Abstract
Background and purpose
To estimate target and cardiac doses from breast cancer radiotherapy in Denmark and in the Stockholm and Umeå areas of Sweden during 1977–2001.
Methods
Representative samples of irradiated women were identified from the databases of the Danish Breast Cancer Cooperative Group and the Swedish Nationwide Cancer Registry. Virtual simulation, computed tomography planning and manual planning were used to reconstruct radiotherapy regimens on a typical woman. Estimates of target dose and various measures of cardiac dose were derived from individual radiotherapy charts.
Results
Doses were estimated in 681 Danish and 130 Swedish women. Mean heart dose for individual women varied from 1.6 to 14.9 Gray in Denmark and from 1.2 to 22.1 Gray in Sweden. In Denmark, mean target doses averaged across women increased from 40.6 to 53.8 Gray during 1977–2001 but, despite this, mean heart dose averaged across women remained around 6 Gy for left-sided and 2–3 Gray for right-sided radiotherapy. In Sweden mean target dose averaged across women increased from 38.7 to 46.6 Gray during 1977–2001, while mean heart dose averaged across women decreased from 12.0 to 7.3 Gray for left-sided and from 3.6 to 3.2 Gray for right-sided radiotherapy. Temporal trends for mean biologically effective dose [BED] to the heart, mean dose to the left anterior descending coronary artery, the right coronary artery and the circumflex coronary artery were broadly similar.
Conclusions
Cardiac doses in Denmark were low relative to those in Sweden. In both countries, target dose increased during 1977–2001. Despite this, cardiac doses remained constant in Denmark and decreased in Sweden.
Keywords: Breast cancer radiotherapy, Radiation-induced heart disease, Cardiac doses
Radiotherapy for early breast cancer reduces the risk of local recurrence and can reduce the risk of death from breast cancer. However, treatment usually involves some unwanted irradiation of the heart and this may result in an increased risk of heart disease. The most recent overview of the long-term follow-up of data from the randomised trials of radiotherapy for early breast cancer found that there was a highly significant 27% increase in relative risk of mortality from heart disease in women randomised to radiotherapy [1]. Cardiac radiation doses are usually higher in left-sided than in right-sided breast cancer and observational studies following women in the general population after a diagnosis of breast cancer have also found higher cardiac mortality in women with left-sided cancers compared with women with right-sided cancers [2,3].
Radiotherapy techniques have changed over the past few decades enabling improved dose distributions to be delivered to target tissues. However, current radiotherapy regimens still deliver some dose to cardiac structures [4,5]. In some women with left-sided breast cancer the target tissue coverage is currently being compromised due to the limits that have been set on cardiac dose, and it is not known whether such limits have at present been set at the most appropriate levels. For other women, the cardiac dose is lower and does not limit the dose to the target tissue, but it is not at present known whether such women will in the future incur any increase in cardiac risk. Appropriate levels for constraints during radiotherapy planning and also reliable estimation of the future risk of radiation-induced heart disease for individual women both require accurate estimates of the dose–response relationship for radiation-induced heart disease. As part of a programme of work to derive such relationships, detailed dosimetry based on the original radiotherapy charts has been carried out to estimate individual heart and coronary artery doses and also target doses for a large number of women who were irradiated in Denmark during the period 1977–2001. The present paper describes the method used to estimate these doses.
The first uniform nationwide protocol for breast cancer radiotherapy in Denmark was issued in 1977 [6]. This protocol was revised in 1982, in 1989 and again in 1999. The present paper, therefore, presents target and cardiac doses for a representative sample of women who were irradiated in Denmark for breast cancer in the periods covered by these protocols. As part of the same research programme, cardiac doses have also been estimated using similar methodology for a large number of women who were irradiated in the Stockholm and Umeå areas of Sweden [7] and the present paper also presents doses for a representative sample of women irradiated in this area of Sweden during the same time periods.
Methods
Selection of women
The database of the Danish Breast Cancer Cooperative Group (DBCG) [8] was used to identify all women irradiated for breast cancer in Denmark during 1977–2001 at ages <75 years. Within this population, women developing or dying from ischaemic heart disease were identified from the Danish hospital inpatient and outpatient registers and from death certificates. For each of the women with heart disease (i.e., each ‘case’ woman), 2 control women were selected. Doses were reconstructed for all the cases and for all the controls using the method described below.
A total of 681 women were selected as controls and this paper presents the estimated doses for these 681 women. The method used to select these control women was as follows. For each case, all the women in the study population were identified who had been diagnosed with breast cancer during the same 5-year calendar period as the case, who were in the same 5-year age group as the case, and who had not developed heart disease by the time the case woman had developed heart disease (or had died, for cases who were identified from death certificates). From the pool of eligible controls for each case, two were selected at random. The selection was with replacement, so that some women were chosen as controls for more than one case, which accounts for the fact that the total number of women selected as controls is not precisely equal to twice the number of case women. A consequence of this method of selection is that within each calendar period, the women selected as controls had an age distribution reflecting that of the women who subsequently developed heart disease, rather than that of the population of irradiated breast cancer patients. To examine the consequence of this, average mean heart doses were calculated separately for women aged 20–49, 50–59, 60–69 & 70–79 at breast cancer diagnosis for left-sided and right-sided cancers for calendar periods 1977–1981, 1982–1988 & 1989–2001. Values for the different age groups were similar, with no suggestion of any trend. We, therefore, conclude that, for each calendar period, the estimated doses are representative of the doses delivered to the entire population of irradiated breast cancer patients in Denmark.
The method of selection of the 130 Swedish women presented in this paper was similar to that used for the 681 Danish women. The method used to reconstruct the doses of the Swedish women has been described elsewhere [7].
Categorisation of radiotherapy charts
Each woman’s radiotherapy chart, including a photograph of the treatment fields and a dose-plan (if available) was copied. In the rare cases (less than 1% of women) where the chart was not available, information on the regimen used was sought from the woman’s medical notes. The charts were used to extract details on regimens used to irradiate the breast, chest wall and internal mammary chain for each woman. Details of regimens used to irradiate only the supraclavicular fossa or axilla were not obtained since their contribution to heart dose is usually an order of magnitude lower than from breast, chest wall and internal mammary chain radiotherapy [9]. Each woman’s radiotherapy chart was categorised according to the regimen she received. This included details of radiotherapy technique(s) used, laterality, target dose(s) and fraction size(s) and whether she received either supraclavicular fossa or axillary irradiation.
Set-up of typical radiotherapy regimens
Each radiotherapy regimen was reconstructed using a technique based on virtual simulation and computed tomography (CT) based 3-dimensional treatment planning. All the regimens were reconstructed on the CT planning scan of a typical patient of average build who had been selected from the radiotherapy planning database of a UK radiotherapy department. The heart and coronary arteries were contoured. The cranial limit of the heart included the right atrium and excluded the pulmonary trunk, ascending aorta and superior vena cava. The lowest contour of the heart was the caudal myocardial border. The heart contour included heart muscle and circulating blood volume; it did not include the pericardium. The left main coronary artery arises from the aorta and branches into the left anterior descending (LAD) and circumflex coronary arteries. Due to its short length, its contour was included with that of the LAD coronary artery. The circumflex coronary artery was contoured from the branch point of the left main coronary artery. The right coronary artery was contoured from its origin at the aorta.
Beam modalities employed were 6 MV, cobalt-60 and 10 MeV electrons. Dose calculations for 100 and 250 keV fields were carried out using manual planning. The algorithms used for the dose calculations were the pencil beam [10,11] and the collapsed cone superposition convolution algorithms [12,13]. The former was used for all regimens and the latter for a selection of regimens that involved regions of substantial tissue inhomogeneity. Agreement between algorithms was within 2% of dose for all regimens.
Dose calculations
For each regimen, dose–volume histograms (DVHs) were generated for the heart and for each of the three main coronary arteries. These were used to estimate mean dose and biologically effective dose (BED) to each cardiac structure using the linear quadratic model: BED = [nd(1 + d/(α/β)] where n is number of fractions and d is dose per fraction in Gray (Gy). An alpha–beta ratio of 2 Gy was used as previously described [7]. Other measures of dose, such as maximum dose to each structure were estimated but not presented since they are likely to vary substantially from patient to patient.
Left-sided supraclavicular fossa and/or axillary irradiation has previously been estimated to deliver around 0.5 Gy mean dose to the heart and to each of the three main coronary arteries [9]. Therefore, for women who received left-supraclavicular fossa or axillary radiotherapy, 0.5 Gy dose was added to the mean heart and coronary artery doses calculated from irradiation of the other targets. Right-sided supraclavicular fossa and axillary fields are further from the heart and would be expected to deliver lower doses to cardiac structures. Therefore, adjustments were not carried out for women treated with right-supraclavicular fossa or axillary radiotherapy. BEDs to the heart and coronary arteries depend on dose and dose per fraction. Therefore, it was not possible to adjust cardiac BEDs for the use of supraclavicular fossa or axillary radiotherapy.
The approximate target dose(s) for each woman were calculated according to the same definition in both Denmark and Sweden, in order to allow comparison between countries. They were derived by calculating the dose at the centre of the breast or chest wall for tangential irradiation, at the approximate depth of the tumour bed for direct orthovoltage chest wall fields and at the approximate depth of the internal mammary chain for orthovoltage internal mammary fields. For other direct or oblique chest wall or internal mammary fields, target dose was assumed to be 90% of the given dose. For the 6 MV lateral thorax (and supraclavicular fossa) field, target dose was assumed to be 100% of the given dose. For further details of target dose calculation from Swedish regimens, see Taylor et al. [7].
Average cardiac doses during different time periods were estimated as follows. Mean heart dose was calculated for each of the 681 women evaluated. The women were then divided according to time period of irradiation: 1977–1981, 1982–1988 or 1989–2001. The average of the mean heart doses was calculated for each period. This was repeated for doses to the coronary arteries. Average target dose was estimated in the same way, taking account of all of the anatomical regions irradiated.
Results
Breast cancer radiotherapy regimens
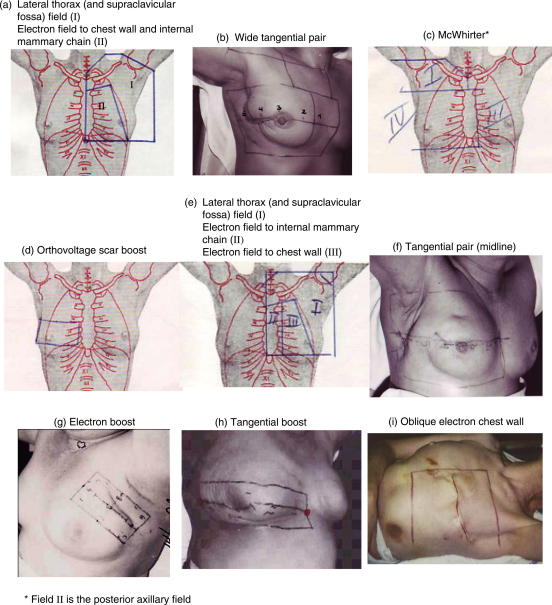
Information on radiotherapy regimen was sought for 681 Danish women. Radiotherapy charts were available for 677 of the 681 women (99%) and radiotherapy regimen was ascertained for all 681 women. The total number of different breast cancer radiotherapy regimens found between the years 1977 and 2001 in Denmark was 36. Twenty-two of these regimens (11 left-sided and 11 right-sided) were received by five or more of the 681 women. Details of these regimens are summarised on Table 1 and Fig. 1.
Table 1.
Estimated cardiac doses from common breast cancer radiotherapy regimens used to irradiate the breast, chest wall and internal mammary nodes in Denmark from 1977 to 2001.
| Description | Usual given or target dose (Gy)A | Usual dose per fraction (Gy) | Field arrangement | Usual beam energy | Number women | Heart |
LADD |
RCAE | CircF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean dose (Gy)B |
Mean BED (Gy2)C |
Mean dose (Gy)B |
Mean BED (Gy2)C |
Mean dose (Gy) |
Mean dose (Gy) |
||||||||||||
| Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | ||||||
| Regimens common during 1977–1981G | |||||||||||||||||
| Lateral thorax (and supraclavicular fossa) field (a)H,I | 45.0 | 3.75 | Oblique anterior | 8–18 MV | 173 | 3.0 | 1.9 | 4.5 | 2.7 | 9.0 | 0.8 | 14.9 | 0.9 | 2.9 | 5.4 | 1.5 | 0.5 |
| Electron field to chest wall and internal mammary chain | 40.7 | 3.39 | Direct anterior | 10 MeV | |||||||||||||
| Wide tangential pair to chest wall (b) | 40.7 | 1.85 | Wide tangential pair | 6 MV | 58 | 8.5 | 2.7 | 14.9 | 3.8 | 23.5 | 1.6 | 45.7 | 2.0 | 3.3 | 11.0 | 3.6 | 1.0 |
| McWhirter (c) | 23.6–36.0 | 1.80 | Wide tangential pair | 250–300 keVJ | 46 | 14 | 9 | 25 | 13 | 43 | 5 | – | – | 10 | 19 | 7 | 4 |
| Lateral thorax (and supraclavicular fossa) field (a) | 45.0 | 3.75 | Oblique anterior | 4 MV | 22 | 4 | 2 | 5 | 3 | 6 | 1 | – | – | 2 | 2 | 2 | 1 |
| Orthovoltage scar boost (d) | 36.0 | 3.00 | Direct anterior | 100 keVJ | |||||||||||||
| Regimens common during 1982–1988G | |||||||||||||||||
| Lateral thorax (and supraclavicular fossa) field (e) | 50.0–55.0 | 2.00–2.20 | Oblique anterior | 4–10 MV | 114 | 4.5 | 3.0 | 6.0 | 3.9 | 12.8 | 1.1 | 18.1 | 1.2 | 5.3 | 8.9 | 2.0 | 0.7 |
| Electron field to internal mammary chain | 54.0 | 2.16 | Direct anterior | 9–14 MeV | |||||||||||||
| Electron field to chest wall | 50.0 | 2.00 | Direct anterior | 6–10 MeV | |||||||||||||
| Tangential pair to breast (midline) (f) | 50.0 | 2.00 | Tangential pair | 6–10 MV | 6 | 6.0 | 1.7 | 9.2 | 1.9 | 23.5 | 1.4 | 41.6 | 1.7 | 2.2 | 3.2 | 3.0 | 1.1 |
| Regimens common during 1989–2001G | |||||||||||||||||
| Tangential pair to breast (midline) (f) | 48.0 | 2.00 | Tangential pair | 6–10 MV | 132 | 6.1 | 1.7 | 11.9 | 1.9 | 24.0 | 1.4 | 58.5 | 1.5 | 2.1 | 3.3 | 3.0 | 1.1 |
| Electron scar boost (g) | 10.8 | 2.16 | Direct oblique | 8–15 MeV | |||||||||||||
| Tangential pair to breast (midline) (f) | 48.0 | 2.00 | Tangential pair | 6–10 MV | 59 | 6.3 | 1.8 | 12.4 | 1.9 | 24.8 | 1.5 | 62.8 | 1.6 | 2.3 | 3.4 | 3.0 | 1.1 |
| Tangential scar boost (h) | 10.0 | 2.00 | Tangential pair | 6–25 MV | |||||||||||||
| Oblique electron field to breast/chest wall (i) | 51.9 | 2.16 | Oblique anterior | 8–15 MeV | 27 | 4.7 | 2.1 | 7.2 | 2.6 | 17.6 | 0.6 | 35.2 | 0.6 | 2.7 | 6.4 | 2.0 | 0.6 |
| Electron scar boost (g) | 10.8 | 2.16 | Direct oblique | 8–18 MeV | |||||||||||||
| Oblique electron field to breast/chest wall (i) | 51.9 | 2.16 | Oblique anterior | 8–15 MeV | 15 | 4.4 | 2.0 | 6.0 | 2.5 | 16.2 | 0.6 | 26.1 | 0.9 | 2.6 | 6.2 | 1.9 | 0.6 |
| Wide tangential pair to breast/chest wall (b) | 48.0 | 2.00 | Wide tangential pair | 6–18 MV | 10 | 10.3 | 3.3 | 18.1 | 4.2 | 29.1 | 1.9 | 59.9 | 1.7 | 3.9 | 13.2 | 4.3 | 1.2 |
| Electron scar boost (g) | 10.8 | 2.16 | Direct oblique | 6–18 MeV | |||||||||||||
Estimates based on typical tumour dose and calculated on a representative patient with typical anatomy.
Each regimen presented was received by ⩾5 of the 681 women included in the study. Nineteen of the 681 women received other, less commonly used, regimens.
Given dose, i.e., dose at Dmax (maximum dose) where different from prescribed dose.
For some regimens, cardiac doses differed slightly from those of the patients described in Fig. 2 since tumour dose varied slightly from patient to patient.
BED (biologically effective dose) calculated using the linear quadratic equation: BED = [nd(1 + d/(α/β)] where n is number of fractions and d is dose per fraction in Gy. α/β was assumed to be 2. BEDs were based on average doses in first 300 women in the study except for wide tangential pair/electron scar boost and McWhirter regimens where there was more than 5 Gy difference between tumour doses for the first 300 women and final 681 women. LAD coronary artery BEDs are not presented for manually planned techniques since they are based on few dose points.
Left anterior descending coronary artery.
Right coronary artery.
Circumflex coronary artery.
New protocols were introduced in these years.
(a), (b) etc. refers to the illustrations in Fig. 1.
Regimen comprises both techniques, i.e., lateral thorax and chest wall & internal mammary chain.
Doses for manually planned regimens are rounded due to the uncertainties involved in planning. All other doses are given to 1 decimal place.
Fig. 1.
Danish breast cancer radiotherapy techniques reconstructed for cardiac dose estimations, ordered according to time period of irradiation.
Information on Swedish breast cancer regimens used during this time period can be found in Taylor et al. 2009 [7].
Heart dose from Danish breast cancer regimens
From 1977 until 1988 most women irradiated for breast cancer received an electron field to the chest wall +/− the internal mammary chain (Fig. 1a and e, Table 1). Most women also received megavoltage irradiation of the lateral thorax and supraclavicular fossa (Fig. 1a and e, Table 1). The energy of the direct or oblique electron fields depended on each woman’s soft-tissue thickness. The greater the soft-tissue thickness, the greater the energy of electrons used. These regimens usually delivered estimated mean heart dose of around 3–5 Gy (BED ∼4–6 Gy2) for left-sided and around 2–3 Gy (BED ∼2–4 Gy2) for right-sided radiotherapy (Table 1). Some women in the study received bolus to the chest wall field, others were treated without bolus. The difference that 1 cm bolus made to estimated mean heart dose was 1 Gy for left-sided irradiation, and less than this for right-sided irradiation. A small volume of the heart anteriorly received between 20 Gy and 50 Gy. Most of the heart volume received less than 10 Gy due to the rapid decrease in dose from the electron fields. The megavoltage field used to irradiate the lateral part of the thorax was distant from the heart and contributed only 0.8 Gy estimated mean heart dose for left-sided and 0.6 Gy for right-sided radiotherapy (data not shown).
In some parts of Denmark there was no access to megavoltage or electron treatment in the 1970s and early 1980s. Instead, tangential orthovoltage irradiation of the chest wall and internal mammary chain was used (McWhirter technique) (Fig. 1c) [14]. This delivered mean estimated doses of 14 Gy (BED = 25 Gy2) to the heart for left-sided and 9 Gy (BED = 13 Gy2) for right-sided radiotherapy (Table 1). These high heart doses are partly explained by lateral scatter and partly by the depth–dose characteristics of an orthovoltage beam.
During the period 1989–2001, an increasing number of women received breast-conserving surgery with postoperative megavoltage tangential irradiation of the conserved breast (Fig. 1f). Most women also received a boost to the tumour bed with electrons (Fig. 1g) or with tangential beams (Fig. 1h) if the tumour was deep-seated. Standard tangential irradiation of the whole breast or chest wall (Fig. 1f) +/− boost radiotherapy usually resulted in estimated mean heart doses of around 6 Gy (BED ∼10 Gy2) for left-sided and 2 Gy (BED ∼2 Gy2) for right-sided radiotherapy (Table 1).
Coronary artery doses from Danish breast cancer regimens
Dose to the LAD coronary artery was greater for left-sided than for right-sided regimens due to its anatomical position close to the left breast and left internal mammary chain. The artery tended to receive the highest radiation doses of more than 20 Gy estimated mean dose (BED ∼40–60 Gy2) and around 50 Gy estimated maximum dose (data not shown) from left-tangential radiotherapy (Figs. 1b,c,f, Table 1) since parts of the artery were close to, or included in, the radiation fields. LAD coronary artery dose from left electron radiotherapy was around 9–18 Gy (BED ∼15–35 Gy2), with maximum doses of between 44 and 52 Gy (data not shown). There is likely to be considerable interpatient variability in maximum dose to the LAD coronary artery since it is usually close to the edge of the fields.
The right coronary artery is located anteriorly, slightly to the right of midline and it received the highest doses from right-sided regimens. The circumflex coronary artery is located posteriorly, distant from the breast and internal mammary chain and for most regimens it received lower dose than the heart or the other two coronary arteries.
Heart and coronary artery doses for Danish women
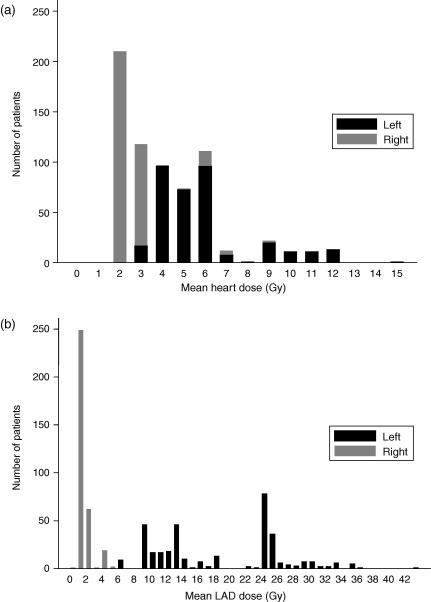
Estimated heart dose for the 681 Danish women varied from 1.6 to 14.9 Gy mean dose (Fig. 2a) and from 1.9 to 25.3 Gy2 mean BED. Estimated coronary artery mean doses in these women varied from 0.4 to 42.8 Gy for the LAD coronary artery (Fig. 2b), from 1.8 to 19.4 Gy for the right coronary artery and from 0.1 to 6.8 Gy for the circumflex coronary artery.
Fig. 2.
Estimated mean heart (panel a) and left anterior descending coronary artery (LAD) (panel b) doses in Danish breast cancer patients irradiated between 1977 and 2001. Dose for the left-sided patients is represented by black bars and dose for the right-sided patients, by grey bars. Estimates are based on individual patient doses. Regimens were reconstructed on a typical patient with average anatomy.
Trends in cardiac dose in Denmark and Sweden 1977–2001
Heart doses in Denmark have been consistently low over the past few decades due to the widespread use of techniques that spare the heart, namely electron irradiation of the chest wall and internal mammary chain [15,16], and tangential irradiation with the medial border on midline. The estimated average mean heart dose remained fairly constant between 1977 and 2001 at around 5–6 Gray for left-sided and 2–3 Gray for right-sided irradiation (Table 2).
Table 2.
Cardiac dose estimates for Danish women identified using the Danish Breast Cancer Group database and irradiated for breast cancer since 1977, based on individual radiotherapy charts.
| Year of radiotherapya | Number of women evaluated | Average mean dose (standard deviation) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target dose (Gy) | Heart dose (Gy) |
Heart BEDb (Gy2) |
LADc dose (Gy) |
RCAd dose (Gy) |
Circe dose (Gy) |
|||||||
| Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | |||
| 1977–1981 | 199 | 40.6 | 6.1 | 2.9 | 10.2 | 4.6 | 16.4 | 1.5 | 4.2 | 7.7 | 3.1 | 1.1 |
| (6.3) | (3.3) | (1.6) | (7.9) | (3.7) | (9.7) | (1.2) | (1.9) | (4.0) | (1.4) | (0.9) | ||
| 1982–1988 | 187 | 48.4 | 5.7 | 2.9 | 8.4 | 3.9 | 16.3 | 1.4 | 4.2 | 8.2 | 2.8 | 0.9 |
| (6.6) | (2.3) | (1.1) | (5.9) | (2.5) | (7.2) | (0.8) | (1.5) | (3.3) | (1.0) | (0.6) | ||
| 1989–2001 | 295 | 53.8f | 5.8 | 2.1 | 10.1 | 2.4 | 20.9 | 1.3 | 3.0 | 5.3 | 2.8 | 0.9 |
| (5.1) | (1.2) | (0.5) | (3.2) | (0.8) | (5.3) | (0.3) | (1.3) | (2.8) | (0.6) | (0.2) | ||
Regimens were reconstructed on a representative patient with typical anatomy.
Women were grouped according to the years that breast cancer protocols changed in Denmark. The DBCG77 protocol was mainly used between 1977 and 1981; the DBCG82 protocol between 1982 and 1988 and the DBCG89 protocol between 1989 and 2001.
The biologically effective dose (BED) takes into account the fraction size as well as dose and is given by BED = [nd(1 + d/(α/β)] where n is number of fractions and d is dose per fraction in Gy. α/β was assumed to be 2 Gray. It was possible to calculate BEDs for 97% of the women studied. It was not possible to calculate BED for the other 3% women who received unusual techniques such as iridium wire radiotherapy.
Left anterior descending coronary artery.
Right coronary artery.
Circumflex coronary artery.
Target dose includes boost radiotherapy.
There was a slight increase in LAD coronary artery dose from left-sided radiotherapy from 16.4 Gy in 1977–1981 to 20.9 Gy in 1989–2001. This can be partly explained by the increasing use of tangential radiotherapy which can cause hotspots of high radiotherapy dose in the anterior part of the heart and partly by the increase in average target dose from 40.6 Gy in 1977–1981 to 53.8 Gy in 1989–2001. Doses to the right coronary artery from both left- and right-sided irradiation have reduced between 1977 and 2001. There was little change in estimated LAD coronary artery dose from right-sided irradiation and in circumflex coronary artery doses during this period (Table 2).
In Sweden, mean heart dose for individual women varied from 1.2 to 22.1 Gray, and the estimated average mean heart dose in 1977–1981 was 12.0 Gy for left-sided radiotherapy and 3.6 Gy for right-sided radiotherapy (Table 3). These doses fell to 7.3 Gy and 3.2 Gy in 1989–2001 for left-sided and right-sided radiotherapy, respectively. Similar dose reductions were seen in doses to each of the three coronary arteries for left-sided irradiation. There was little change in doses to the LAD, right and circumflex coronary arteries from right-sided irradiation during this time period.
Table 3.
Cardiac dose estimates for Swedish women identified using the Swedish nationwide cancer register and irradiated for breast cancer since 1977, based on individual radiotherapy charts.
| Year of radiotherapya | Number of women evaluated | Average mean dose (standard deviation) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target dose (Gy) | Heart dose (Gy) |
Heart BEDb (Gy2) |
LADc dose (Gy) |
RCAd dose (Gy) |
Circe dose |
|||||||
| Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | |||
| 1977–1981 | 36 | 38.7 | 12.0 | 3.6 | 20.8 | 4.5 | 23.6 | 1.5 | 8.7 | 9.5 | 7.7 | 0.8 |
| (10.2) | (5.2) | (3.0) | (10.0) | (4.0) | (7.6) | (0.8) | (5.4) | (9.1) | (3.4) | (0.3) | ||
| 1982–1988 | 51 | 44.6 | 8.0 | 3.7 | 11.6 | 5.1 | 21.1 | 1.9 | 6.9 | 11.1 | 4.2 | 0.9 |
| (8.8) | (5.8) | (2.4) | (10.2) | (3.9) | (9.0) | (1.0) | (7.1) | (9.9) | (2.9) | (0.4) | ||
| 1989–2001 | 43 | 46.6 | 7.3 | 3.2 | 10.5 | 4.2 | 18.8 | 2.1 | 5.7 | 8.4 | 3.9 | 1.3 |
| (8.5) | (7.2) | (2.2) | (11.2) | (3.2) | (11.7) | (0.7) | (6.2) | (9.5) | (3.3) | (0.3) | ||
Regimens were reconstructed on a representative patient with typical anatomy.
Women were grouped according to the years that breast cancer protocols changed in Denmark to enable comparison with Danish women in Table 2.
The biologically effective dose (BED) takes into account the fraction size as well as dose and is given by BED = [nd(1 + d/(α/β)] where n is number of fractions and d is dose per fraction in Gy. α/β was assumed to be 2 Gray. It was possible to calculate BEDs for women who received computer planned technique combinations or a single manually planned technique (91% of the women studied). It was not possible to calculate BED for the other women who received two techniques, one of which was manually planned (9% of the women studied).
Left anterior descending coronary artery.
Right coronary artery.
Circumflex coronary artery.
Discussion
The present study has estimated doses to the heart and coronary arteries from breast cancer radiotherapy regimens used in Denmark between 1977 and 2001. Twenty-two different breast cancer radiotherapy regimens were commonly used and these resulted in radiation doses of between 1.7 and 14 Gy to the heart and 0.5 and 43 Gy to the coronary arteries. Women irradiated for left-sided breast cancer generally received higher doses than those irradiated for right-sided cancer.
Trends in cardiac dose in Denmark and Sweden 1977–2001
Over the past few decades, indications for breast cancer radiotherapy, the radiotherapy techniques used and beam energies available have changed substantially throughout the world. Surgical techniques have also evolved, with the increasing use of breast conserving surgery since the 1980s. In many countries, these changes have resulted in reductions in dose to the heart [4,7,17,18]. The risk of radiation-induced heart disease in breast cancer survivors is known to be related to radiation dose [19–22]. This risk is, therefore, likely to have reduced in countries such as Sweden where cardiac doses reduced substantially between 1977 and 2001, but to have stayed fairly constant in Denmark where there was little trend in the magnitude of cardiac doses.
Since the year 2001 doses to all cardiac structures from tangential radiotherapy in Denmark are likely to have reduced due to the increasing use of CT planned radiotherapy [14,16]. CT based treatment planning provides detailed information about coverage of the target(s), dose homogeneity and doses to organs at risk. It has been found to improve the dose distribution in the target(s) and to reduce mean heart dose from around 4.5 Gy with standard left-tangential irradiation to 3.0 Gy with CT-based conformal left-tangential irradiation [23].
Strengths and limitations of the study
Information on typical cardiac and target doses was derived by studying the radiotherapy charts of women irradiated for breast cancer. Cardiac doses based on individual radiotherapy regimens were available for all Danish women and for 99% of Swedish women and our sample contained more than 600 women in Denmark and more than 100 women in Sweden irradiated between 1977 and 2001. These women are likely to be representative of the general irradiated breast cancer populations in Denmark and Sweden.
Since 1977, radiotherapy in all regions of Denmark has been delivered according to the DBCG protocols. Information concerning the different treatment regimens for this study was obtained both from the DBCG protocols and from the radiotherapy charts or oncology notes of 681 Danish women. These two sources provided detailed and consistent information. For most Danish radiotherapy techniques, there was little geographical or interpatient variation in parameters such as field borders or patient treatment position. Therefore, reconstruction of regimens on our typical patient is likely to be representative of reconstruction in previous decades. However, for boost radiotherapy, the DBCG protocols indicated that the field should encompass the surgical scar with a 3 cm margin [14]. The radiotherapy charts revealed substantial variability in boost field size and position. Estimated cardiac doses from boost radiotherapy are, therefore, subject to a higher degree of variability than doses from other techniques. However, heart dose from boost irradiation was always low relative to other techniques because of the low given dose of 6–20 Gy.
The women included in the study were not CT-planned, therefore, their heart doses needed to be calculated retrospectively. Retrospective dosimetry is subject to several sources of variability. The cardiac doses received by an individual woman are likely to vary according to her anatomical characteristics and according to the circumstances of her radiotherapy, such as her treatment position and the linear accelerator used. Most of these sources of variability would apply to any retrospective dosimetry method. They are described and quantified by Taylor et al. [9]. The most important source of dose variability was found to be the effect of differing patient anatomy. Nevertheless differences in heart dose are usually likely to be greater for different regimens than for different women [9].
Electron beam energy was usually chosen according to the thickness of soft tissue for the Danish women in this study. Beam energies varied from 4 to 22 MeV for breast or chest wall radiotherapy and from 9 to 18 MeV for internal mammary radiotherapy. The internal mammary chain and chest wall electron regimens reconstructed on our typical patient were 10 MeV, based on her soft tissue thickness. Tailoring of electron energy in individual women in the study is likely to have reduced the effect of differing patient anatomy on cardiac doses since higher energy beams were used for patients with thicker soft tissue.
Variation in cardiac doses for Danish women
The current study has estimated cardiac doses for women irradiated in Denmark between 1977 and 2001. For individual women in the study, there was a wide range of cardiac doses of between 1.6 and 14.9 Gy mean heart dose and between 0.1 and 42.8 Gy mean coronary artery dose. These dose variations occurred firstly because women irradiated for left-sided cancers usually received higher cardiac doses than those irradiated for right-sided cancers. Secondly, although most Danish women received around 5 Gy mean heart dose for left-sided and 2 Gy for right-sided radiotherapy (Fig. 2), at least some women in each decade received regimens that delivered higher heart doses. For example, in this study some women in the 1970s and 80s received McWhirter radiotherapy, which delivered around 14 Gy for left-sided and 9 Gy for right-sided radiotherapy, and some women in the 1990s received wide tangential pair and electron boost irradiation, which delivered around 10 Gy and 3 Gy for left- and right-sided radiotherapy, respectively.
However, the majority of women in each decade received electron radiotherapy or standard tangential pair radiotherapy, which delivered lower heart doses of around 4–6 Gy for left-sided and 2 Gy for right-sided radiotherapy. Therefore, there was little variation in cardiac doses received by the irradiated breast cancer population as a whole from decade to decade. This suggests that we may expect little variation in the risk of radiation-induced heart disease from decade to decade between 1977 and 2001. These doses will enable assessment of the risks of radiation-induced heart disease experienced by Danish breast cancer survivors irradiated during 1977–2001, and contribute toward prediction of the future risks of heart disease.
Funding
Cancer Research UK, Medical Research Council (core funding to Clinical Trial Service Unit). Carolyn Taylor, Paul McGale and Sarah Darby acknowledge support from the British Heart Foundation Core Research Funding to CTSU (grant CH/96001) and Centre of Research Excellence, Oxford (grant RE/08/004). Department of Health, London (project grant RRX 108). The European Commission (contract: 012796(FI6R); project: RACE). The study sponsors had no role in study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the paper for publication.
Conflicts of interest statement
None.
Acknowledgements
The authors gratefully acknowledge Dymphna Hermans and Sam James for their help with this work.
References
- 1.Early Breast Cancer Trialists Collaborative Group Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and on 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.Darby S.C., McGale P., Taylor C.W., Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300 000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 3.Darby S.C., McGale P., Peto R., Granath F., Hall P., Ekbom A. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: nationwide cohort study of 90 000 Swedish women. BMJ. 2003;326:256–257. doi: 10.1136/bmj.326.7383.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor C.W., Povall J.M., McGale P. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys. 2008;72:501–507. doi: 10.1016/j.ijrobp.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 5.Lohr F., El-Haddad M., Dobler B. Potential effect of robust and simple IMRT approach for left-sided breast cancer on cardiac mortality. Int J Radiat Oncol Biol Phys. 2009;74:73–80. doi: 10.1016/j.ijrobp.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Danish Breast Cancer Cooperative Group (DBCG) http://www.dbcg.dk/; 2010 [accessed 10.12.10].
- 7.Taylor C.W., Nisbet A., McGale P. Cardiac doses from Swedish breast cancer radiotherapy since the 1950s. Radiother Oncol. 2009;90:127–135. doi: 10.1016/j.radonc.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 8.Moeller S., Jensen M.-B., Ejlertsen B. The clinical database of the Danish Breast Cancer Cooperative Group (DBCG); its 30-year experience and future promise. Acta Oncol. 2008;47:506–524. doi: 10.1080/02841860802059259. [DOI] [PubMed] [Google Scholar]
- 9.Taylor C.W., Nisbet A., McGale P., Darby S.C. Cardiac exposures in breast cancer radiotherapy: 1950s to 1990s. Int J Radiat Oncol Biol Phys. 2007;69:1484–1495. doi: 10.1016/j.ijrobp.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Ahnesjö A., Saxner M., Trepp A. A pencil beam model for photon dose calculation. Med Phys. 1992;19:263–273. doi: 10.1118/1.596856. [DOI] [PubMed] [Google Scholar]
- 11.Ahnesjö A. Analytic modelling of photon scatter from flattening filters in photon therapy beams. Med Phys. 1994;21:1227–1235. doi: 10.1118/1.597205. [DOI] [PubMed] [Google Scholar]
- 12.Ahnesjö A. Collapsed cone convolution of radiant energy for photon dose calculation in heterogeneous media. Med Phys. 1989;16:577–592. doi: 10.1118/1.596360. [DOI] [PubMed] [Google Scholar]
- 13.Saxner M., Ahnesjö A. Implementation of the collapsed cone method for clinical beam qualities. Med Phys. 1998;25:A185. [Google Scholar]
- 14.Overgaard M., Christensen J.J. Postoperative radiotherapy in DBCG during 30 years. Techniques, indications and clinical radiobiological experience. Acta Oncol. 2008;47:639–653. doi: 10.1080/02841860802078085. [DOI] [PubMed] [Google Scholar]
- 15.Hojris I., Overgaard M., Christensen J.J., Overgaard J. Morbidity and mortality of ischaemic heart disease in high-risk breast-cancer patients after adjuvant postmastectomy systemic treatment with or without radiotherapy: analysis of DBCG 82b and 82c randomised trials. Lancet. 1999;354:1425–1430. doi: 10.1016/s0140-6736(99)02245-x. [DOI] [PubMed] [Google Scholar]
- 16.Thomsen M.S., Berg M., Nielsen H.M. Post-mastectomy radiotherapy in Denmark: From 2D to 3D treatment planning guidelines of The Danish Breast Cancer Cooperative Group. Acta Oncol. 2008;47:654–661. doi: 10.1080/02841860801975000. [DOI] [PubMed] [Google Scholar]
- 17.Gyenes G., Gagliardi G., Lax I. Evaluation of irradiated heart volumes in Stage I breast cancer patients treated with postoperative adjuvant radiotherapy. J Clin Oncol. 1997;15:1348–1353. doi: 10.1200/JCO.1997.15.4.1348. [DOI] [PubMed] [Google Scholar]
- 18.Fuller S.A., Haybittle J.L., Smith R.E.A., Dobbs H.J. Cardiac doses in post-operative breast irradiation. Radiother Oncol. 1992;25:19–24. doi: 10.1016/0167-8140(92)90190-6. [DOI] [PubMed] [Google Scholar]
- 19.Gagliardi G., Lax I., Ottolenghi A., Rutqvist L.E. Long-term cardiac mortality after radiotherapy of breast cancer–application of the relative seriality model. Br J Radiol. 1996;69:839–846. doi: 10.1259/0007-1285-69-825-839. [DOI] [PubMed] [Google Scholar]
- 20.Schultz-Hector S., Trott K.-R. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 21.http://www.asco.org/ASCOv2/MultiMedia/Virtual+Meeting?&vmview=vm_session_presentations_view&confID=47&sessionID=2267. 2010; [accessed 19.12.10].
- 22.Gagliardi G., Constine L.S., Moiseenko V. Radiation dose–volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010;76:S77–S85. doi: 10.1016/j.ijrobp.2009.04.093. [DOI] [PubMed] [Google Scholar]
- 23.Muren L.P., Maurstad G., Hafslund R., Anker G., Dahl O. Cardiac and pulmonary doses and complication probabilities in standard and conformal tangential irradiation in conservative management of breast cancer. Radiother Oncol. 2002;62:173–183. doi: 10.1016/s0167-8140(01)00468-6. [DOI] [PubMed] [Google Scholar]