Abstract
Current anticoagulants target coagulation Factors upstream from fibrin assembly and polymerization (i.e. formation of fibrin clot). While effective, this approach requires constant patient monitoring since pharmacokinetics and pharmacodynamics vary from patient to patient. To address these limitations, we developed an alternative anticoagulant that effectively inhibits fibrin polymerization. Specifically, we investigated PEGylated fibrin knob `A' peptides, evaluating the effect of both PEG chain length (0, 2, 5, 10, 20, and 30 kDa) and knob peptide sequence (GPRPAAC, GPRPFPAC, and GPRPPERC) on inhibiting fibrin polymerization (i.e. clot formation). Thrombin-initiated clotting assays with purified fibrinogen were performed to compare clot formation with each peptide-PEG conjugate. Results indicated a biphasic effect of PEG chain length whereby active-PEG conjugates demonstrated increasingly enhanced inhibition of fibrin polymerization from 0 to 5 kDa PEG. However, the anticoagulant activity diminished to control levels for PEG chains above 5 kDa. Ultimately, we observed a 10-fold enhancement of anticoagulant activity with active peptides PEGylated with 5 kDa PEG compared to non-PEGylated knob peptides. The sequence of the active peptide significantly influenced the anticoagulant properties only at the highest 1:100 molar ratio where GPRPFPAC-5 kDa PEG and GPRPPERC-5 kDa PEG demonstrated significantly lower percent clottable protein than GPRPAAC-5 kDa PEG. Moreover, human plasma treated with the active 5 kDa PEG conjugate exhibited delayed the prothrombin time to within the therapeutic range specified for oral anticoagulants. Collectively, this study demonstrated the utility of PEGylated fibrin knob peptides as potential anticoagulant therapeutics.
Keywords: polyethylene glycol, fibrinogen, coagulation
Introduction
The body initiates the coagulation cascade following trauma or vascular injury stimulating platelet aggregation and the formation of a fibrin mesh that binds to cell surface receptors on activated platelets (Davie et al. 1991). While these events help maintain hemostasis, complications such as occlusions and embolisms may occur if coagulation is over stimulated or induced to pathological levels. To prevent these adverse side effects, patients typically require anticoagulant interventions.
The coagulation cascade consists of a number of zymogens and proteins that work in concert to convert soluble plasma protein fibrinogen to fibrin monomers thereby generating a fibrin clot (Figure 1A) (Davie et al. 1991). Ultimately, the extrinsic and intrinsic coagulation pathways converge onto Factor X to generate active Factor X (FXa). FXa further forms the prothombinase complex with activated Factor V (FVa) and calcium ion (Ca2+) on a phospholipid membrane to catalyze the conversion of prothrombin to thrombin. Activated thrombin is then free to initiate the conversion of fibrinogen to fibrin. Specifically, thrombin binds to fibrinogen at the centrally located amino-termini and cleaves the fibrinopeptides to expose fibrin `knobs' (Figure 1B) (Doolittle 1984; Lord 2007). Fibrin self-assembly then occurs through non-covalent interactions between the newly exposed knobs and the polymerization `holes' distally located on the carboxyl-termini of fibrin(ogen) (Everse et al. 1995). Factor XIII in fibrin is catalyzed and activated in the presence of thrombin to covalently crosslink fibrin fibers within the clot.
Figure 1. Coagulation Cascade.
(A) Intrinsic and extrinsic coagulation pathways and the targets of common anticoagulant drugs. (B) Thrombin exposes fibrin knob domains that in turn initiate fibrin assembly via knob:hole interactions. (C) Fibrin knobs interfere with fibrin monomer assembly and polymerization by competing for fibrin hole domains.
Current FDA-approved anticoagulants primarily target FXa or thrombin through either direct or indirect mechanisms (Dvorak et al. 2010; Orfeo et al. 2010). The most common anticoagulant for acute applications, heparin, forms a complex with antithrombin III that in turn inhibits thrombin and to some extent FXa. However, the pharmacodynamics and pharmacokinetics of unfractionated heparin are largely unpredictable and vary from batch to batch (Dvorak et al. 2010). Low molecular weight heparins (e.g. fondaparinux) offer improved half-life and decreased side affects compared to unfractionated heparin; however, inter-individual variability in pharmacodynamics and pharmacokinetics has recently been reported for low molecular weight heparins (Hacquard et al. 2011). Vitamin K antagonists (e.g. warfarin, Coumadin®) remain the only FDA-approved oral anticoagulant available for chronic/long-term anticoagulation therapy; these antagonists diminish vitamin K-dependent carboxylation of Factors II, VI, IX and X. However, such drugs are largely affected by diet, drug interaction, and patient variability necessitating frequent monitoring of coagulation activity (e.g. measurements of bleed and clotting times). Consequently, alternative targets used separately or in conjunction with current therapies may decrease the risks for pathological thrombi.
The dynamics of fibrin assembly and modifications to assembly kinetics have long been studied (Blomback 1958). For instance, variations in ionic strength, calcium concentration, and fibrinogen and/or thrombin concentrations dramatically affect fibrin fiber assembly and end clot structure (Carr et al. 1987; Carr et al. 1985). Additionally, synthetic fibrin knob `A' peptides have been shown to interrupt fibrin assembly through direct competition for fibrin polymerization holes (Laudano and Doolittle 1978; Laudano and Doolittle 1980). Based on this inherent binding mechanism, we investigated the anticoagulant properties of fibrin knob `A' peptides alone or covalently bound to linear polyethylene glycol (PEG) chains of varying molecular weights (MWs; Figure 1C). PEG is an FDA approved polymer commonly used as a carrier for low molecular weight drugs and enables covalent modification of other biological macromolecules (See Review (Pasut and Veronese 2007)). In general, PEGylated conjugates exhibit delayed in vivo recognition and clearance compared to non-PEGylated molecules, thereby increasing the half-life of drugs and biomolecules (Bowen et al. 1999). In addition to these potential favorable in vivo properties, we hypothesize that the PEGylated knob peptides will exhibit enhanced clotting inhibition when compared to the non-PEGylated knob peptides presumably through increased steric hindrance contributed by the PEG chain preventing native fibrin knobs to compete the knob peptides off of the polymerization holes. Moreover, we anticipate that both the PEG MW and concentration will play a critical role in inhibiting coagulation. Additionally, we previously investigated the binding kinetics of fibrin knobs peptides to the polymerization holes and reported a novel peptide sequence (GPRPFPAC) with enhanced affinity compared to the gold standard, GPRPAAC (Stabenfeldt et al. 2010). Therefore, we hypothesized that knob peptides with enhanced binding affinities will translate to higher functionality of inhibiting fibrin polymerization. We tested this hypothesis by comparing the anticoagulation properties of three different fibrin `A' knob peptides PEGylated with a 5kDa PEG chain.
By targeting the final fibrin assembly step, the anticoagulant is not dependent on concentration of coagulation factors upstream of end clot formation. Our approach effectively exploits the native fibrin assembly mechanisms and offers an alternative to the current anticoagulants on the market. Consequently, this fibrin knob-based anticoagulant may be used alone or in conjunction with current anticoagulants.
Materials and Methods
Peptide PEGylation
Fibrin knob peptides were PEGylated via maleimide-sulfhydryl chemistry. Heterofunctionalized PEG chains with methoxyl and maleimide groups (mPEG-MAL; JenKem Technology, Allen, TX) were reacted with cysteine terminating fibrin knob peptides (GPRPAAC, GPRPFPAC, or GPRPPERC) or control peptides (GPSPAAC or GPSPFPAC; GenScript, Piscataway, NJ). Specifically, a solution of the appropriate size mPEG-MAL (2, 5, 10, 20, or 30 kDa) was incubated with the desired peptide at a 1:2 molar ratio (mPEG-MAL:peptide) in 100 mM phosphate buffer pH 7.2, 150 mM sodium chloride, 10 mM EDTA for 4 hours at room temperature with gentle agitation. Peptide-PEG conjugates underwent overnight dialysis (Slidalyzer Dialysis Cassettes, MWCO 1kDa; Pierce, Rockford, IL) against ultrapure deionized water (diH2O) to remove excess unreacted peptides. Samples were then lyophilized and stored at −20°C. Prior to use, samples were resuspended in diH2O and final conjugate concentrations were determined with a previously published iodine-PEG assay (Gong et al. 2007) and a FluoroProfile® Protein Assay (Sigma Aldrich, St. Louis, MO). These assays also verified the end 1:1 molar ratio of PEG:peptide of each conjugate sample.
Thrombin-Initiated Fibrin Polymerization Assays
Thrombin-initiated fibrin polymerization assays were used to evaluate anticoagulant activity. For all assays, fibrin clots were prepared with final concentrations of human fibrinogen at 1 mg/mL (plasminogen-, fibronectin-, von Willebrand Factor-depleted; Enzyme Research Laboratories, Inc., South Bend, IN), human α-thrombin at 1 NIH U/mL (ERL), activated human factor XIII at 1 U/mL in a HEPES-buffered solution supplemented with calcium chloride (25 mM HEPES + 150 mM NaCl + 5 mM CaCl2; HBSC). Experimental groups with knob peptides or PEGylated peptide conjugates were evaluated at fibrinogen:conjugate molar ratios of 10:1, 1:1, 1:10, and 1:100. Prior to initiating polymerization, 50 μL of fibrinogen or fibrinogen + peptide/conjugate were incubated at room temperature for 30 min in a transparent 96-well plate. Polymerization was initiated by adding 50 μL of thrombin + FXIIIa to each well. Turbidity curves were generated from absorbance measurements recorded every minute for 60 min (350nm; SpectraMax M2 Microplate Reader, Molecular Devices, Sunnyvale, CA). Post-assay analysis of turbidity curves included the peak absorbance and thrombin clotting time (Supplemental Data).
Percent Clottable Protein Assay
Upon completing turbidity assays, the resulting fibrin clots were removed using standard 200 μL pipette tips leaving behind the remaining soluble protein (i.e. the clot liquor). The soluble protein content in the clot liquor was quantified using a Quant-iT™ protein assay (Invitrogen; Carlsbad, CA). Data were presented as percent clottable protein, the amount of initial protein minus soluble protein in the clot liquor all divided by the initial protein.
Confocal Microscopy
Confocal microscopy was used to evaluate the fibrin fiber structure. Briefly, fibrin clots were prepared as described above with addition of 5% fluorescently labeled fibrinogen (Alexa-555 nm, Invitrogen). Upon initiating polymerization with thrombin and FXIIIa, 100 μL was immediately transferred to a glass slide with 300 μm spacers and capped with a cover slide. Clots were imaged 60 min after polymerization. Five random 10 μm z-stack sections of each clot were imaged with a Zeiss Laser Scanning Microscope (LSM 510 VIS; Zeiss, Inc., Thronwood, NY). At least four individual clots were imaged per group. Image analysis and 3-D projections were performed with ZEN 2009 imaging software.
Prothombin Time
Prothrombin time (PT) was measured to evaluate clotting of human plasma initiated via the extrinsic coagulation pathway. Blood samples collected in citrated collection tubes were spun at 1800g for 30 min to acquire cell-depleted plasma (generously donated by Dr. Shannon Barker). Plasma was used within 9 h from collection time and stored at room temperature prior to use. PT was evaluated with the Innovin kit (Siemens Dade; Deerfield, IL). Briefly, plasma was diluted at 1:10 with HBS (25 mM HEPES + 150 mM NaCl ) or HBS + peptide/peptide-PEG and incubated for 30 min at room temperature. Plasma samples (50 μL) were then transferred to a transparent 96-well plate and incubated at 37°C for 1.5 min. Pre-warmed Innovin reagent (37°C, 100 μL) was added to each well and the absorbance was immediately recorded every 2 sec for 1 min (350nm; SpectraMax Plate reader). All sample groups were performed in triplicate. The resulting turbidity curve was analyzed at three phases: (1) lag phase, (2) reaction phase, and (3) plateau phase to determine the PT values (Supplemental Data) (Gogstad et al. 1986). The International Normalization Ratio (INR) was calculated normalize the data to control plasma (Supplemental Data).
Statistical Analysis
Data are presented as the mean ± one standard deviation. Results were analyzed by the appropriate one- or two-way ANOVA, followed by pair-wise comparisons with the appropriate Tukey's or Bonferroni post-hoc test (Prism 5, GraphPad Software, Inc.; La Jolla, CA). A 95% confidence level and corresponding p-value <0.05 was considered significant.
Results
In this study, we investigated (1) the effect of the molecular weight of the linear PEG chain (0, 2, 5, 10, 20, and 30 kDa) conjugated to an active knob peptide (GPRPAAC) or inactive control peptide (GPSPAAC) and (2) influence of the active fibrin knob peptide sequence (GPRPAAC, GPRPFPAC, and GPRPPERC) on fibrin polymerization. All experimental groups were also tested at four molar ratios (10:1, 1:1, 1:10, and 1:100 fibrinogen:conjugate).
Peak Absorbance
Looking at the 10:1 fibrinogen:conjugate molar ratio of varied PEG MW, no significant differences in peak absorbance were observed between any of the groups (Figure 2A). At a ratio of 1:1, active peptide (GPRPAAC)-PEG conjugates of 0 and 2 kDa showed no significant difference compared to controls (GPSPAAC) whereas the active 5 and 10 kDa PEG conjugates demonstrated a 20% decrease in peak absorbance (p < 0.05; Figure 2B). PEG chains of 20 and 30 kDa eliminated this effect. At the 1:10 ratio, a biphasic response was observed as the peak absorbance of the active-PEG conjugates from 0 kDa to 5 kDa decreased proportionally to nearly 75% of the control groups (p < 0.001; Figure 2C). The peak absorbance increased step-wise to control levels with active-PEG conjugates of 10, 20, and 30 kDa. A similar, but more drastic biphasic trend was observed with the 1:100 fibrinogen:conjugate molar ratio (Figure 2D). Specifically, the active-PEG conjugates from 0 to 5 kDa decreased the peak absorbance to 90% of the control groups. The peak absorbance for active-PEG conjugates from 10 to 30 kDa increased step-wise to control levels.
Figure 2. Peak Absorbance with Varying PEG MW.
In all subfigures, the abscissa represents PEG size (kDa) with the 0 kDa mark indicating the associated peptide in non-PEGylated form. Peak absorbance represents the maximum O.D. over the course of the 60min assay. Data for GPSPAAC (•) and GPRPAAC (□) formulations at four molar ratios of fibrinogen:conjugate are displayed, (A) 10:1, (B) 1:1, (C) 1:10, and (D) 1:100. (Mean ± SD; n= 6–9; # denotes p < 0.05, * denotes p < 0.01, and $ denotes p < 0.001).
The peak absorbance results presented in Figure 3 exemplify the effect of PEGylating the active knob peptides (GPRPxxxx-5). For the non-PEGylated peptides, only at the highest concentration (1:100 molar ratio) did we observe a significant decrease in the peak absorbance for the active peptides (GPRPxxxx-0; ~ 35% decrease) compared to the control peptide (GPSPFPAC-0, Figure 3A; p < 0.001). In contrast, for the 5 kDa PEGylated active knob peptides (GPRPxxxx-5; Figure 3B), a statistically significant 75% decrease compared to the control peptide (GPSPFPAC-5) was observed at the 1:10 molar ratio (p < 0.001). This trend continued at the 1:100 ratio where the peak absorbance the active 5 kDa PEG conjugates significantly decreased by 85% compared to the control (p < 0.001). No significant differences were observed between any of the active-PEGylated groups at any of the tested concentrations.
Figure 3. Peak Absorbance with Varying Knob Peptides.
In all subfigures, the abscissa represents the molar ratio of fibrinogen:conjugate. Peak absorbance represents the maximum O.D. over the course of the 60min assay. (A) Non-PEGylated peptides and (B) PEGylated peptides were evaluated; GPSPFPAC (●), GPRPAAC (□), GPRPFPAC (▾), and GPRPPERC (◇). (Mean ± SD; n= 6–9; # denotes p < 0.05, * denotes p < 0.01, and $ denotes p < 0.001).
Thrombin Clotting Time
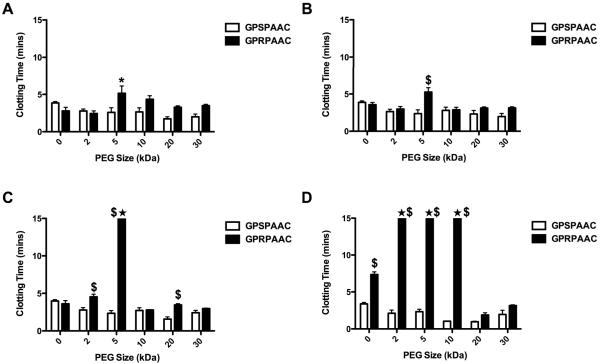
Thrombin clotting time is a measure of fibrin polymerization kinetics. The reported clotting times were normalized to a fibrin only control to account for variations in peak absorbance values; the clotting time absorbance threshold was defined as half the peak absorbance of a control fibrin clot with no supplements. At the 10:1 and 1:1 fibrinogen:conjugate molar ratio (Figure 4A–B), only the active-PEG conjugate (GPRPAAC) at 5 kDa significantly increased clotting time (2-fold) compared to all of the other groups (p < 0.01). At the 1:10 molar ratio (Figure 4C), no significant difference was observed with the non-PEGylated active peptide (GPRPAAC-0), but the active-PEG conjugate at 2 kDa displayed a significant 2-fold increase in clotting time (p < 0.001). The active-PEG conjugate at 5 kDa was defined as “no clot” as the turbidity never reached the half-max threshold defined by the fibrin only control. Above 5 kDa PEG, a significant increase in clotting time was only observed with the active-PEG conjugate at 20 kDa (p < 0.001). When increasing the molar ratio to 1:100 (Figure 4D), we observed a significant 2-fold increase in clotting time with the active non-PEGylated peptide (0 kDa) as compared to the control (p < 0.001) confirming previous reports (Laudano and Doolittle 1980). Active-PEG conjugates with 2, 5, and 10 kDa were defined as no clot; however, no significant difference was observed with 20 and 30 kDa PEG conjugates.
Figure 4. Thrombin Clotting Time with Varying PEG MW.
Thrombin clotting times for GPSPAAC and GPRPAAC with varying PEG MWs. Sample groups denoted with ★ indicate “No Clot” as measured over the course of the 60min assay. (A) 10:1, (B) 1:1, (C) 1:10, and (D) 1:100 molar ratio. (Mean ± SD; n= 6–9; # denotes p < 0.05, * denotes p < 0.01, and $ denotes p < 0.001).
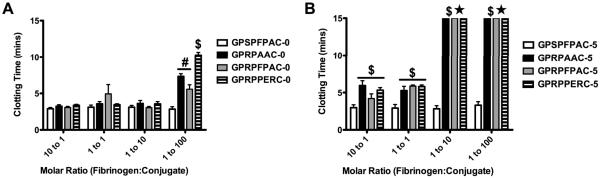
The thrombin clotting time of the active non-PEGylated peptides (GPRPxxxx-0) remained near control levels (GPSPFPAC-0) for 10:1, 1:1, and 1:10 fibrinogen:conjugate molar ratios. At the highest 1:100 molar ratio, the active non-PEGylated peptides demonstrated a 2- to 3-fold increase compared to the control (p < 0.001; Figure 5A). Amongst the active groups at this 1:100 molar ratio, the clotting time for GPRPPERC was significantly longer than GPRPAAC and GPRPFPAC (p < 0.01). In contrast, for the 5 kDa PEGylated active peptide groups (GPRPxxxx-5), a significant increase in clotting time was observed with all active groups beginning at the lowest molar ratio (10:1) and continued through the highest molar ratio (1:100) (Figure 5B; p < 0.001). More strikingly, all active groups were defined as “no clot” at 1:10 and 1:100 molar ratios.
Figure 5. Thrombin Clotting Time for Varying Knob Peptides.
Thrombin clotting times for control GPSPFPAC and active knob peptides (GPRPAAC, GPRPFPAC, GPRPPERC). (A) Non-PEGylated peptides and (B) PEGylated peptides were evaluated. Sample groups denoted with ★ indicate “No Clot” as measured over the course over the 60min assay. (Mean ± SD; n= 6–9; # denotes p < 0.05, * denotes p < 0.01, and $ denotes p < 0.001).
Percent Clottable Protein
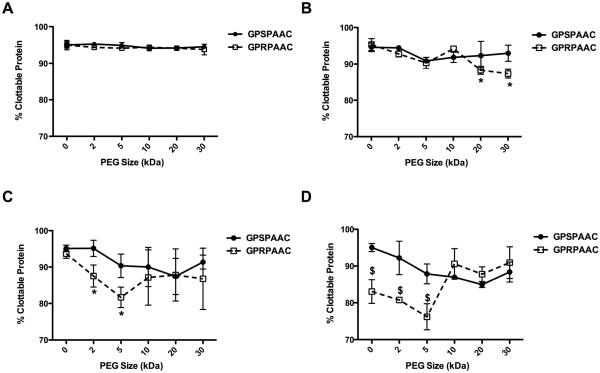
The percent clottable protein was also evaluated following clot formation using a standard protein quantification assay as illustrated in Figure 6. At the 10:1 fibrinogen:conjugate molar ratio, the percent clottable protein values of the active (GPRPAAC) peptides did not significantly deviate from the control (GPSPAAC) groups across all PEG MWs (Average: 94.14 ± 0.71%; Figure 6A). At the ratio of 1:1 (Figure 6B), the percent clottable protein of the active groups mirrored the controls except for the 20 kDa and 30 kDa PEG groups, where the percent clottable protein significantly decreased compared to controls (p < 0.01). At the 1:10 ratio (Figure 6C), a biphasic trend emerged as the percent clottable protein significantly decreased with the addition of the 2 kDa and 5 kDa active conjugates compared to matched controls (p < 0.01) whereas the active-PEG conjugates at 10, 20 and 30 kDa remained near control levels. We also noted a decreasing trend in the percent clottable protein for the control-PEG groups as PEG MW increased, potentially indicating a non-specific effect of PEG on protein interactions as the percent mass of the PEG increased. At the 1:100 molar ratio (Figure 6D), the biphasic trend continued as the percent clottable protein significantly decreased proportionally with the active non-PEGylated peptide (0 kDa) and with the 2 kDa and 5 kDa active conjugates compared to matched controls (p < 0.001). However, no significant differences in percent clottable protein were observed with the 10, 20, and 30 kDa active groups.
Figure 6. Percent Clottable Protein with varying PEG MW.
In all subfigures, the abscissa represents PEG size (kDa) with the 0 kDa mark indicating the associated peptide in non-PEGylated form. Percent clottable protein was quantified from the remaining protein in solution after clot formation. Data for GPSPAAC (●) and GPRPAAC (□) formulations at four molar ratios of fibrinogen:conjugate are displayed, (A) 10:1, (B) 1:1, (C) 1:10, and (D) 1:100. (Mean ± SD; n= 6–9; # denotes p < 0.05, * denotes p < 0.01, and $ denotes p < 0.001).
In comparing the percent clottable protein for the non-PEGylated peptide groups (Figure 7A), a significant decrease was only observed at the highest 1:100 fibrinogen:conjugate molar ratio (p < 0.001). No statistical difference was observed among the active non-PEGylated peptides at any of the ratios. In contrast, the active 5 kDa PEGylated (GPRPxxxx-5) groups demonstrated a significant decrease compared to the control beginning at the 1:1 ratio (Figure 7B; p < 0.05). This trend continued for the 1:10 molar ratio as the active-PEG groups significantly decreased from the control (p < 0.001); however, no significance among the active-PEG groups was observed. At the 1:100 molar ratio, all active-PEG groups remained significantly lower than the control (p < 0.001). We also observed a significant difference among the active-PEG groups as the percent clottable protein for GPRPFPAC-5 and GPRPPERC-5 were significantly lower than GPRPAAC-5 (p < 0.01). The influence of active peptide sequence on percent clottable protein appeared significant only at the highest concentration we evaluated where GPRPFPAC-5 and GPRPPERC-5 were significantly more effective than GPRPAAC-5.
Figure 7. Percent Clottable Protein with Varying Knob Peptides.
In all subfigures, the abscissa represents the molar ratio of fibrinogen:conjugate. Percent clottable protein was quantified from the remaining protein in solution after clot formation. (A) Non-PEGylated peptides and (B) PEGylated peptides were evaluated; GPSPFPAC (●), GPRPAAC (□), GPRPFPAC (▾), and GPRPPERC (◇).(Mean ± SD; n= 6–9; # denotes p < 0.05, * denotes p < 0.01, and $ denotes p < 0.001).
Clot Fiber Structure
We used confocal microscopy to visually evaluate the fiber structure of fibrin clots polymerized in the presence of an active knob peptide (GPRPFPAC) in non-PEGylated and PEGylated form (5 kDa PEG; Figure 8). The native fibrin clot demonstrates the heterogeneity of the fibrin fibers including a distribution of fiber thickness and the presence of fiber bundles (Figure 8A). In comparison, the clot supplemented with a non-PEGyated active peptide at 1:10 molar ratio appeared to contain thinner fibers and decreased fiber bundles compared to the control (Figure 8B). A more pronounced effect was observed with active-PEG conjugate as the fiber thickness and density dramatically decreased compared to the control and non-PEGylated active peptide groups supporting the results from the turbidity assays (Figure 8C). Additionally, a greater degree of terminated fibers was observed with the active-PEG conjugated.
Figure 8. Fibrin Clot Structure.
Representative confocal micrographs projections of 10μm z-stacks of (A) control fibrin clot, (B) fibrin clot supplemented with GPRPFPAC, and (C) fibrin clot supplemented with GPRPFPAC-5k PEG both at a 1:10 fibrinogen:conjugate molar ratio. Objective = 63×; Scale bars = 10μm.
Plasma Clotting Assay: Prothombin Time
Extending this study further, we evaluated the prothombin time (PT) of human plasma treated with control peptide (GPSPFPAC) or active peptide (GPRPFPAC) in non-PEGylated and PEGylated form (5 kDa PEG). Data were reported as PT (Figure 9A) and the International Normalized Ratio (INR; Figure 9B). INR is commonly used to monitor patients on oral coagulation therapy (e.g. warfarin). We used this ratio to compare the anticoagulant properties of experimental groups compared to HBS control. The results indicated that the PT significantly increased in the presence of the active and control non-PEGylated peptides, most likely due to increase in ionic strength contributed by the peptides (Figure 9A). More strikingly, the active-PEG conjugate significantly increased the PT 2-fold compared to the HBS control and was significantly greater than all other groups (p < 0.001). Similar trends were observed with the INR values, where the presence of peptide or peptide-PEG conjugates increased the INR value from the HBS control (Figure 9B), but the greatest (~2-fold) increase was still observed with the active-PEG conjugate (p < 0.001). Moreover, an INR above 2.0 is considered within the therapeutic range for oral anticoagulant therapies (Gogstad et al. 1986; Hacquard et al. 2011).
Figure 9. Prothrombin Time Assay.
Prothrombin time (PT) was evaluated with fresh human plasma in the presence or absence of the control GPSPFPAC or active knob GPRPFPAC in non-PEGylated and PEGylated form. (A) PT values for plasma + HBS and GPSPFPAC and GPRPFPAC with and without 5 kDa PEG. (B) INR values for corresponding experimental and control groups. (Mean ± SD; n= 3; * denotes p < 0.01 and $ denotes p < 0.001).
Discussion
The coagulation cascade involves an intricate sequence of signaling events that lead to the formation of a fibrin clot. Current FDA-approved anticoagulants primarily target factors upstream of fibrin monomer activation and assembly. In this study, we evaluated the potential of PEGylated fibrin knob peptides as anticoagulants via inhibiting the assembly and polymerization of fibrin, a downstream stage in the coagulation cascade. Knob peptides conjugated to 5 kDa PEG chain exhibited the highest anticoagulant potential. Moreover, we demonstrated that adding the 5 kDa PEG chain to three different knob mimics afforded a 10-fold increase in anticoagulant activity compared to the non-PEGylated peptides. This increase is indicated by the similar levels of percent clottable protein observed at 1:10 molar ratio of fibrinogen:conjugate as those levels observed with the 1:100 molar ratio of fibrinogen:peptide.
PEGylating small molecules and peptide sequences has been shown to increase the half-life and reduce immunogenicity issues in vivo (See Reviews (Esposito et al. 2003; Pasut and Veronese 2007)). Such characteristics are very attractive for future translational applications of any clinical product. In addition, we hypothesized that the increased mass contributed by the PEG chain would increase the steric hindrance near/around the fibrin polymerization hole thereby decreasing the propensity of native fibrin knobs to compete off the knob peptide conjugates. Our results indicated a critical size was necessary for this inhibition to occur. As the PEG MW increased from 0k to 5kDa, we observed a significant decrease in fibrin polymerization as determined by peak absorbance, clotting time and percent clottable protein. However, PEG MWs above 5 kDa had diminished effects on inhibiting fibrin polymerization. We speculate that at 10 kDa and above the increased mass may have actually prevented the knob peptide from interacting efficiently with the fibrin polymerization pocket. Similar bioactive dependencies on PEG MW have been previously reported (Bailon et al. 2001; Bowen et al. 1999; Zhang et al. 2008). For example, Bowen et al. reported an inverse correlation between PEG MW and the in vitro bioactivity of PEGylated nartograstim, a granulocyte colony-stimulating factor (Bowen et al. 1999). However in vivo, the increased benefit of reduced clearance and thus increased plasma residence time appeared to overcome any diminished bioactivity since the PEGylated nartograstim far out-performed the non-PEGylated nartograstim (Bowen et al. 1999). Similarly, PEGylation of interferon-2α was shown to decrease bioactivity in vitro, but the in vivo performance with PEGylated interferon significantly increased compared to non-PEGylated interferon-2α, as the half-life increased 7-fold and the plasma residence time increased 20-fold (Bailon et al. 2001; Reddy et al. 2002). Collectively, these studies demonstrate the benefits of PEGylating bioactive molecules particularly as a means to increase the bulk size of the protein in order to reduce clearance. Our study demonstrated that in vitro PEGylation of a small 7–8 residue peptide maintains activity up to 5 kDa where we reached a point of diminishing returns in PEG size thereafter. However, future in vivo studies are needed to evaluate the pharmacokinetics of the various PEG MWs to determine the optimal PEG chain size for in vivo activity. We cannot dismiss the possibility that the increased circulation half-life due to larger PEG chains may outweigh the apparently lower activity of the peptides in the context of larger PEG chains.
Fibrin knob peptides have been shown to inhibit fibrin polymerization at high concentrations (>100-fold molar excess) (Laudano and Doolittle 1978). Variations in functionality have also been related to the amino acid composition. For example, the sequence Gly-Pro-Arg (GPR) represents the minimum binding sequence required for fibrin knob peptides to interact with the fibrin polymerization holes as a mutation of either the 1Gly or 3Arg residues dramatically decreases this binding interaction (Laudano and Doolittle 1980). Doolittle also showed that the mutant Gly-Pro-Arg-Pro (GPRP) has significantly higher binding affinity to the polymerization holes compared to the native Gly-Pro-Arg-Val (GPRV) (Laudano and Doolittle 1980). We recently investigated how residues downstream from GPRP influence the binding properties of GPRPxxxx peptides (Stabenfeldt et al. 2010). We demonstrated backbone chain flexibility and molecular orientation of the 3Arg side group played a critical role in determining the affinity to the polymerization holes. Here, we evaluated the translation of molecular binding affinities to functionally inhibiting fibrin assembly and polymerization. The results indicated that at equal and 1:10 fibrinogen:conjugate molar ratios, variations in peptide binding affinities did not influence inhibition and the increased mass contributed by PEG appeared to dominant potential differences in molecular recognition. However at 100-fold molar excess, PEGylation of higher affinity peptides, GPRPFPAC and GPRPPERC, exhibited enhanced anticoagulant activity as compared to GPRPAAC-5 kDa PEG. Ultimately, the combination of high affinity knob peptides and a linear 5 kDa PEG chain enhances the anticoagulant activity at least 10-fold over the non-PEGylated knob peptides.
Coagulation in vivo depends on numerous coagulation factors and co-factors, which can vary from person-to-person. For this reason, we performed a clinical clotting assay with fresh human plasma to evaluate the performance of the PEGylated knob peptides under physiologically relevant conditions. The results demonstrated that with a 10-fold molar excess of active-PEG conjugate, we observed a significant increase in PT (i.e., prolonged clotting time). Moreover, the International Normalization Ratio (INR) of plasma treated with the active-PEG was within the therapeutic range defined for patients receiving oral anticoagulant therapy (i.e. warfarin). Therefore, active-PEG conjugates extended the clotting time under physiological conditions.
Anticoagulant therapies currently focus on inhibiting or decreasing the activity of coagulation factors upstream from fibrin assembly. Commonly these therapies inhibit one or possibly two factors in the cascade resulting in a one-dimensional approach. Recent studies have investigated a combination of complementary therapies to further enhance individual anticoagulant effects. For example, employing an antithrombin inhibitor, r-hirudin, that inhibits soluble and fibrin-bound thrombin with a direct FXa inhibitor has been shown to enhance clotting times in vitro (Meddahi and Samama 2009). Similarly, PEGylated knob peptides may be used in conjunction with an alternative anticoagulant to target the coagulation cascade at multiple phases. Therefore, if the concentration of co-delivered anticoagulant drops below functional levels, the PEGylated knob peptides will prevent converted fibrin monomers from assembling and polymerizing into a fibrin clot. This approach may address the potentially adverse effect of inhibiting multiple clotting factors such as increased risk of bleeding.
Conclusion
In conclusion, we demonstrated that fibrin knob peptides PEGylated with 5 kDa linear PEG chains resulted in a 10-fold increase in anticoagulant activity over non-PEGylated peptides. At the highest concentration tested, the knob peptide sequence significantly influences the anticoagulant properties as measured by percent clottable protein where GPRPFPAC-5 kDa PEG and GPRPPERC-5 kDa PEG emerged as the most potent inhibitors. Looking forward to translating into the clinical setting, PEGylation affords the added benefit of potentially increasing the half-life and reducing the clearance rate of the knob peptides potentially leading to enhanced activity due to increase plasma residence time.
Supplementary Material
Acknowledgements
This work was supported by the NIH (1R01EB011566 and 1R01NS065109; T.H.B.) and the NIH IRACDA Post-doctoral Fellowship (K12 GM000680; S.E.S.). The authors acknowledge Dr. Shannon Barker for the generous donation of human plasma.
References
- Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung WJ, Porter JE, Ehrlich GK, Pan W, Xu ZX, Modi MW, et al. Rational design of a potent, long-lasting form of interferon: a 40 kDa branched polyethylene glycol-conjugated interferon alpha-2a for the treatment of hepatitis C. Bioconjug Chem. 2001;12(2):195–202. doi: 10.1021/bc000082g. [DOI] [PubMed] [Google Scholar]
- Blomback B. Studies on fibrinogen: its purification and conversion into fibrin. Acta Physiol Scand Suppl. 1958;43(148):1–51. [PubMed] [Google Scholar]
- Bowen S, Tare N, Inoue T, Yamasaki M, Okabe M, Horii I, Eliason JF. Relationship between molecular mass and duration of activity of polyethylene glycol conjugated granulocyte colony-stimulating factor mutein. Exp Hematol. 1999;27(3):425–32. doi: 10.1016/s0301-472x(98)00051-4. [DOI] [PubMed] [Google Scholar]
- Carr ME, Gabriel DA, Mcdonagh J. Influence of Factor-Xiii and Fibronectin on Fiber Size and Density in Thrombin-Induced Fibrin Gels. Journal of Laboratory and Clinical Medicine. 1987;110(6):747–752. [PubMed] [Google Scholar]
- Carr ME, Kaminski M, Mcdonagh J, Gabriel DA. Influence of Ionic-Strength, Peptide Release and Calcium on the Structure of Reptilase and Thrombin Derived Fibrin Gels. Thrombosis and Haemostasis. 1985;54(1):159–159. [Google Scholar]
- Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30(43):10363–10370. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- Doolittle RF. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- Dvorak M, Vlasin M, Dvorakova M, Rauser P, Lexmaulova L, Gregor Z, Staffa R. Heparin and its derivatives in the treatment of arterial thrombosis: a review. Veterinarni Medicina. 2010;55(11):523–546. [Google Scholar]
- Esposito P, Barbero L, Caccia P, Caliceti P, D'Antonio M, Piquet G, Veronese FM. PEGylation of growth hormone-releasing hormone (GRF) analogues. Adv Drug Deliv Rev. 2003;55(10):1279–91. doi: 10.1016/s0169-409x(03)00109-1. [DOI] [PubMed] [Google Scholar]
- Everse SJ, Pelletier H, Doolittle RF. Crystallization of Fragment-D from Human Fibrinogen. Protein Science. 1995;4(5):1013–1016. doi: 10.1002/pro.5560040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogstad GO, Dahl KH, Christophersen A, Bjerke A. Turbidimetric determination of prothrombin time by clotting in a centrifugal analyzer. Clin Chem. 1986;32(10):1857–62. [PubMed] [Google Scholar]
- Gong XW, Wei DZ, He ML, Xiong YC. Discarded free PEG-based assay for obtaining the modification extent of pegylated proteins. Talanta. 2007;71(1):381–384. doi: 10.1016/j.talanta.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Hacquard M, Perrin J, Lelievre N, Vigneron C, Lecompte T. Inter-individual variability of effect of 7 low molecular weight antithrombin-dependent anticoagulants studied in vitro with Calibrated Automated Thrombography. Thromb Res. 2011;127(1):29–34. doi: 10.1016/j.thromres.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Laudano AP, Doolittle RF. Synthetic peptide derivatives that bind to fibrinogen and prevent the polymerization of fibrin monomers. Proc Natl Acad Sci U S A. 1978;75(7):3085–9. doi: 10.1073/pnas.75.7.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudano AP, Doolittle RF. Studies on synthetic peptides that bind to fibrinogen and prevent fibrin polymerization. Structural requirements, number of binding sites, and species differences. Biochemistry. 1980;19(5):1013–9. doi: 10.1021/bi00546a028. [DOI] [PubMed] [Google Scholar]
- Lord ST. Fibrinogen and fibrin: scaffold proteins in hemostasis. Current Opinion in Hematology. 2007;14(3):236–241. doi: 10.1097/MOH.0b013e3280dce58c. [DOI] [PubMed] [Google Scholar]
- Meddahi S, Samama MM. Is the inhibition of both clot-associated thrombin and factor Xa more clinically relevant than either one alone? Blood Coagul Fibrinolysis. 2009;20(3):207–14. doi: 10.1097/MBC.0b013e3283273529. [DOI] [PubMed] [Google Scholar]
- Orfeo T, Butenas S, Brummel-Ziedins KE, Gissel M, Mann KG. Anticoagulation by factor Xa inhibitors. J Thromb Haemost. 2010;8(8):1745–53. doi: 10.1111/j.1538-7836.2010.03917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasut G, Veronese FM. Polymer-drug conjugation, recent achievements and general strategies. Progress in Polymer Science. 2007;32(8–9):933–961. [Google Scholar]
- Reddy KR, Modi MW, Pedder S. Use of peginterferon a1fa-2a (40 KD) (Pegasys (R)) for the treatment of hepatitis C. Advanced Drug Delivery Reviews. 2002;54(4):571–586. doi: 10.1016/s0169-409x(02)00028-5. [DOI] [PubMed] [Google Scholar]
- Stabenfeldt SE, Gossett JJ, Barker TH. Building better fibrin knob mimics: an investigation of synthetic fibrin knob peptide structures in solution and their dynamic binding with fibrinogen/fibrin holes. Blood. 2010;116(8):1352–1359. doi: 10.1182/blood-2009-11-251801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Han B, Lin X, Wu X, Yan H. Modification of antimicrobial peptide with low molar mass poly(ethylene glycol) J Biochem. 2008;144(6):781–8. doi: 10.1093/jb/mvn134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.