Abstract
Purpose
Peptide receptor radionuclide therapy (PRRT) using radiolabelled somatostatin analogues is a treatment option for patients with disseminated neuroendocrine tumours (NET). A combination treatment using the high-energy 90Y beta emitter for larger lesions and the lower energy 177Lu for smaller lesions has been postulated in the literature.The aim of the study was to evaluate combined 90Y/177Lu-DOTATATE therapy in comparison to 90Y-DOTATATE alone.
Methods
Fifty patients with disseminated NET were included in the study prospectively and divided into two groups: group A (n = 25) was treated with 90Y-DOTATATE, whereas group B (n = 25) received the 1:1 90Y/177Lu-DOTATATE. The administered activity was based on 3.7 GBq/m2 body surface area in three to five cycles, with amino acid infusion for nephroprotection.
Results
The median overall survival time in group A was 26.2 months while in group B median survival was not reached. Overall survival was significantly higher in group B (p = 0.027). Median event-free survival time in group A was 21.4 months and in group B 29.4 months (p > 0.1). At the 12-month follow-up, comparison of group A vs group B showed stable disease (SD) in 13 vs 16 patients, disease regression (RD) in 5 vs 3 patients and disease progression (PD) in 3 vs 4 patients; 4 and 2 patients died, respectively. The 24-month follow-up results were SD in nine vs ten patients, RD in one patient vs none and PD in four patients in both groups; three and four patients died, respectively. Side effects were rare and mild.
Conclusion
The results indicate that therapy with tandem radioisotopes (90Y/177Lu-DOTATATE) provides longer overall survival than with a single radioisotope (90Y-DOTATATE) and the safety of both methods is comparable.
Keywords: Somatostatin receptor, Peptide receptor radionuclide therapy, Neuroendocrine tumours, 90Y-DOTATATE, 177Lu-DOTATATE
Introduction
The choice of an appropriate treatment option as far as patients with inoperable or metastatic neuroendocrine tumours (NET) are concerned is limited. The response rate of chemotherapy is only at the level of 20–35% [1], effective in a minority of patients with poorly differentiated NETs and is completely ineffective in the majority of well-differentiated NETs. “Cold” somatostatin analogues exert a good clinical effect, decreasing flushing, diarrhoea and other symptoms of carcinoid syndrome [2, 3].
Worth mentioning is the fact that a prospective randomized study of the effect of octreotide LAR in the control of tumour growth in patients with metastatic midgut NETs (PROMID study) showed that octreotide LAR inhibits tumour growth in patients with well-differentiated metastatic midgut NETs [4].
Peptide receptor radionuclide therapy (PRRT) with radiolabelled somatostatin analogues has become an established method of treatment of disseminated NET. [111In-diethylenetriaminepentaacetic acid (DTPA)0]octreotide firstly was used in some clinical trials, with regression observed in 8% of patients. Because of the physical characteristics and short range of the Auger electrons of 111In, the best results were observed in patients with small tumours and relatively high tumour uptake [5]. Recently, new somatostatin analogues that can be labelled with beta-emitting radionuclides have been developed. Currently 90Y and 177Lu are used. 90Y emits beta particles with a high maximum energy (Emax 2.27 MeV) and long maximum particle range in tissues (10 mm), whereas 177Lu has lower energy (Emax 0.497 MeV) and a shorter particle range in tissues (maximum 2–4 mm).
This isotope has a small γ-emission which makes it suitable for post-therapeutic scintigraphic imaging [133 keV (6.5%); 208 keV (11%)]. Studies that evaluated the efficacy of total doses of 7.4–20.2 GBq [90Y-DOTA0,Tyr3]octreotide treatment have demonstrated a good response in 10–34% of patients [6–9]. Another large trial (MAURITIUS) which evaluated response post 90Y-DOTA-(D)Na1-lanreotide (90Y-DOTALAN) in 154 patients has shown a partial response (PR) and disease stabilization in 14 and 41% of patients, respectively [10].
Recent advances in somatostatin analogues have paved the way to the development of octreotate, which can be labelled with both 177Lu and 90Y radionuclides and is characterized by a higher affinity for somatostatin receptor type 2 leading to high tumour uptake.
The first clinical results of [177Lu-DOTA0,Tyr3,Thr8]octreotate (DOTATATE) therapy with a cumulative dose of 22.2–29.6 GBq were described in 34 patients with gastroenteropancreatic (GEP) NET. The patients were followed up 3–6 months after receiving the final dose [11]. At 3 months after the final administration, complete response (CR) was found in 1 patient (3%), PR in 12 patients (35%), stable disease (SD) in 14 patients (41%) and progressive disease (PD) in 7 patients (21%). The side effects of PRRT were few and mostly transient, with mild bone marrow suppression and nephrotoxicity, which were the most common finding.
de Jong et al. were the first to have described the use of combination treatment consisting of 50% 177Lu-DOTATATE and 50% 90Y-DOTATOC in rats, demonstrating that survival rates were three times longer [12, 13]. To our knowledge, there are no studies in the literature that evaluated the use of this combination treatment in human subjects.
The aim of this study was to evaluate combined 90Y/177Lu-DOTATATE therapy in patients with tumours of various sizes and non-homogeneous receptor distribution in comparison to 90Y-DOTATATE alone, using overall survival (OS) and progression-free survival (PFS) as primary clinical end points.
Materials and methods
The study was approved by the Ethics Committee of the Medical University of Warsaw. All patients gave written informed consent.
Patients
Fifty patients with histological confirmation of metastatic NETs (WHO II) were enrolled in the study. At the time of treatment all patients showed PD confirmed by CT examination, somatostatin receptor scintigraphy (SRS) and/or increasing blood concentration of chromogranin A (CgA).
Patients were divided into two groups. Group A consisting of 25 patients (11 men and 14 women) with a mean age (± SD) of 57.3 ± 10.6 years was treated with 90Y-DOTATATE. Group B consisting of 25 patients (9 men and 16 women) with a mean age (± SD) of 56 ± 11.6 years was treated with a combination treatment including 90Y and 177Lu in one i.v. application.
The major criterion for inclusion into this study was the benefit from PRRT therapy. Because of the fact that it was the first PRRT study in Poland, patients had extended disease. The Karnofsky index was used for estimating the patients’ general condition.
The following inclusion criteria for therapy were used:
SRS-positive disease with uptake in the tumour and metastases higher that the liver, evaluated within 3 months before inclusion (qualitative analysis)
Histological confirmation of NET; inoperable or metastatic disease
Haemoglobin level (Hb) ≥ 10 g/dl; leucocytes (WBC) ≥ 2×109/l; thrombocytes (PLT) ≥ 90×109/l
Calculated creatinine clearance (CrCl) > 40 ml/min
Karnofsky performance status ≥ 60
Life expectancy > 3 months
No pregnancy or lactation
All patients underwent blood tests (full blood count, kidney, liver functions tests and CgA) and staging prior to therapy with the use of CT and SRS using 99mTc-HYNIC-TATE (99mTc-HYNIC-Tyr3-octreotate) [14].
For patients receiving cold long-acting somatostatin analogues, the radionuclide therapy was performed 5 weeks after the completion of octreotide (Sandostatin LAR, Novartis) and 3 weeks after lanreotide (Somatuline, Ipsen). Patients who received chemotherapy were not treated with PRRT until after a period of 3 months.
Patients were given a follow-up examination in the clinic post PRRT at 3, 6, 12, 24 and 36 months using blood tests, CT and 99mTc-HYNIC-TATE following the same protocols as the ones used prior to therapy.
Radiopharmaceuticals
99mTc-HYNIC-TATE: the detailed method of kit preparation and labelling with 99mTc was presented before [14, 15]. Briefly, the peptide conjugate HYNIC-Tyr3-octreotate (piChem, Graz, Austria) was prepared in the dried kit form under aseptic conditions (Institute of Atomic Energy POLATOM, Poland) after adding commercially available stannous chloride, mannitol, tricine, and N,N′-ethylenediaminediacetic acid. Quality control was performed by instant thin-layer chromatography silica gel (ITLC-SG) strips (Pall) developed in 0.9% saline for the determination of non-peptide-bound radioactivity.
The labelling yield efficiency exceeded 90% in all cases. Tests for sterility and bacterial endotoxins were routinely performed.
90Y-DOTATATE and 90Y/177Lu-DOTATATE dried kits containing 100 μg (DOTA-Tyr3-octreotate) (piChem, Graz, Austria) and 50.0 mg of commercially available ascorbic acid were prepared under aseptic conditions. For labelling the content of each kit vial was dissolved in not more than 0.5 ml of 90Y no-carrier-added chloride of desired radioactivity (max. 6.5 GBq per kit) or 177Lu carrier-added (specific activity of around 555 GBq/mg Lu) chloride solution, both isotopes provided by the Institute of Atomic Energy POLATOM, Poland. In case of low volume 0.9% NaCl was used to fill up to 0.5 ml. Incubation was carried out at 95°C for 25 min. After cooling down to room temperature, the volume was increased up to 1.5–2.0 ml with 0.9% NaCl. The preparation was sterilized by filtration with 0.22-μm filters (Millipore) to sterile glass vials followed by filter wash with 50 mg/ml ascorbic acid to the final radioactive concentration of 740 MBq/ml. The radiochemical purity (RCP) was assessed by HPLC (column: Synergy 4 Fusion RP 80A 150 × 4.6 mm, flow rate 0.6 ml/min, UV detection at 220 nm and radiometric detection, solvent A: 0.1% trifluoroacetic acid in water, solvent B: acetonitrile, gradient: 0 min 18% B; 9 min 60% B; 12 min 60% B; 15 min 18% B; 21 min 18% B) and Sep-Pak C18 (Waters) mini-column separation according to the supplier’s instructions. The limit for RCP was over 99.0% for each 90Y- and 177Lu-labelled DOTATATE. The specific activities obtained were on average 74.7 GBq/μmol DOTATATE (in the range from 55.86 to 98.89 GBq/μmol) and 38.20 GBq/μmol (in the range from 23.55 to 44.83 GBq/μmol) for 90Y- and 177Lu-labelled DOTATATE, respectively. 90Y-DOTATATE was administered as such while the mixed doses of 90Y/177Lu-DOTATATE were prepared from each 90Y- and 177Lu-labelled DOTATATE solution with equal radioactivity (1 GBq 177Lu/1 GBq 90Y).
Treatment
Therapy was performed on an outpatients basis. Mixed amino acid (1,000 ml Vamin 18, Fresenius Kabi) and Ringer’s solutions (500 ml) were infused over 8 h for kidney protection, with infusion of 200 ml prior to administration of the treatment [16–19]. Before administration of the radiopharmaceutical, ondansetron (8 mg, Zofran, Glaxo Wellcome, Atossa, Anpharm SA) was injected intravenously to prevent nausea and vomiting.
Treatment sessions were repeated, up to a total calculated dose of 7.4 GBq/m2. The injected activity per one course was 2.2–3.7 GBq. With respect to 90Y/177Lu-DOTATATE treatment, 50% activity of 90Y-DOTATATE and 50% activity of 177Lu-DOTATATE were injected. The median period between the treatment courses was 40 and 49 days, respectively.
Post-therapy imaging
A whole-body scan (256 × 256 matrix, 8-min acquisition per cm) and single photon emission computed tomography (SPECT) acquisition of the abdomen (128 × 128 matrix, 6º × 60 s) were performed using the dual-head Varicam camera (GE) with parallel-hole, high-energy, general purpose collimators [energy window centred on 177Lu photopeak (216 keV) and a ±10% window width].
Bremsstrahlung imaging whole-body scans in 256 × 256 matrix, 8 cm/min was performed in ten patients 24 h post 90Y-DOTATATE therapy again using the dual-head Varicam camera (GE) with a parallel-hole, low-energy, high-resolution collimator (energy window centred on 100 keV with a window width of ±35%) [20].
Evaluation of results and assessment of clinical benefit
The staging of disease and treatment response were evaluated at 3, 6, 12, 24 and 36 months follow-up. Blood tests were repeated every 7 days, others between 14 and 21 days after each cycle of therapy, and finally 3, 6, 12, 24 and 36 months after the completion of the therapy. Response to treatment on CT was defined according to Response Evaluation Criteria in Solid Tumors (RECIST). Side effects were scored according to the WHO criteria.
Event-free survival (EFS) was defined as the time from PRRT to the first evidence of progression or relapse, or to death. OS was defined as the time from PRRT to death from any cause.
The following progression criteria were defined:
In patients with clinical symptoms of carcinoid syndrome, aggravation of flushing, diarrhoea or lacrimation occurrence
Increased level of CgA of more than 50% above a previously measured value on follow-up (3, 6 and 12 months following therapy)
The increase of two-dimensional tumour diameters of more than 30% on enhanced three-phase CT
The detection of new tumour foci on SRS with 99mTc-HYNIC-TATE on planar whole-body and SPECT imaging of the abdomen (if needed, SPECT of the chest)
Statistical methods
Means and standard deviations, medians and quartiles or frequencies depending on the parameters’ distribution were used to summarize patient characteristics. The difference between comparable parameters was checked on the basis of Mann-Whitney and Wilcoxon tests.
OS, EFS and probability of 24-month survival were calculated on Kaplan-Meier estimator and compared via the log-rank test [21]. The multiple proportional hazards regression model (Cox analysis) was used for the analysis of OS and EFS. The association of chosen clinical predictors with OS and EFS was verified using the multiple proportional hazards regression model. No model reduction was performed to allow the adjustment of the effect of quality measurements for the effect of possible clinical predictors.
Model assumptions were tested on the basis of Schoenfeld residuals and in cases of violation the stratified model was fitted [22].
Calculations were done in Stata v.10.1 software (Stata Statistical Software: Release 10,Stata Corporation LP, College Station, TX, USA).
Results
Patient characteristics
In the group treated with 90Y-DOTATATE alone, 12 patients were diagnosed with foregut NETs (9 pancreas NET: 3 gastrinoma, 1 insulinoma, 5 non-functioning tumours; 3 bronchial NET), 11 patients with midgut NETs (8 carcinoid, 3 small intestinal), 1 patient with hindgut NET (rectal NET) and 1 patient with unknown primary tumours.
In the group treated with a combination of 90Y/177Lu-DOTATATE, 13 patients were diagnosed with foregut NETs (9 pancreas NET: 1 glucagonoma, 4 gastrinoma, 4 non-functioning tumours; 3 bronchial NET, 1 epiglottal NET), 8 patients with midgut NETs (7 carcinoids, 1 small intestinal), 1 patient with hindgut NET (rectal NET), 2 patients with other tumours [1 patient with multiple endocrine neoplasia (MEN 1), 1 patient with von Hippel-Lindau syndrome] and 1 patient with unknown primary tumours.
There was no statistical difference in comparable parameters (age, sex, origin of tumours, size of metastases on CT, CgA, number and localization of metastases and treatment before PRRT). A Karnofsky performance status ≥ 80 was seen in the majority of patients; only two patients in both groups had a status ≥ 60.
Detailed patient characteristics are listed in Table 1.
Table 1.
Patient characteristics
| 90Y-DOTATATE (n = 25) | 90Y/177Lu-DOTATATE (n = 25) | |
|---|---|---|
| Age range | 37–75 | 31–73 |
| Mean ± SD | 57.3 ± 10.6 | 55 ± 11.5 |
| Sex: female | 14 (56%) | 16 (64%) |
| Female age range | 37–75 | 39–73 |
| Mean ± SD | 58.2 ± 2.9 | 58.5 ± 12.4 |
| Male age range | 37–70 | 39–64 |
| Mean ± SD | 54.3 ± 10.8 | 53.7 ± 9.3 |
| Foregut | 12 (48%) | 13 (52%) |
| Midgut | 11 (44%) | 8 (32%) |
| Hindgut | 1 (4%) | 1 (4%) |
| MEN 1 | 0 | 1 (4%) |
| von Hippel-Lindau syndrome | 0 | 1 (4%) |
| Without primary | 1 (4%) | 1 (4%) |
| Size of lesion (CT in mm), range | 10–166 | 16–155 |
| Median (25%, 75%) | 50 (39, 91) | 47 (30, 75) |
| Number of lesions | ||
| 1 | 2 (8%) | 0 |
| 1–10 | 9 (36%) | 8 (32%) |
| >10 | 14 (56%) | 17 (68%) |
| Site of metastases | ||
| Liver | 19 (76%) | 20 (80%) |
| Bones | 4 (16%) | 7 (28%) |
| Lymph node | 10 (40%) | 7 (28%) |
| CgA (U/l ) | ||
| Range | 5.1–11,477 | 24–4,572 |
| Median (25%, 75%) | 423 (272.6, 1,000) | 179 (71, 417.8) |
| Surgery | 20 | 19 |
| Chemotherapy | 8 | 12 |
| “Cold” somatostatin analogues | 9 | 6 |
Post-therapeutic imaging
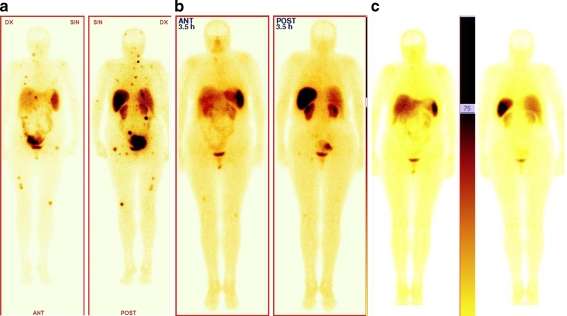
Post-therapeutic scans were performed to evaluate the biodistribution of tracer. In all cases increased uptake in the tumours and metastases was observed, compared to the liver background on qualitative analysis. The results of post-therapeutic images in all cases were comparable with the distribution of disease on 99mTc-HYNIC-TATE imaging (Figs. 1 and 2). Following post-therapeutic scans uptake in the majority of patients was unchanged. Only in two patients (one in group A and one in group B) were decreasing uptake and decrease in size of the liver metastatic lesions observed.
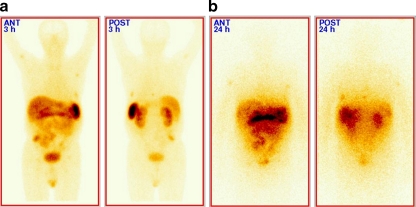
Fig. 1.
A 58-year-old man with multiple liver and peritoneum metastases of gastrinoma. a SRS with 99mTc-HYNIC-TATE before treatment with uptake in metastatic lesions in liver and peritoneum. b 24-h post-therapeutic image after administration of 90Y-DOTATATE using bremsstrahlung
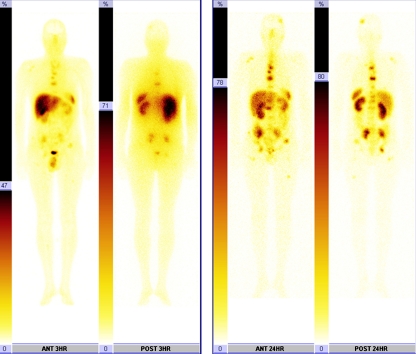
Fig. 2.
A 42-year-old man with multiple bone metastases of atypical bronchial carcinoid tumour without hormonal activity. a SRS with 99mTc-HYNIC-TATE before treatment with uptake in metastatic lesions in bone. b 24-h post-therapeutic image after administration of 90Y/177Lu-DOTATATE using 177Lu photopeak
Results of therapy
The median follow-up observation period in group A was 37.7 months and the interquartile range (IQR) for the observation period was 20.5–46.7 months. In group B the median follow-up observation period was 34.6 months and the IQR was 25.2–39.5 months.
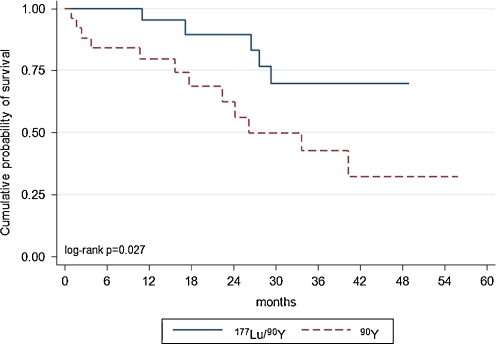
In the 90Y-DOTATATE group of patients OS was 26.2 months. In patients treated with 90Y/177Lu-DOTATATE, median survival was not reached, because less than 50% patients died. The survival plot depicting OS and the log-rank test (p < 0.027) demonstrate that the OS in the group treated with 90Y/177Lu-DOTATATE was statistically significantly longer (p < 0.027) (Fig. 3). The calculated probability of 24-month survival was 62% in the group treated with 90Y-DOTATATE and 89% in those treated with 90Y/177Lu-DOTATATE.
Fig. 3.
Kaplan-Meier estimators of OS
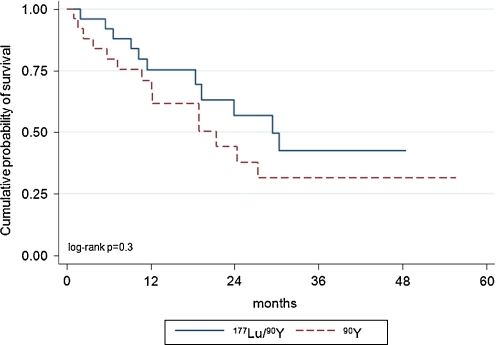
Median EFS in the 90Y-DOTATATE and 90Y/177Lu-DOTATATE groups was 21.4 and 29.4 months, respectively; the difference in EFS was not statistically significant (Fig. 4). The probability of 24-month PFS was 44 and 57%, respectively.
Fig. 4.
Kaplan-Meier estimators of EFS
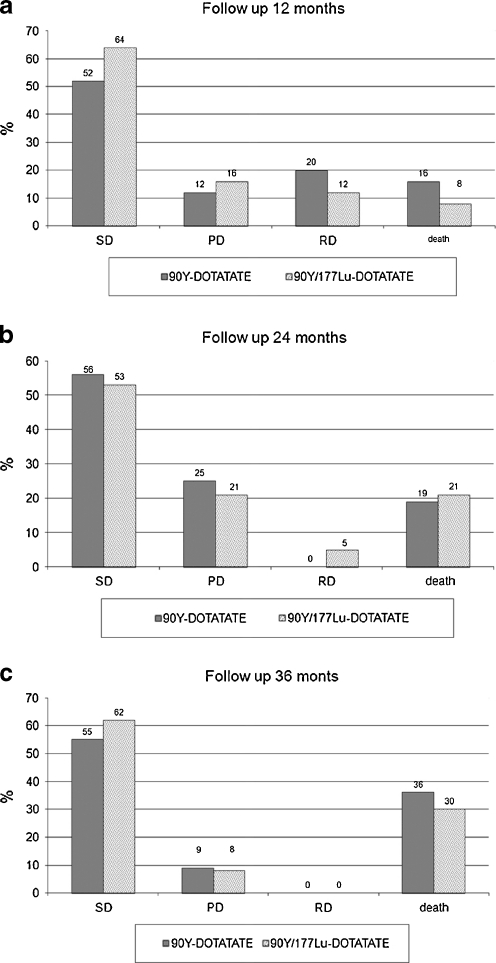
At the 12-month follow-up in the 90Y-DOTATATE group vs the 90Y/177Lu-DOTATATE group, the following was observed: SD in 13 vs 16 patients, disease regression (RD) in 5 vs 3 patients, and PD in 3 vs 4 patients; 4 and 2 patients died, respectively (Fig. 5a).
Fig. 5.
Comparison of the PRRT results (SD, RD, PD and patients who died): a 12 months, b 24 months, c 36 months follow-up
At the 24-month follow-up: SD was observed in nine vs ten patients, RD in patients in the group treated with 90Y/177Lu-DOTATATE and progression in four patients in both groups; five and four patients died, respectively (Fig. 5b).
At the 36-month follow-up, SD was noted in six vs eight patients and PD in one patients in both groups; four and four patients died, respectively (Fig. 5c).
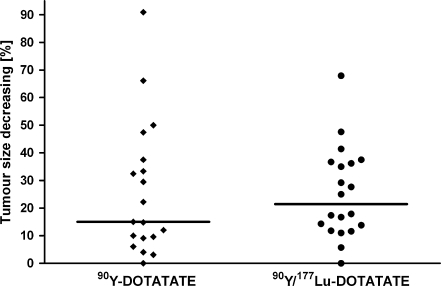
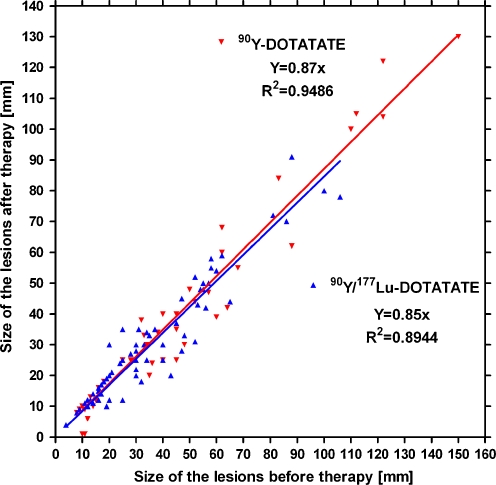
CT showed regression in size of the biggest lesions in the group treated with 90Y-DOTATATE from median 50 to 44 mm and in the group treated with 90Y/177Lu-DOTATATE from 47 to 33 mm (Fig. 6). The changes in both groups are not statistically significant. Correlation between tumour size before and after therapy is shown in Fig. 7.
Fig. 6.
Changes on CT in the size of the biggest tumours 3–6 months after completed therapy. The line represents median reduction of tumour size in % for both groups
Fig. 7.
Correlation between tumour size and type of therapy before and 3–6 months after therapy
The patients in our study were not randomized to either group A or B but were treated first with 90Y-DOTATATE (group A) and patients who presented later were treated with 90Y/177Lu-DOTATATE. For this reason we used the Cox analysis to evaluate the effects of the relevant prognostic factors on the survival times in each group of patients and the independence of these variables.
In the proportional hazards regression model for OS the radionuclides used, age of patients, sex, CgA level, number of lesions and size of maximal lesion were included. The model for OS was stratified by number of lesions due to a violated proportional hazards assumption.
Treatment is statistically significantly associated with OS, but not with EFS (Tables 2 and 3). Statistical analysis led to lower probability of death in the group treated with tandem 90Y/177Lu-DOTATATE than with 90Y-DOTATATE alone [hazard ratio (HR) = 5.74, 95% confidence interval (CI) 1.63–20.2, p = 0.006] (Table 2). Effects of treatment on SRS are shown in Fig. 8.
Table 2.
Cox proportional hazards regression model of OS
| OS | HR (95% CI) | p |
|---|---|---|
| 177Lu/90Y vs 90Y | 5.74 (1.63–20.2) | 0.006 |
| Age ≥ 56 vs < 56 | >0.1 | |
| Sex M vs F | >0.1 | |
| Size of lesion ≥ median vs < median | >0.1 | |
| Number of lesions > median vs < median | >0.1 | |
| CGA > median vs < median | >0.1 |
Table 3.
Cox proportional hazards regression model of EFS
| EFS | HR (95% CI) | p |
|---|---|---|
| 177Lu/90Y vs 90Y | >0.1 | |
| Age ≥ 56 vs < 56 | >0.1 | |
| Sex M vs F | >0.1 | |
| Size of lesion ≥ median vs < median | >0.1 | |
| Number of lesions > median vs < median | 7.2 (1.76–29.4) | 0.006 |
| CGA > median vs < median | >0.1 |
Fig. 8.
A 62-year-old woman with multiple bone and liver metastases of pancreas neuroendocrine carcinoma without hormonal activity. a SRS with 99mTc-HYNIC-TATE before treatment with uptake in metastatic lesions in bone and liver. b 12-month follow-up shows reduced number of metastases, with only a few visible in bone. c 24-month follow-up shows nearly complete response to treatment
Side effects of treatment with 90Y-DOTATATE and 90Y/177Lu-DOTATATE
The treatment was well tolerated. No severe adverse events occurred. Nausea and vomiting during administration of treatment and amino acids were observed in 20 of 50 patients (40%) with the same frequency in both groups. All cases of nausea and vomiting were successfully treated with ondansetron. Mild pain (no treatment required) at the site of the tumour was observed in 4 of 25 patients (16%) treated with 90Y-DOTATATE within the first 48 h post-treatment and in 3 of 25 patients (12%) treated with 90Y/177Lu-DOTATATE within the first 72 h after treatment. In 2 of 50 patients (4%) chest pain immediately after administration was observed (1 patient treated with 90Y-DOTATATE and 1 patient treated with 90Y/177Lu-DOTATATE). Liver tests were stable. According to WHO haematological toxicity criteria, haematological toxicity grade 3 was seen only in one patient treated with 90Y-DOTATATE (patient received chemotherapy before PRRT). Toxicity grades 1 and 2 were seen with nearly the same frequency in both groups (grade 1 in six patients in the group treated with 90Y-DOTATATE vs seven patients in the group treated with 90Y/177Lu-DOTATATE and grade 2 in three patients vs four patients, respectively) and without clinical symptoms. The decrease of WBC, RBC and PLT after therapy was similar in both groups. During treatment CrCl was stable in 15 patients treated with 90Y-DOTATATE and in 13 patients treated with 90Y/177Lu-DOTATATE. Deterioration in kidney function was observed in three patients in each group, measured by creatinine level and calculated CrCl (the maximum observed changes in CrCl in the group treated with 90Y-DOTATATE was from 111 ml/min prior to therapy which declined to 54 ml/min at the 36-month follow-up and in the group treated with 90Y/177Lu-DOTATATE from 106 ml/min prior to therapy to 67 ml/min at the 36-month follow-up). These patients received chemotherapy (etoposide+cisplatin) prior to PRRT. In two patients treated with 90Y/177Lu-DOTATATE transient deterioration in kidney function was observed.
Totally, for all patients at the 36-month follow-up the mean creatinine level increase was 0.07 mg/dl per year and CrCl decrease 5.1 ml/min per year.
Discussion
PRRT using radiolabelled somatostatin analogues is a promising new treatment option for patients with metastatic or inoperable somatostatin receptor-positive NETs. Clinical trials and radiochemistry examinations have demonstrated that both 177Lu and 90Y are suitable beta-emitting radionuclides for PRRT. It is speculated in the literature that 90Y emits beta particles with longer path lengths and higher energies which may be preferable for larger tumours, while 177Lu with shorter beta particle range and longer half-life may be preferable for small tumours. In our study we did not observe an influence of beta particle energy on size response of tumours (Figs. 6 and 7). Based on the fact that 177Lu has a longer half-life, it will take longer to deliver the same dose as 90Y. Therefore, in patients with tumours of various sizes and non-homogeneous receptor distribution, a possible solution might be the use of a combination of radionuclides [19, 23, 24].
Another option is a sequential administration of these analogues, e.g. initial administration of 90Y-labelled analogue to treat the larger tumours, followed by 177Lu-labelled analogue in the next treatment cycle(s) for treatment of smaller metastases.
In our study we used OS and EFS as primary end points to assess the effect of 90Y-DOTATATE only in comparison to combined 90Y/177Lu-DOTATATE therapy. As we know, this the first study in the literature that evaluated the role of combined PRRT in disseminated NETs. The OS after treatment in our patients was longer in the patients treated with 90Y/177Lu-DOTATATE in comparison to treatment with 90Y-DOTATATE only and longer than has previously been reported in the literature [25].
We observed longer EFS in the group treated with 90Y/177Lu-DOTATATE compared to treatment with 90Y-DOTATATE alone, but this was not statistically significant. This difference might be significant if the period of follow-up is longer. In addition, patients were not randomized, as group A was treated first and then group B at a later date.
A study by Kwekkeboom et al. about treatment with 177Lu-DOTATATE [26] reported that median PFS was 33 months and median OS was 46 months. This may be related to a longer period of follow-up.
No serious adverse events occurred after treatment with either 90Y/177Lu-DOTATATE or 90Y-DOTATATE. The toxicity as assessed according to the WHO criteria was limited and without clinical manifestations in both groups of patients. Therefore, we conclude that treatment with 90Y/177Lu-DOTATATE in disseminated or inoperable patients with NETs is feasible and safe. Clinical improvement could be observed and most patients have benefited from the treatment. We have demonstrated that this therapy is superior to 90Y-DOTATATE alone with a longer period of OS.
In addition the labelling and administration of DOTATATE in combined therapy was straightforward, and its application was safe.
The major limitation of this analysis, like other published PRRT studies, is the lack of randomization. CT treatment response is limited to anatomical changes and it is not sensitive enough to depict functional changes. That is why, for better inclusion and evaluation of therapy response, positron emission tomography (PET)/CT techniques with 68Ga-DOTATATE should be used, which was not available in our department during the study.
Conclusion
The results indicate that therapy with tandem radioisotopes (90Y/177Lu-DOTATATE) provides longer OS than with a single radioisotope (90Y-DOTATATE) and the safety of both methods is comparable. It seems that OS time and EFS time rather than WHO criteria for determining tumour burden should be used to assess the response to therapy.
However, more extensive studies with a larger number of patients are required both to establish the proportion of each isotope to be used in this dual therapy and to evaluate EFS over a longer period of follow-up.
Acknowledgments
This study was supported by Research Grant (6 P05 2004 C/6453 and 4/85195/1210/529) from the Ministry of Health and Ministry of Education. The results of this project presented at the 56th American Society of Nuclear Medicine (SNM) Annual Meeting, Toronto, Canada received (1) Young Investigator Award American Society of Nuclear Medicine (SNM) Molecular Imaging and (2) Award Nuclear Oncology Council Young Investigator, and Award EANM Eckert & Ziegler Abstract Award at the congress of the European Association of Nuclear Medicine (EANM) Barcelona.
Special thanks for Imene Zerizer from Imperial College Healthcare NHS Trust, London for English correction.
Conflicts of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Eriksson B, Annibale B, Bajetta E, Mitry E, Pavel M, Platania M, et al. ENETS Consensus Guidelines for Standards of Care in Neuroendocrine Tumors: chemotherapy in patients with neuroendocrine tumors. Neuroendocrinology. 2009;90:214–219. doi: 10.1159/000225950. [DOI] [PubMed] [Google Scholar]
- 2.Dharmsathaphorn K, Sherwin RS, Cataland S, Jaffe B, Dobbins J. Somatostatin inhibits diarrhea in the carcinoid syndrome. Ann Intern Med. 1980;92:68–69. doi: 10.7326/0003-4819-92-1-68. [DOI] [PubMed] [Google Scholar]
- 3.Vinik A, Moattari AR. Use of somatostatin analog in management of carcinoid syndrome. Dig Dis Sci. 1989;34(Suppl 3):14S–27S. doi: 10.1007/BF01536042. [DOI] [PubMed] [Google Scholar]
- 4.Rinke A, Muller H-H, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients wit metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 5.Kwekkeboom D, Krenning EP, de Jong M. Peptide receptor imaging and therapy. J Nucl Med. 2000;41:1704–1713. [PubMed] [Google Scholar]
- 6.Waldherr C, Pless M, Maecke HR, Haldemann A, Mueller-Brand J. The clinical value of [90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol. 2001;12:941–945. doi: 10.1023/A:1011160913619. [DOI] [PubMed] [Google Scholar]
- 7.Paganelli G, Bodei L, Handkiewicz Junak D, Rocca P, Papi S, Lopera Sierra M, et al. 90Y-DOTA-D-Phe1-Try3-octreotide in therapy of neuroendocrine malignancies. Biopolymers. 2002;66:393–398. doi: 10.1002/bip.10349. [DOI] [PubMed] [Google Scholar]
- 8.Otte A, Herrmann R, Heppeler A, Behe M, Jermann E, Powell P, et al. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med. 1999;26:1439–1447. doi: 10.1007/s002590050476. [DOI] [PubMed] [Google Scholar]
- 9.Waldherr C, Pless M, Maecke H, Schumacher T, Crazzolara A, Nitzsche EU, et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq (90)Y-DOTATOC. J Nucl Med. 2002;43:610–616. [PubMed] [Google Scholar]
- 10.Virgolini I, Britton K, Buscombe J, Moncayo R, Paganelli G, Riva P. In- and Y-DOTA-lanreotide: results and implications of the MAURITIUS trial. Semin Nucl Med. 2002;32:148–155. doi: 10.1053/snuc.2002.31565. [DOI] [PubMed] [Google Scholar]
- 11.Kwekkeboom D, Bakker W, Kam BLR, Teunissen JJ, Kooij PP, de Herder WW, et al. Treatment of patients with gastro-entero-pancreatic (GEP) tumours with the novel radiolabelled somatostatin analogue [177Lu-DOTA(0),Tyr3]octreotate. Eur J Nucl Med Mol Imaging. 2003;30:417–422. doi: 10.1007/s00259-002-1050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Jong M, Bernard HF, Breeman WA, et al. Combination of 90Y- and 177Lu-labeled somatostatin analogs is superior for radionuclide therapy compared to 90Y- or 177Lu-labeled analogs only. J Nucl Med. 2002;43:123P–124P. [PubMed] [Google Scholar]
- 13.de Jong M, Breeman WA, Valkema R, Bernard BF, Krenning EP. Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. J Nucl Med. 2005;46(Suppl 1):13S–17S. [PubMed] [Google Scholar]
- 14.Hubalewska-Dydejczyk A, Fröss-Baron K, Mikołajczak R, Maecke HR, Huszno B, Pach D, et al. 99mTc-EDDA/HYNIC-octreotate scintigraphy, an efficient method for the detection and staging of carcinoid tumours: results of 3 years’ experience. Eur J Nucl Med Mol Imaging. 2006;33:1123–1133. doi: 10.1007/s00259-006-0113-7. [DOI] [PubMed] [Google Scholar]
- 15.Storch D, Béhé M, Walter MA, Chen J, Powell P, Mikolajczak R, et al. Evaluation of [99mTc/EDDA/HYNIC0]octreotide derivatives compared with [111In-DOTA0,Tyr3,Thr8]octreotide and [111In-DTPA0]octreotide: does tumor or pancreas uptake correlate with the rate of internalization? J Nucl Med. 2005;46:1561–1569. [PubMed] [Google Scholar]
- 16.Behr TM, Goldenberg DM, Becker W. Reducing the renal uptake of radiolabeled antibody fragments and peptides for diagnosis and therapy: present status, future prospects and limitations. Eur J Nucl Med. 1998;25:201–212. doi: 10.1007/s002590050216. [DOI] [PubMed] [Google Scholar]
- 17.Rolleman EJ, Valkema R, de Jong M, Kooij PP, Krenning EP. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur J Nucl Med Mol Imaging. 2003;30:9–15. doi: 10.1007/s00259-002-0982-3. [DOI] [PubMed] [Google Scholar]
- 18.Jamar F, Barone R, Mathieu I, Walrand S, Labar D, Carlier P, et al. 86Y-DOTA(0)-D-Phe1-Tyr3-octreotide (SMT487)—a phase 1 clinical study: pharmacokinetics, biodistribution and renal protective effect of different regimens of amino acid co-infusion. Eur J Nucl Med Mol Imaging. 2003;30:510–518. doi: 10.1007/s00259-003-1117-1. [DOI] [PubMed] [Google Scholar]
- 19.Bernhardt P, Forssell-Aronsson E, Jacobsson L, Skarnemark G. Low-energy electron emitters for targeted radiotherapy of small tumours. Acta Oncol. 2001;40:602–608. doi: 10.1080/028418601750444141. [DOI] [PubMed] [Google Scholar]
- 20.Shen S, DeNardo GL, Yuan A, DeNardo DA, DeNardo SJ. Planar gamma camera imaging and quantitation of yttrium-90 bremsstrahlung. J Nucl Med. 1994;35(8):1381–1389. [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 22.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. doi: 10.1093/biomet/69.1.239. [DOI] [Google Scholar]
- 23.de Jong M, Breeman WA, Bernard BF, Bakker WH, Schaar M, van Gameren A, et al. [177Lu-DOTA(0),Tyr3] octreotate for somatostatin receptor-targeted radionuclide therapy. Int J Cancer. 2001;92:628–633. doi: 10.1002/1097-0215(20010601)92:5<628::AID-IJC1244>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 24.Capello A, Krenning EP, Breeman WA, Bernard BF, Konijnenberg MW, de Jong M. Tyr3-octreotide and Tyr3-octreotate radiolabeled with 177Lu or 90Y: peptide receptor radionuclide therapy results in vitro. Cancer Biother Radiopharm. 2003;18:761–768. doi: 10.1089/108497803770418300. [DOI] [PubMed] [Google Scholar]
- 25.Kwekkeboom DJ, Mueller-Brand J, Paganelli G, Anthony LB, Pauwels S, Kvols LK, et al. Overview of results of peptide receptor radionuclide therapy with 3 radiolabeled somatostatin analogs. J Nucl Med. 2005;46(Suppl 1):62S–66S. [PubMed] [Google Scholar]
- 26.Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]