
Incidence of ischemic strokes and intracranial hemorrhages in clinical trials of bevacizumab-containing regimens for recurrent malignant gliomas conducted by the Neuro-Oncology Branch at the NIH
| Clinicaltrials.gov identifier | Drugs | No. of patients | No. of ischemic strokes | No. of intracranial hemorrhages |
|---|---|---|---|---|
| NCT00271609 | Bevacizumab | 88 | 1 | 1 |
| NCT00559923 | Bevacizumab and enzastaurin | 73 | 2 | 2 |
| Total | 161 | 3 (1.9%) | 3 (1.9%) |
All IS and ICH diagnoses were confirmed by neuroimaging. Classic stroke symptoms and syndromes were not required for the diagnosis of IS or ICH, because the baseline neurological abnormalities of malignant glioma patients make the clinical diagnosis of stroke difficult. We defined IS as an area of diffusion-weighted imaging (DWI) hyperintensity not attributable to the T2-weighted image ‘shine-through’ phenomenon, with corresponding apparent diffusion coefficient (ADC) hypointensity. The IS area needed to be outside of the established tumor volume, because gliomas treated with AT often exhibit restricted diffusion within the tumor volume. Additionally, diffusion-restricted lesions needed to fit an arterial vascular distribution. In most cases, subsequent scans were obtained to demonstrate resolution of these lesions in accordance with typical IS patterns, thereby ensuring that such lesions did not instead represent new tumor foci. Brain MRI perfusion using dynamic susceptibility contrast (DSC) was analyzed in a subset of patients with IS. Episodes of IS were classified by trial of org 10172 in acute stroke treatment (TOAST) criteria [9]. Duration of AT was defined as the continuous period of any anti-VEGF/VEGFR therapy immediately preceding the cerebrovascular event (CVE).
Statistical analysis
All statistical calculations were performed using STATA v10.0 (StataCorp: College Station, TX). To account for variable AT durations, we calculated the IS and ICH rates per 100 person-months. The Kaplan–Meier method and log-rank test were used for survival analysis and for comparison of AT duration preceding IS versus ICH. Follow-up extended through October 30, 2010. All patients provided written informed consent to the NCI IRB-approved protocol “Natural History Study of Patients with CNS Tumors” (clinicaltrials.gov identifier: NCT00458640) and individual therapeutic trials.
Results
The 22 patients with IS or ICH included in our study generally reflected the larger malignant glioma population in terms of their demographics (Tables 3, 4). Twelve of 22 patients were enrolled on NCI trials for recurrent malignant glioma using VEGFR TKIs (Table 1) or bevacizumab (Table 2) at the time of IS or ICH; the other 10 patients developed either IS or ICH while receiving bevacizumab off clinical trial. The median age of all 22 patients was 53 years; 17 patients (77%) were men; and 13 patients (59%) had GBM. Eight patients developed IS, and 14 experienced ICH while on AT for malignant glioma recurrence. All subjects had undergone radiotherapy, at a median of 19.4 months prior to the CVE. Patients had received a median of 2 anti-glioma regimens before initiation of the AT regimen associated with the CVE.
Table 3.
Malignant glioma patients diagnosed with ischemic strokes (IS) while on antiangiogenic therapy
| Patient | Age at diagnosis/ gender |
Type of IS | Histology | Tumor location |
Risk factors for IS |
No. of prior anti- glioma regimens |
Prior anti- VEGF/ VEGFR regimens |
Time on anti-VEGF/ VEGFR prior to IS (mos.) |
Time from tumor diagnosis to IS (mos.) |
Regimen at time of IS |
Symptoms at diagnosis if IS |
IS Location | Survival after IS (mos.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39/M | Lacunar | AA | R frontal | HTN, DVT/PE | 2 | Bevacizumab, enzastaurin |
6.0 | 15.4 | Bevacizumab | AMS, Dysarthria |
L brainstem | 7.8 |
| 2 | 54/F | Lacunar | GBM | R temporal | None | 4 | Sunitinib | 16.2 | 42.2 | Bevacizumab | Hemiparesis, Sensory |
R basal ganglia |
2.0+ |
| 3t | 61/M | Lacunar | GBM | L frontal | HTN, Dyslipidemia |
2 | None | 20.6 | 35.1 | Bevacizumab, enzastaurin |
Asymptomatic | R basal ganglia |
10.9+ |
| 4t | 67/F | Lacunar | GBM | L frontal | Smoking | 1 | None | 19.1 | 27.4 | Bevacizumab, enzastaurin |
Hemiparesis, Dysarthria |
R basal ganglia Intern. capsule |
7.6+ |
| 5 | 42/M | Cardioembolic | GBM | R frontal | DVT/PE | 3 | Bevacizumab, tandutinib |
23.3 | 29.3 | Bevacizumab | AMS | Bilateral pancortical |
1.6 |
| 6 | 75/M | Cardioembolic | GBM | R parietal- occipital, corpus callosum |
A-fib, HTN, Smoking, Dyslipidemia |
2 | Bevacizumab | 14.6 | 22.4 | Bevacizumab, carboplatin |
Sensory | R parietal | 5.2 |
| 7 | 69/F | Large artery | GBM | L parietal | DVT/PE, smoking |
2 | Bevacizumab, irinotecan |
19.6 | 23.9 | Bevacizumab | AMS | R occipital | 3.9+ |
| 8t | 62/M | Unclassified | GBM | R occipital | Unspecified Arrhythmia |
2 | None | 2.5 | 16.2 | Bevacizumab | AMS | Brainstem | 0.2 |
IS ischemic stroke, HTN hypertension, DVT deep vein thrombosis, PE pulmonary embolism, A-fib atrial fibrillation, GBM glioblastoma, AA anaplastic astrocytoma, AMS altered mental status, t patients with IS while on clinical trial
Table 4.
Malignant glioma patients diagnosed with intracranial hemorrhages (ICH) while on antiangiogenic therapy
| Patient | Age at diagnosis/ gender |
Type of ICH | Tumor histology | Location of tumor |
Risk factors for ICH |
No. of prior anti- glioma regimens |
Prior anti-VEGF/ VEGFR Regimens |
Time on anti- VEGF/ VEGFR prior to ICH (mos.) |
Time from tumor Diagnosis to ICH (mos.) |
Regimen at time of Event |
Symptoms at diagnosis of ICH |
Survival after ICH (mos.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1t | 34/M | Intratumoral | Anaplastic oligoastrocytoma |
R frontal/ temporal |
HTN | 2 | None | 0.9 | 124.9 | Bevacizumab, enzastaurin |
Asymptomatic | 2.4+ |
| 2t | 36/M | Intratumoral | Anaplastic oligodendroglioma |
Bilateral temporal/ parietal/ occipital, corpus callosum |
Anticoagulant use | 6 | None | 2.2 | 50.8 | Vandetanib | Hemiparesis, AMS |
1.5 |
| 3t | 46/M | Intratumoral | Gliosarcoma | R frontal | None | 4 | Bevacizumab | 1.3 | 14.7 | Sunitinib | AMS, Vomiting, Headache |
0.7 |
| 4t | 50/M | Intratumoral | GBM | L frontal/parietal | None | 2 | None | 0.9 | 11.3 | Bevacizumab, enzastaurin |
Asymptomatic | 2.6 |
| 5 | 51/M | Intratumoral | GBM | L temporal | None | 1 | None | 2.6 | 16.2 | Bevacizumab | Asymptomatic | 1.5+ |
| 6 | 56/M | Intratumoral | AA | R temporal | None | 5 | Bevacizumab, enzastaurin; Sunitinib, Bevacizumab |
4.4 | 35.0 | Bevacizumab, carboplatin |
Seizure | 1.7 |
| 7t | 59/F | Intratumoral | AA | R frontal | Mild thrombocytopenia |
2 | Bevacizumab, enzastaurin |
4.6 | 19.7 | Sunitinib | Hemiparesis, AMS |
3.7 |
| 8t | 62/M | Intratumoral | GBM | R frontal/ temporal |
HTN | 4 | Bevacizumab, temozolomide; Bevacizumab, irinotecan; Bevacizumab, carboplatin |
12.7 | 18.8 | Sunitinib | AMS | 0.5 |
| 9t | 67/M | Intratumoral | GBM | R temporal | HTN | 1 | None | 9.0 | 18.9 | Vandetinib | Asymptomatic | 7.3 |
| 10 | 51/M | Intratumoral, Subdural |
Gliosarcoma | L frontal/parietal | HTN, Antiplatelet use |
1 | None | 15.9 | 21.2 | Bevacizumab | Language Changes, AMS, Vomiting, Headache |
2.9+ |
| 11 | 52/M | Intratumoral Intraventricular |
Anaplastic oligoastrocytoma |
Bilateral frontal, corpus callosum, cerebellum brainstem |
Anticoagulant use | 2 | None | 0.2 | 168.8 | Bevacizumab, irinotecan |
AMS, Gait Changes |
1.3+ |
| 12t | 60/M | Subdural | AA | Corpus callosum | None | 1 | None | 10.0 | 18.6 | Bevacizumab | AMS, Gait Changes |
4.6 |
| 13 | 37/M | Cavernoma intralesional |
GBM | L fronto- parietal |
None | 1 | None | 0.8 | 96.8 | Bevacizumab, carboplatin |
Asymptomatic | 2.0+ |
| 14t | 47/F | Unspecified intracerebral |
GBM | R parietal/ occipital |
None | 2 | Bevacizumab | 4.5 | 19.5 | Vandetinib | Hemiparesis, AMS |
0.3 |
HTN hypertension, GBM glioblastoma, AA anaplastic astrocytoma, AMS altered mental status, t patients with ICH while on clinical trial
Ischemic stroke (IS)
There were 4 lacunar, 2 cardioembolic, 1 large-vessel, and 1 unclassified IS events (Table 3). We included only patients with IS outside the tumor volume (Fig. 1). 88% of these patients had one or more IS risk factors; additionally, 50% had developed AT-induced hypertension. Altered mental status and lateralizing symptoms were the most common presentations, while one patient had an asymptomatic lacunar infarct. Five patients with IS had available perfusion MRI scans. For four patients, the stroke area defined by DWI and ADC maps showed decreased perfusion; one patient had a lacunar stroke that was too small to be assessed by perfusion MRI. Among these five IS patients, three exhibited restricted diffusion within the initial tumor volume; in all three cases, the initial tumor volume had developed this decreased perfusion during bevacizumab treatment.
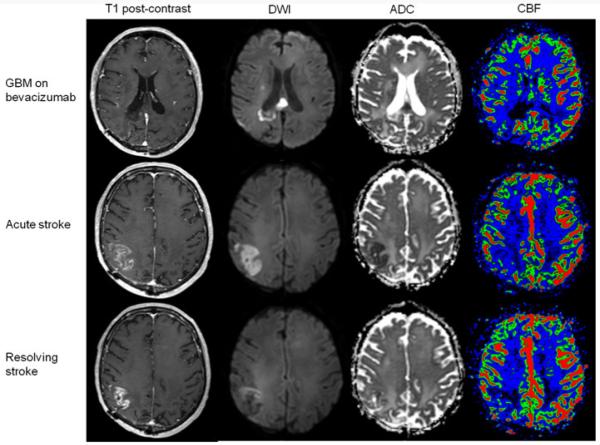
Fig. 1.
Patient with restricted diffusion within the tumor volume related to bevacizumab treatment who developed a right middle cerebral artery ischemic stroke. Upper panel Patient (Table 3, patient 6) with a corpus callosum glioblastoma treated with bevacizumab. This lesion, with minimal enhancement on post-gadolinium T1-weighted images, demonstrated restricted diffusion within the tumor volume on diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC). Additionally, the tumor showed decreased cerebral blood flow (CBF) for several months. Middle panel The same patient was found to have a wedge-shaped enhancing lesion on post-gadolinium T1-weighted images outside the original tumor volume–within the right parietal lobe–on a routine MRI for assessment of his GBM. On exam, he had new left-sided cortical sensory signs. DWI and ADC showed restricted diffusion; CBF was decreased in that area, compared to the contralateral cortex. Lower panel Follow-up MRI four weeks later showed improvement of enhancement on post-gadolinium T1-weighted images, resolution of restricted diffusion on DWI and ADC, and persistence of decreased CBF, all compatible with a resolving ischemic stroke
Prior to the IS, patients had received AT for a median of 16.2 months. At the time of IS diagnosis, six patients had tumor response or stability on AT, while two had tumor progression. There were no cases of IS among 158 patients on VEGFR TKI trials (Table 1). The frequency of IS in bevacizumab trials for recurrent malignant glioma was 1.9% (3 out of 161 patients; Table 2), corresponding to a rate of 0.38 cases per 100 patient-months. Five patients started antiplatelet drugs, and one patient with atrial fibrillation received oral anticoagulation. Patients 5 and 8 died 1.6 and 0.2 months after the IS and did not receive antiplatelets or anticoagulants. Three patients received no further anti-glioma therapy, 2 received cytotoxic chemotherapy, and 3 resumed bevacizumab for 1.5, 2, and 4 months without further CNS vascular events. Four patients had died (2 of tumor progression, 2 of IS); median survival from IS diagnosis was 7.8 months.
Intracranial hemorrhage (ICH)
There were 11 intratumoral (with concurrent subdural or intraventricular in one patient each), 1 distant intraparenchymal, 1 subdural, and 1 unspecified ICH events (Table 4). Nine of 11 intratumoral bleeds occurred at the time of tumor progression. Nine patients (64%) developed hypertension during AT. Five patients (36%) were asymptomatic at diagnosis. Four of these asymptomatic patients had intratumoral bleeds, while 1 had a small hemorrhage within a radiation-induced cavernous malformation. At ICH diagnosis, patients had been on AT for a median of 2.6 months. Eight patients were on bevacizumab, and six were on VEGFR TKIs (sunitinib or vandetanib). There were three cases of ICH (1.9%) among 161 patients on bevacizumab trials (0.38 cases per 100 patient-months; Table 2) and six cases of ICH (3.8%) among 158 patients on trials of VEGFR inhibitors (1.6 cases per 100 patient-months; Table 1). One patient required subdural hematoma decompression; another required ventriculoperitoneal shunt placement for intraventricular hemorrhage and hydrocephalus. Seven patients received no further glioma therapy, six received cytotoxic drugs, and one later resumed bevacizumab for two months but had tumor progression with no further bleeding. Nine patients had died (3 of tumor progression, 2 of ICH, and 4 of unknown causes); median survival from ICH diagnosis was 2.6 months.
Discussion
The incidence of IS and ICH among recurrent malignant glioma patients receiving AT was relatively low. Despite difficulties in comparing complication rates across trials due to variable treatment durations, the ICH rates of 1.9% with bevacizumab and 3.8% with VEGFR TKIs are comparable to a 2.2% ICH rate seen in 184 patients treated with non-antiangiogenic agents for recurrent malignant glioma [10]. Meta-analysis of AT trials for non-CNS malignancies, with most of the trials excluding patients with cerebral metastases, revealed an ICH rate of 0.1 to 0.7% [11, 12]. The higher ICH rates reported in studies of malignant glioma, irrespective of AT, likely reflect the inherent tendency of these tumors to bleed. Notably, 71% of our ICH cases occurred intratumorally in the setting of tumor progression. These ICH cases may not have had a causal relationship with AT because of the propensity of malignant gliomas to bleed at the time of progression.
IS occurred in 1.9% of patients treated on our bevacizumab trials, which is comparable to a 1.7% incidence of IS or transient ischemic attacks among 983 patients treated with bevacizumab for non-CNS cancers [5]. Considering that glioma patients have an increased risk of IS due to radiation vasculopathy and hypercoagulability [8], this low frequency of IS with bevacizumab is somewhat reassuring. However, to establish definitively that AT increases the risk of IS or ICH in glioma patients, large-scale randomized controlled trials are needed. While we await the results of two randomized controlled trials of temozolomide chemoradiotherapy with or without bevacizumab, one can attempt to interpret our IS rate by comparison to historical data. A tertiary cancer center reported an estimated IS frequency of 1.3% in glioma patients treated in the pre-AT era over the full disease course [8]. A review of 7 non-AT trials conducted by an NCI-sponsored brain tumor consortium for recurrent malignant glioma patients [13-19], a population similar to that of our current study, described no cases of IS among 409 patients. While the comparison of absolute IS frequencies is difficult, the absence of IS in these non-antiangionenic agent phase I and II trials may be the result of short trial duration due to early tumor progression and treatment cessation. In addition, the absence of IS could be partially attributable to DWI unavailability at the time these studies were conducted.
Despite the relatively low frequencies of IS and ICH in our study, the survival time of glioma patients after CVEs on AT was short. This observation could be explained by the severity of the CVEs experienced by recurrent malignant glioma patients on AT or by our 25% IS-related acute mortality rate, which is higher than the 7% acute mortality rate seen in the general IS population [20]. Our ICH-related acute mortality was at least 14%, which similarly is higher than the 8% rate in a prior study of primary brain tumors [7]. More likely, however, this short overall survival reflects a population of recurrent malignant glioma patients who were heavily pretreated with a median of 2 anti-glioma regimens before the AT. After CVEs, anti-tumor treatment options were limited, and performance status was usually poor; consequently, almost 50% of patients did not received further anti-glioma therapy.
Interestingly, patients with IS had been on AT much longer than those with ICH (median of 16.2 vs. 2.6 months, P = 0.001). Most patients with recurrent malignant glioma receive AT for only a few months because of early tumor progression, while those with prolonged tumor responses seem particularly at risk for IS. Thus, preliminary data suggesting that bevacizumab has better efficacy than VEGFR TKIs [21] may explain the absence of IS in 158 patients treated with VEGFR TKIs. Additionally, a recent trial of upfront radiation, temozolomide, and bevacizumab for newly diagnosed GBM reported an IS incidence of 9%, [22] with IS occurring at a range of 83–538 days from initial resection. While the extended course of AT preceding IS in this study agrees with our findings, further studies are still needed to determine whether longer bevacizumab treatment duration is associated with an increased risk of IS; whether an increased risk of IS persists after discontinuation of AT; and whether AT potentiates the risk of radiation-induced vasculopathy.
We included only IS outside the glioma volume, because many patients on AT develop intratumoral areas of restricted diffusion on DWI [23]. Importantly, IS-related restricted diffusion areas resolve in weeks; in contrast, intratumoral DWI-hyperintense lesions typically persist throughout AT and may represent hypercellular tumor [24] or tumor necrosis [23]. In our small sample of IS cases, DSC MRI perfusion did not seem useful to distinguish IS from intratumoral DWI diffusion restriction, because perfusion was decreased for both lesions. Another small study also showed decreased perfusion in DWI diffusion-restricted areas within the glioma volume [23]; nevertheless, larger studies are needed to determine whether MRI perfusion is useful to differentiate DWI diffusion-restricted lesions in glioma patients receiving AT. Until the completion of such studies, IS diagnoses in glioma patients should be based on a combination of clinical presentation, neuroimaging characteristics (e.g., compatibility with arterial vascular distribution), and, most importantly, the temporal evolution of the DWI diffusion-restricted lesions. Although regions of transient restricted diffusion outside the tumor volume could be alternatively attributable to post-seizure cortical diffusion abnormalities [25], our patients with cortical IS exhibited wedge-shaped or multifocal lesions that were unlikely to be seizure-related. A prior study of glioma patients not on AT found that IS was the initial clinical diagnosis in only 43% of patients with imaging-confirmed IS and that 15% of IS cases were asymptomatic [8]. Consequently, DWI sequences should be routinely acquired for all glioma patients, as the clinical presentation is often not classic in this population with already significant underlying neurological deficits.
Inhibition of VEGF decreases nitric oxide and prostacyclin production, activates procoagulant pathways, and may elevate blood viscosity, all potentially increasing the risk of arterial ischemic events [26]. Conversely, VEGF inhibition decreases proliferation, migration, and survival of endothelial cells, which can compromise vascular integrity and may predispose to hemorrhage [26]. In addition, arterial hypertension is a common side effect of bevacizumab and VEGFR TKIs [5] that may contribute to the risk of IS and ICH, although the hypertension is usually transient and easily controlled with standard antihypertensives. To analyze properly whether AT-induced hypertension or any other factors predispose to IS or ICH in this patient population, a much larger study is needed.
Because the pathophysiology underlying IS and ICH on AT remains unclear, no specific prophylactic or treatment recommendations can be made. These complications should, therefore, be managed with standard measures whenever appropriate. The patient’s clinical condition and underlying tumor status often guide management of CVEs and further glioma therapy. While clinical trials usually require cessation of AT after IS or ICH, three patients with IS and 1 with ICH in our study population resumed bevacizumab off trial without further vascular complications, as the potential benefit of controlling their tumors was thought to outweigh the risk of CVE recurrence. However, the small number and short follow-up of these patients cannot confirm the safety of this approach. Thus, the potential risk of recurrent CVEs should be carefully assessed and thoroughly discussed with each patient before restarting AT.
Our study has inherent limitations due to its retrospective nature and the small number of CVEs. Our findings cannot determine causality or the effect size of AT on the risk of IS or ICH in malignant glioma patients. Only randomized controlled studies can assess the relative risk of IS and ICH; a completed trial of cediranib, a VEGFR TKI, with or without lomustine versus lomustine alone and two ongoing phase III trials of standard chemoradiation with or without bevacizumab will help clarify these issues. However, because the incidence of IS and ICH is expected to be relatively low, meta-analysis of these large, randomized trials may be needed to define the effect of AT on the risk of CVEs in glioma patients. Moreover, the increased CVE rates seen in control patients due to radiation-induced vasculopathy, hypercoagulability, and spontaneous intra-tumoral hemorrhage, may similarly necessitate meta-analysis of these large studies to achieve sufficient statistical power.
Acknowledgments
NIH Intramural Research Program (1ZIDBC011098-02). T.J.F. is a Fellow in the Clinical Research Training Program, a public–private partnership supported jointly by the NIH and Pfizer Inc (via a grant to the Foundation for NIH from Pfizer Inc). F.M.I. is supported by the National Cancer Institute’s Clinical Investigator Development Program and the NIH Intramural Program (1ZIABC011347-01 and 1ZIABC011348-01).
Contributor Information
Tyler J. Fraum, Neuro-Oncology Branch, National Cancer Institute and National Institute of Neurological Disorders and Stroke, National Institutes of Health, 9030 Old Georgetown Rd., Room 221, Bethesda, MD 20892-8202, USA; Duke University School of Medicine, Durham, NC, USA; tyler.fraum@duke.edu
Teri N. Kreisl, Neuro-Oncology Branch, National Cancer Institute and National Institute of Neurological Disorders and Stroke, National Institutes of Health, 9030 Old Georgetown Rd., Room 221, Bethesda, MD 20892-8202, USA; kreislt@mail.nih.gov
Joohee Sul, Neuro-Oncology Branch, National Cancer Institute and National Institute of Neurological Disorders and Stroke, National Institutes of Health, 9030 Old Georgetown Rd., Room 221, Bethesda, MD 20892-8202, USA; joohee.sul@nih.gov.
Howard A. Fine, Neuro-Oncology Branch, National Cancer Institute and National Institute of Neurological Disorders and Stroke, National Institutes of Health, 9030 Old Georgetown Rd., Room 221, Bethesda, MD 20892-8202, USA; hfine@mail.nih.gov
Fabio M. Iwamoto, Neuro-Oncology Branch, National Cancer Institute and National Institute of Neurological Disorders and Stroke, National Institutes of Health, 9030 Old Georgetown Rd., Room 221, Bethesda, MD 20892-8202, USA
References
- 1.Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol. 2009;5:610–620. doi: 10.1038/nrneurol.2009.159. doi:10.1038/nrneurol.2009.159. [DOI] [PubMed] [Google Scholar]
- 2.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. doi:10.1200/JCO. 2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 3.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. doi:10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quant EC, Norden AD, Drappatz J, Muzikansky A, Doherty L, Lafrankie D, Ciampa A, Kesari S, Wen PY. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11:550–555. doi: 10.1215/15228517-2009-006. doi:10.1215/15228517-2009-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, Bergsland E, Ngai J, Holmgren E, Wang J, Hurwitz H. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. doi:10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 6.Zangari M, Fink LM, Elice F, Zhan F, Adcock DM, Tricot GJ. Thrombotic events in patients with cancer receiving antiangiogenesis agents. J Clin Oncol. 2009;27:4865–4873. doi: 10.1200/JCO.2009.22.3875. doi:10.1200/JCO.2009.22.3875. [DOI] [PubMed] [Google Scholar]
- 7.Navi BB, Reichman JS, Berlin D, Reiner AS, Panageas KS, Segal AZ, DeAngelis LM. Intracerebral and subarachnoid hemorrhage in patients with cancer. Neurology. 2010;74:494–501. doi: 10.1212/WNL.0b013e3181cef837. doi:10.1212/WNL.0b013e3181cef837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreisl TN, Toothaker T, Karimi S, DeAngelis LM. Ischemic stroke in patients with primary brain tumors. Neurology. 2008;70:2314–2320. doi: 10.1212/01.wnl.0000314648.82924.6f. doi:10.1212/01.wnl.0000314648.82924.6f. [DOI] [PubMed] [Google Scholar]
- 9.Adams HP, Jr, Woolson RF, Biller J, Clarke W, TOAST Study Group Studies of Org 10172 in patients with acute ischemic stroke. Haemostasis. 1992;22:99–103. doi: 10.1159/000216301. [DOI] [PubMed] [Google Scholar]
- 10.Wen PY, Yung WK, Lamborn KR, Dahia PL, Wang Y, Peng B, Abrey LE, Raizer J, Cloughesy TF, Fink K, Gilbert M, Chang S, Junck L, Schiff D, Lieberman F, Fine HA, Mehta M, Robins HI, DeAngelis LM, Groves MD, Puduvalli VK, Levin V, Conrad C, Maher EA, Aldape K, Hayes M, Letvak L, Egorin MJ, Capdeville R, Kaplan R, Murgo AJ, Stiles C, Prados MD. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12:4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. doi:10.1158/1078-0432. [DOI] [PubMed] [Google Scholar]
- 11.Carden CP, Larkin JM, Rosenthal MA. What is the risk of intracranial bleeding during anti-VEGF therapy? Neuro Oncol. 2008;10:624–630. doi: 10.1215/15228517-2008-010. doi:10.1215/15228517-2008-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hapani S, Sher A, Chu D, Wu S. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology. 2010;79:27–38. doi: 10.1159/000314980. doi:10.1159/000314980. [DOI] [PubMed] [Google Scholar]
- 13.Prados MD, Lamborn K, Yung WK, Jaeckle K, Robins HI, Mehta M, Fine HA, Wen PY, Cloughesy T, Chang S, Nicholas MK, Schiff D, Greenberg H, Junck L, Fink K, Hess K, Kuhn J. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro Oncol. 2006;8:189–193. doi: 10.1215/15228517-2005-010. doi:10.1215/15228517-2005-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puduvalli VK, Yung WK, Hess KR, Kuhn JG, Groves MD, Levin VA, Zwiebel J, Chang SM, Cloughesy TF, Junck L, Wen P, Lieberman F, Conrad CA, Gilbert MR, Meyers CA, Liu V, Mehta MP, Nicholas MK, Prados M. Phase II study of fenretinide (NSC 374551) in adults with recurrent malignant gliomas: A North American Brain Tumor Consortium study. J Clin Oncol. 2004;22:4282–4289. doi: 10.1200/JCO.2004.09.096. doi:10.1200/JCO.2004.09.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cloughesy TF, Wen PY, Robins HI, Chang SM, Groves MD, Fink KL, Junck L, Schiff D, Abrey L, Gilbert MR, Lieberman F, Kuhn J, DeAngelis LM, Mehta M, Raizer JJ, Yung WK, Aldape K, Wright J, Lamborn KR, Prados MD. Phase II trial of tipifarnib in patients with recurrent malignant glioma either receiving or not receiving enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2006;24:3651–3656. doi: 10.1200/JCO.2006.06.2323. doi:10.1200/JCO.2006.06.2323. [DOI] [PubMed] [Google Scholar]
- 16.Butowski N, Chang SM, Junck L, DeAngelis LM, Abrey L, Fink K, Cloughesy T, Lamborn KR, Salazar AM, Prados MD. A phase II clinical trial of poly-ICLC with radiation for adult patients with newly diagnosed supratentorial glioblastoma: a North American Brain Tumor Consortium (NABTC01–05) J Neurooncol. 2009;91:175–182. doi: 10.1007/s11060-008-9693-3. doi:10.1007/s11060-008-9693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raizer JJ, Abrey LE, Lassman AB, Chang SM, Lamborn KR, Kuhn JG, Yung WK, Gilbert MR, Aldape KA, Wen PY, Fine HA, Mehta M, Deangelis LM, Lieberman F, Cloughesy TF, Robins HI, Dancey J, Prados MD. A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro Oncol. 2010;12:95–103. doi: 10.1093/neuonc/nop015. doi:10.1093/neuonc/nop015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang SM, Wen P, Cloughesy T, Greenberg H, Schiff D, Conrad C, Fink K, Robins HI, De Angelis L, Raizer J, Hess K, Aldape K, Lamborn KR, Kuhn J, Dancey J, Prados MD. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–361. doi: 10.1007/s10637-005-1444-0. doi:10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 19.Iwamoto FM, Lamborn KR, Kuhn JG, Wen PY, Yung WK, Gilbert MR, Chang SM, Lieberman FS, Prados MD, Fine HA. A phase I/II trial of the histone deacetylase inhibitor, romidepsin, for adults with recurrent malignant glioma: North American Brain Tumor Consortium Study 03-03. Neuro Oncol. 2011 doi: 10.1093/neuonc/nor017. doi:10.1093/neuonc/nor017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. doi:10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 21.Iwamoto FM, Lamborn KR, Robins HI, Mehta MP, Chang SM, Butowski NA, Deangelis LM, Abrey LE, Zhang WT, Prados MD, Fine HA. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06–02) Neuro Oncol. 2010;12:855–861. doi: 10.1093/neuonc/noq025. doi:10.1093/neuonc/noq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai A, Tran A, Nghiemphu PL, Pope WB, Solis OE, Selch M, Filka E, Yong WH, Mischel PS, Liau LM, Phuphanich S, Black K, Peak S, Green RM, Spier CE, Kolevska T, Polikoff J, Fehrenbacher L, Elashoff R, Cloughesy T. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29:142–148. doi: 10.1200/JCO.2010.30.2729. doi:10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rieger J, Bahr O, Muller K, Franz K, Steinbach J, Hattingen E. Bevacizumab-induced diffusion-restricted lesions in malignant glioma patients. J Neurooncol. 2010;99:49–56. doi: 10.1007/s11060-009-0098-8. doi:10.1007/s11060-009-0098-8. [DOI] [PubMed] [Google Scholar]
- 24.Gerstner ER, Frosch MP, Batchelor TT. Diffusion magnetic resonance imaging detects pathologically confirmed, nonenhancing tumor progression in a patient with recurrent glioblastoma receiving bevacizumab. J Clin Oncol. 2010;28:e91–e93. doi: 10.1200/JCO.2009.25.0233. doi:10.1200/JCO.2009.25.0233. [DOI] [PubMed] [Google Scholar]
- 25.Hormigo A, Liberato B, Lis E, DeAngelis LM. Nonconvulsive status epilepticus in patients with cancer: imaging abnormalities. Arch Neurol. 2004;61:362–365. doi: 10.1001/archneur.61.3.362. doi:10.1001/archneur. 61.3.362. [DOI] [PubMed] [Google Scholar]
- 26.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. doi:10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]