Abstract
Objective
We wanted to evaluate the clinical value of intraoperative ultrasonography for real-time guidance when performing microneurosurgical resection of small subcortical lesions.
Materials and Methods
Fifty-two patients with small subcortical lesions were involved in this study. The pathological diagnoses were cavernous hemangioma in 25 cases, cerebral glioma in eight cases, abscess in eight cases, small inflammatory lesion in five cases, brain parasite infection in four cases and the presence of an intracranial foreign body in two cases. An ultrasonic probe was sterilized and lightly placed on the surface of the brain during the operation. The location, extent, characteristics and adjacent tissue of the lesion were observed by high frequency ultrasonography during the operation.
Results
All the lesions were located in the cortex and their mean size was 1.3 ± 0.2 cm. Intraoperative ultrasonography accurately located all the small subcortical lesions, and so the neurosurgeon could provide appropriate treatment. Different lesion pathologies presented with different ultrasonic appearances. Cavernous hemangioma exhibited irregular shapes with distinct margins and it was mildly hyperechoic or hyperechoic. The majority of the cerebral gliomas displayed irregular shapes with indistinct margins, and they often showed cystic and solid mixed echoes. Postoperative imaging identified that the lesions had completely disappeared, and the original symptoms of all the patients were significantly alleviated.
Conclusion
Intraoperative ultrasonography can help accurately locate small subcortical lesions and it is helpful for selecting the proper approach and guiding thorough resection of these lesions.
Keywords: Intraoperative ultrasonography, Microneurosurgery, Subcortex
INTRODUCTION
Accurate location of subcortical small lesions is a challenge in neurosurgery. In the earlier years, computed 546tomography (CT) and magnetic resonance imaging (MRI) played a major role for preoperatively evaluating brain abnormalities, because they can aid in both the characterization and localization of lesions. However, they are complicated, they involve radioactivity, they cannot support real-time navigation and they do not provide positioning marks for later surgery (1). In the past decade, neuronavigation has been developed to assist neurosurgeons in performing surgery more safely, efficaciously, and cost effectively. Neuronavigation helps the neurosurgeon to appropriately plan the operation and to rapidly and accurately locate the lesion and so improve the operation. However, it cannot avoid positioning drift caused by the loss of cerebrospinal fluid, which reduces the accuracy of the positioning (2). Using intraoperative ultrasound enables accurate location of small subcortical lesions, and it enables neurosurgeons to make appropriate treatment decisions and adjust the operation at any time (3). Over the past four years, we have successfully used intraoperative ultrasound when resecting 52 small subcortical lesions via microneurosurgery. We have retrospective analyzed our data and here we report our results.
MATERIALS AND METHODS
Clinical Data
Fifty-two patients (M:F = 28:24; age range, 17-70 years; mean age, 39.4 years) were enrolled in the study from January 2005 and February 2009. Informed consent was received from all the patients and we are in compliance with the requirements of our institutional review board. The lesions were located in different places: 17 in the frontal lobe, 13 in the temporal lobe, 11 in the parietal lobe and 11 in the occipital lobe. The patients exhibited differing clinical symptoms such as epilepsy, headache, vertigo, vomiting, tottering, twitching and anoxia. The pathological diagnoses were cavernous hemangioma in 25 cases, cerebral glioma in eight cases (based on the 2000 WHO nervous system classification (4), three cases were low grades of cerebral gliomas, five cases were high grades of cerebral gliomas), abscess in eight cases, small inflammatory lesions in five cases, brain parasite infection in four cases and the presence of an intra-cranial foreign body in two cases. All the patients had undergone CT or MRI examination to show that the lesions were located in the cortex, the size was less than 2 cm diameter and the depth was between 0.2 cm and 3 cm (mean depth, 1.3 cm). Some of the lesions were surrounded by edema. The pathological characteristics of all the lesions were confirmed after the operation.
Equipment and Methods
We used the high (5-10 MHz) frequency ultrasonic probes of a SonoSite 180Plus (SonoSite, Bothell, WA) or an Aloka SSD-α10 (Aloka, Mitaka-shi, Tokyo, Japan) to observe the lesions during the operation. The probe was insulated with sterile gum and gel, and it was lightly placed either on the dura or directly on the surface of the cortex depending on the operation procedure. For superficial lesions, a high-frequency probe (to detect a depth of 2-5 cm) was used to observe the location, extent and characteristics of the lesions and the adjacent tissues, and to measure the depth of the lesion from the cortex. Color Doppler was used to observe the blood supply of the lesion and the relationship between the lesion and the important adjacent blood vessels. Real-time ultrasonography was used to guide the resection, evaluate the residual tumor tissue and reveal the optimal surgical approach.
Operational Methods
All the patients received general anesthetics and they underwent microneurosurgical resection. Immediately after the scalp had been shaved but before opening up the dura mater, we used ultrasound to detect the location of the lesion. The probe was coated with the coupling agent (sterile gum or gel), the dura was perfused with physiological saline and then the probe was placed lightly on the surface of the dura. We scanned in multiple planes such as coronal, sagittal and transverse planes to determine the location of the lesion and the best approach for resection. After opening the dura mater, we used ultrasound again to assess the lesion size and location, its relationship with the surrounding tissue and to start the color Doppler function to reveal the blood supply to the lesion and the surrounding tissue. The lesion or foreign body was then removed by microneurosurgery, that is, using a microscope and cutting the cortex according to the gyri and sulci on the surface of the brain.
RESULTS
During the operation, all the types of small lesions were displayed clearly and located accurately by intraoperative ultrasonography. The size of the lesion, its depth in the cortex and its relationship with the deep ventricle were revealed. All the lesions were completely resected and post-operative imaging confirmed that the lesions had been completely resected. In all the patients, their original symptoms were significantly alleviated. For the small or deeply located lesions, intraoperative ultrasonography provided accurate information for the neurosurgeons. Residuals of the tumor or hematoma in eight cases were identified and these were confirmed prior to the completion of surgery.
Intraoperative ultrasonography permitted accurate and thorough characterization of the normal brain tissue and all the lesions. For the normal brain cortex, it is only slightly hypoechoic when compared with the normally echoless fluid, and the normal brain cortex was hyperechoic and is less echogenic compared to the meninges around the brain. Color Doppler showed grid-like blood flow signals in these tissues.
The relatively characteristic intraoperative ultrasound findings of the different lesions are described in Table 1. The representative images were shown in Figures 1-5. The ultrasonic characteristics of parasitic lesions were similar to those of inflammatory lesions, but after abscess formation they were similar to those of abscesses. However, a scolex within the multiple cysts was the characteristic intraoperative ultrasound findings for the cerebral cysticercosis (Fig. 5). There were two cases of intracranial foreign bodies located in the superficial cortex and these intracranial foreign bodies demonstrated hyperechoic characteristics.
Table 1.
Intraoperative Ultrasonic Characteristics of Different Pathologic Lesions
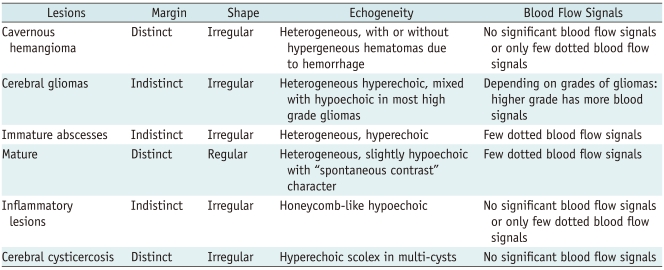
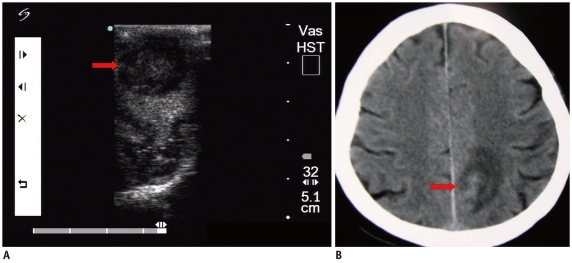
Fig. 1.
Cavernous hemangioma.
A. Intraoperative ultrasonic imaging of cavernous hemangioma (arrow). It shows hyperechoic characteristics with irregular shape and distinct margin. B. T2 sagittal MRI shows lesion (arrow) was located in frontal lobe.
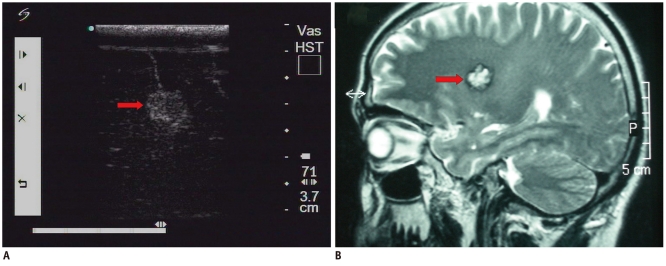
Fig. 5.
Cerebral cysticercosis.
A. Intraoperative ultrasonic imaging of cerebral cysticercosis lesion (arrow). Note hyperechoic scolex in multi-cysts. B. T1 axial MRI shows lesion was located at temporal lobe (arrow). Note there were multiple lesions shown on MRI.
Brain edema was a common phenomenon in most of the lesions, except the cavernous hemangioma. The edema was more echogenic than the normal brain tissue, but it was less echogenic than all the lesions in the present study. However, for most of the abscesses and the inflammation lesions, the edema was slightly difficult to distinguish from the lesions due to the lesser difference of the echogeneity. For the cerebral glioma, the higher grade cerebral glioma demonstrated more severe edema.
There was one case of cerebral glioma that was located at the frontal lobe and the patient demonstrated epilepsy. He developed paralysis of the limbs after resection of the lesion, but he was back to normal one week after the procedure.
DISCUSSION
The main difficulty in resecting small brain lesions lies in locating the lesion during the operation. Small errors can result in unnecessary brain damage, and especially if the lesion is located in an important functional area and this can cause serious complications. Accurate location of the lesion is also a key step in its total removal. This directly influences the success rate of the surgery and the patient's prognosis. Generally, locating intracranial lesions relies on preoperative CT and MRI followed by spatial reconstruction according to the brain surface anatomy. This is straightforward for larger lesions, and it has a low error rate (5). However, small subcortical lesions have been more problematic. With the development of neuronavigation systems, surface brain lesions can be accurately positioned. However, drift in the navigation coordinates caused by the loss of cerebrospinal fluid reduces the accuracy of locating the lesion, and especially for the locating small subcortical lesions.
In the past, because the adult skull can absorb sound waves, and due to sound wave scattering and other factors, transcranial ultrasound diagnosis was used in infants and young children more than in adults. With the technological advances, intraoperative ultrasonography can now show the lesion's location, size, extent and its relationship with the surrounding tissue, and intraoperative ultrasonography can also reveal the lesion's blood supply, it can measure the rate and direction of arterial blood flow and it can differentiate the arterial blood supply of the lesion (6, 7). Intraoperative ultrasonic imaging was first applied as a real-time navigation system in neurosurgery in 1980 (8), after eliminating the influence of the skull on the sound waves. In 1992, Becker et al. (9) first used intraoperative ultrasonic imaging to detect intracranial lesions, arteriovenous malformation, subarachnoid hemorrhage, intracranial hemorrhage, cerebral aneurysms and other pathologies. As the ultrasound technology advances, the improved resolution, flexibility and image quality will increase its range of application still further. In neurological practice, ultrasound is routinely used as a real time navigation system, such as to guide the resection of tumors in the brain and spinal cord. Lunardi et al. (10) reported the usefulness of real-time ultrasound guidance and location after successfully guiding 20 cavernous hemangioma operations with intraoperative ultrasonography. Research has shown that application of intraoperative ultrasonography can help neurosurgeons understand the characteristics of intracranial lesions, such as their size, their borders and their nature, and so this improves the precision of the operation and it reduces the risk and complications of the operation, with widespread clinical application (11).
We summarized the data from 52 patients with small subcortical lesions that were located accurately with intraoperative ultrasound before microneurosurgical resection. When using intraoperative ultrasonography as a guide, the probe must be completely sterilized and carefully handled. The results showed that intraoperative ultrasound could clearly show intracranial lesions, and especially the subcortical ones, and provide an accurate location. It can also detect the relationship between the lesion and the blood vessels to facilitate complete removal of the lesion. In 19 cases we also performed location by neuronavigation. During the operation, we found that the neuronavigation positioning was consistent with the intraoperative ultrasound positioning before cutting though the dura mater. However, after cutting through the dura mater, as a result of the loss of cerebrospinal fluid and brain movement, neuronavigation proved inaccurate in seven cases. By comparison, real-time intraoperative ultrasound can continually adjust during the operation process. Therefore, it can identify residual tumor tissue during surgery, improve the rate of total resection of the lesion and reduce the likelihood of postoperative complications.
The results of this study show that intraoperative ultrasonography facilitates real-time scanning, without the use of radiation, to examine a lesion's location, size and relationship with the surrounding tissue and blood vessels. Intraoperative ultrasonography can be used for tumor resection and evaluation of vessels with color flow Doppler. It can reduce the surgery's time and cost, determine the best surgical approach and reduce postoperative complications. In particular, intraoperative ultrasonography plays an important role for microneurosurgical resection of small subcortical lesions and it is suitable for wide clinical application.
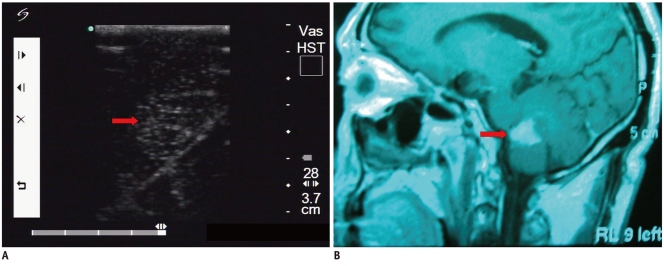
Fig. 2.
Cerebral glioma.
A. Intraoperative ultrasonic imaging of one cerebral glioma (arrow). It demonstrates heterogeneous characteristics with irregular shape and indistinct margin. B. T1 sagittal MRI shows lesion (arrow) was located in frontal lobe.
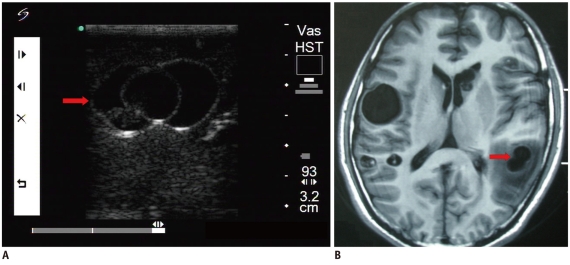
Fig. 3.
Mature abscess.
A. Intraoperative ultrasonic imaging of mature abscess (arrow). It shows hypoechoic characteristics with regular margins and heterogeneous internal echo. B. Axial CT shows lesion (arrow) was located in parietal lobe.
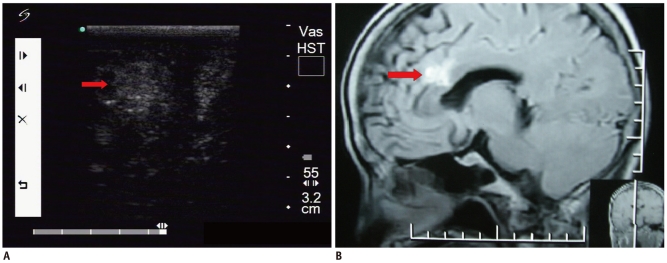
Fig. 4.
Inflammatory lesion.
A. Intraoperative ultrasonic imaging of inflammation lesion (arrow). It demonstrates honeycomb-like hypoechoic characteristics with irregular shapes and indistinct margins (pathological diagnosis: infiltration of lymphocytes). B. T1 sagittal contrast enhanced MRI shows lesion (arrow) was located in cerebellum.
References
- 1.Moller-Hartmann W, Herminghaus S, Krings T, Marquardt G, Lanfermann H, Pilatus U, et al. Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology. 2002;44:371–381. doi: 10.1007/s00234-001-0760-0. [DOI] [PubMed] [Google Scholar]
- 2.Kawamata T, Iseki H, Hori T. [Navigation systems for neurosurgery at present and in the future] No Shinkei Geka. 2003;31:609–618. [PubMed] [Google Scholar]
- 3.Erdogan N, Tucer B, Mavili E, Menku A, Kurtsoy A. Ultrasound guidance in intracranial tumor resection: correlation with postoperative magnetic resonance findings. Acta Radiol. 2005;46:743–749. doi: 10.1080/02841850500223208. [DOI] [PubMed] [Google Scholar]
- 4.Shen TZ, Zhang YL, Chen XR. Advances of WHO classification of brain tumors. Chin Comput Med Imag. 2000;6:219–231. [Google Scholar]
- 5.van Velthoven V. Intraoperative ultrasound imaging: comparison of pathomorphological findings in US versus CT, MRI and intraoperative findings. Acta Neurochir Suppl. 2003;85:95–99. doi: 10.1007/978-3-7091-6043-5_13. [DOI] [PubMed] [Google Scholar]
- 6.Cokluk C, Iyigun O, Senel A, Celik F, Rakunt C. The guidance of intraoperative ultrasonography in the surgical treatment of arteriovenous malformation. Minim Invasive Neurosurg. 2003;46:169–172. doi: 10.1055/s-2003-40737. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Liu X, Hou WH, Dong G, Wei Z, Zhou H, et al. The relationship between intra-operative ultrasonography and pathological grade in cerebral glioma. J Int Med Res. 2008;36:1426–1434. doi: 10.1177/147323000803600632. [DOI] [PubMed] [Google Scholar]
- 8.Janson M, Michael K, Berg J, Anderson J. The role of intraoperative sonography in neurosurgery. J Diagn Med Sono. 2005;21:148–151. [Google Scholar]
- 9.Becker G, Perez J, Krone A, Demuth K, Lindner A, Hofmann E, et al. Transcranial color-coded real-time sonography in the evaluation of intracranial neoplasms and arteriovenous malformations. Neurosurgery. 1992;31:420–428. doi: 10.1227/00006123-199209000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Lunardi P, Acqui M. The echo-guided removal of cerebral cavernous angiomas. Acta Neurochir (Wien) 1993;123:113–117. doi: 10.1007/BF01401865. [DOI] [PubMed] [Google Scholar]
- 11.Sun H, Zhao JZ. Application of intraoperative ultrasound in neurological surgery. Minim Invasive Neurosurg. 2007;50:155–159. doi: 10.1055/s-2007-985146. [DOI] [PubMed] [Google Scholar]