Abstract
Objective
We wanted to assess the safety and efficacy of performing radiofrequency ablation (RFA) in patients with non-colorectal liver metastases.
Materials and Methods
In this retrospective study, 25 patients with 40 hepatic metastases (M:F = 17:8; mean age, 57 years; tumor size, 0.5-5.0 cm) from a non-colorectal origin (stomach, biliary, breast, pancreas, kidney and skin) were treated with RFA. The RFA procedures were performed using either an internally cooled electrode or a clustered electrode under ultrasound or CT guidance. Contrast-enhanced CT scans were obtained immediately after RFA and follow-up CT scans were performed within three months after ablation and subsequently at least every six months. The intrahepatic disease-free interval was estimated and the overall survival from the time of the initial RFA was analyzed using the Kaplan-Meier method.
Results
No intraprocedural deaths occurred, but four major complications developed, including abscesses (n = 3) and pneumothorax (n = 1). Technical effectiveness was determined on the initial follow-up images. During the follow-up period (range, 5.9-68.6 months; median time, 18.8 months) for 37 tumors in 22 patients where technical effectiveness was achieved, 12 lesions (32%, 12 of 37) showed local tumor progression and new intrahepatic metastases occurred in 13 patients (59%, 13 of 22). The median intrahepatic disease-free interval was 10.1 months. The 1-year, 3-year and 5-year overall survival rates after RFA were 86%, 39% and 19%, respectively.
Conclusion
RFA showed intermediate therapeutic effectiveness for the treatment of non-colorectal origin liver metastases.
Keywords: Liver, Interventional procedures, Radiofrequency ablation, Preliminary clinical study
INTRODUCTION
Radiofrequency ablation (RFA) has recently received considerable attention as a minimally invasive treatment for tumor. The technique has been increasingly used for the treatment of hepatocellular carcinoma (HCC) and colorectal liver metastases. In patients with a solitary HCC and Child-Pugh class A cirrhosis, RFA had been shown to be associated with an excellent 5-year survival rate that is comparable to surgical resection (1-6). Furthermore, for the patients with colorectal liver metastases, the 3-year and 5-year survival rates after RFA are in the range of 42-64% and 31-44%, respectively (7-12). Several studies have demonstrated that for patients with small solitary colorectal metastases, the survival rates after surgical resection and RF ablation might be comparable (13-15). Therefore, RFA has been increasingly used as an alternative modality to surgery for patients with small liver metastases from colorectal cancer and who have limited hepatic metastatic disease.
Some parts of eastern Asia, including Korea and Japan, have a very high incidence of gastric cancer (16, 17), and the patients suffering from tumor recurrence in the liver after primary cancer resection are common. Cholangiocarcinoma is the second most common primary liver cancer after HCC (18). Although many studies have demonstrated the therapeutic efficacy and survival of RFA for the treatment of liver metastases (19-33), there have been only limited studies on using RFA for treating liver metastases from non-colorectal cancers such as gastric cancers (19-21) and cholangiocarcinomas (22). The purpose of this study was to assess the safety and efficacy of curative intent RFA for the treatment of metachronous isolated liver metastases from non-colorectal cancers, including gastric cancers and cholangiocarcinomas.
MATERIALS AND METHODS
A review of our radiofrequency ablation (RFA) database identified that 74 treatment sessions for 58 patients were performed for the management of liver metastases from non-colorectal cancer between September 2002 and March 2008. We only included the procedures performed for a curative intent of hepatic metastases. The inclusion criteria included the following. 1) On the images obtained prior to the RFA procedure, the primary cancer was controlled, 2) inoperable hepatic metastases because of bilobar disease, comorbidity, a previous history of liver resection or the patient's refusal, 3) the patients had no detectable extrahepatic metastases, 4) the number of metachronous hepatic metastases was five or less, 5) the tumor size was 5 cm or less, and 6) the patients had at least one follow-up image. We conducted a retrospective review of the imaging findings and medical records. The Institutional Review Board approved this retrospective study and the requirement for patient informed consent was waived.
Patients
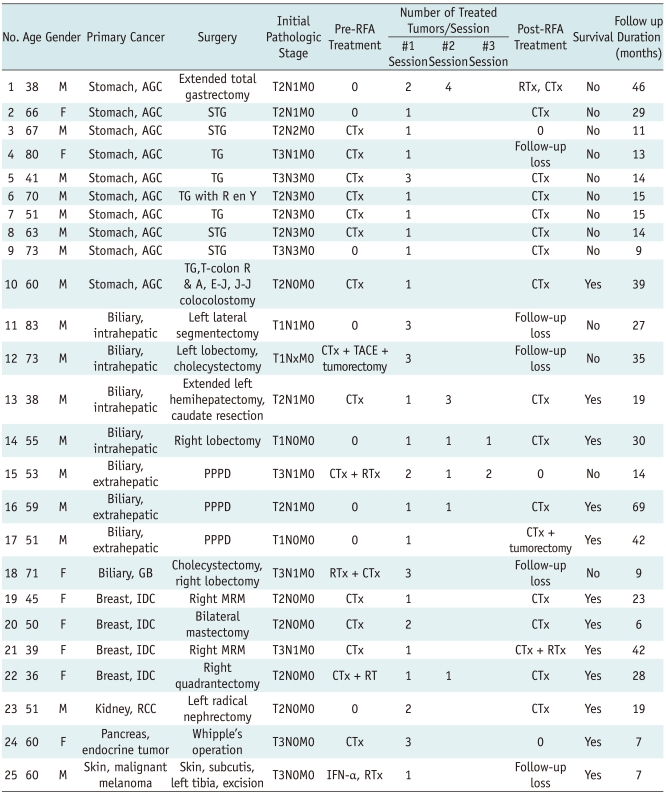
Over a 6-year-period, we selected 28 RFA sessions in 25 patients (M:F = 17:8; age range, 36-83 years; mean age, 57 years) that were performed with a curative intent for hepatic metastases. The histological diagnosis of the primary cancers and the types of treatment for the primary cancers are listed in Table 1. The size of the treated tumors ranged from 0.5 cm to 5 cm in diameter (mean ± standard deviation [SD], 2.0 ± 1.0 cm). The total number of RFA treated tumors was 40 with a range of 1-4 per one treatment session (mean ± SD, 1.6 ± 0.8).
Table 1.
Patient Characteristics
Note.-AGC = advanced gastric cancer, CTx = chemotherapy, E-J = esophagojejunal anastomosis, GB = gallbladder, IDC = intraductal infiltrating carcinoma, J-J = jejunojejunal anastomosis, MRM = modified radical mastectomy, PPPD = pylorus-preserving pancreatoduodenectomy, R en Y = Roux-en Y, RCC = renal cell carcinoma, RFA = radiofrequency ablation, RTx = radiation therapy, STG = subtotal gastrectomy, T-colon R & A = transverse colon resection and anastomosis, TACE = transarterial chemoembolization, TG = total gastrectomy
In our institution, the diagnostic work-up included a dynamic abdominal CT examination that was performed within one month before the RFA procedure. Laboratory tests such as a liver function test, a coagulation test and complete blood tests were also performed. All of the tumors were 5 cm or less in size. For RFA, the metastases should be less than 5 cm in diameter and the tumors should be located remote from large vessels, the bowel or diaphragm to minimize the risk of complications (34, 35). A platelet count of more than 50 × 103/µL and an INR (international normalized ratio) of less than 1.5 were required before the procedure.
Radiofrequency Ablation Procedure
The patients underwent RFA treatment after 12 hours of fasting. For all the patients, RFA therapy was performed with conscious sedation and analgesia, which were achieved by means of intravenous administration of 1-2 mg of midazolam (Dormicum; Roche, Basel, Switzerland) and 50-100 g of fentanyl citrate (Fentanyl; Myengmun, Seoul, Korea). A nurse and the attending physician continuously monitored the vital signs during the procedure and for one hour following the procedure.
Percutaneous RFA was performed by one of the attending physicians with 3-10 years experience in RFA and these procedures were mostly done under sonographic guidance and monitoring, except for three metastases that were not visualized on a sonographic examination and so they were treated under CT guidance. RFA was performed using a 500-KHz monopolar RF generator (either a CC-1, Integra Radionics, Burlington, MA or a multi-channel RF generator, Taewoong, Koyang, Korea). A single cool tip electrode (Cool Tip Electrode; Valleylab, Boulder, CO) or a clustered electrode (Valleylab) was used based on the target tumor size. A single cool tip electrode was used for tumors smaller than 3 cm and a clustered electrode was used for tumors larger than 3 cm.
Radiofrequency was initially applied for 12 minutes and for subsequent ablations for 6 to 12 minutes. An impedance-controlled automated pulsed RF algorithm was used (upper limit, 80 ohms) with a maximum peak current of 1000-2000 mA and 50-200 W. A peristaltic pump (Watson-Marlow, Paris, France) was used for cooling of the electrode with saline solution (0℃) at a rate that was sufficient to maintain an electrode temperature below 20℃. Constant monitoring of the temperature at the tip of the needle was also performed. After ablation, the electrode was withdrawn with cauterizing the tract.
Treatment Assessment and Follow Up
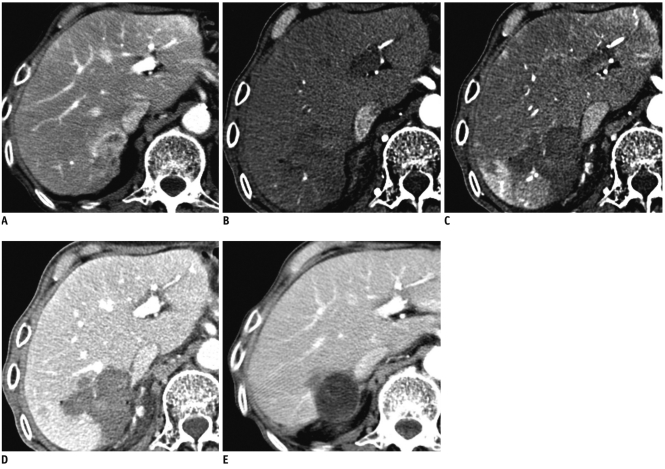
Immediately after the completion of the radiofrequency ablation procedure, a three-phase dynamic enhanced CT study (120 kVp, 200 mAs, 1.5 ml/body weight of nonionic contrast) was performed to evaluate the presence of residual tumor at the treated site and any immediate complications such as hemorrhage and bowel injury. A lack of enhancement of the ablated zone that covered the tumor area with no evidence of irregular peripheral enhancement was considered complete ablation of the macroscopic tumor and a technically successful RFA procedure (Fig. 1). Four metastases were seen as residual lesions on the immediate post-RFA enhanced CT images and additional RFA treatment was performed within 24 hours after the initial RFA procedure. After additional sonography-guided or CT-guided RFA, technically successful RFA was performed for all of the treated lesions.
Fig. 1.
Imaging findings are shown for approximately 3.6 cm sized gastric cancer liver metastasis that was completely ablated (technical success) without recurrence.
A-E. Pre-ablation contrast-enhanced CT in equilibrium phase (A) shows 3.6 cm sized peripheral enhancing mass located at segment 7. Immediate-ablation dynamic contrast-enhanced CT demonstrates complete necrosis of metastasis with lack of enhancement on early arterial (B), late arterial (C) and portal venous (D) phase. After eight months, follow-up contrast-enhanced CT image (E) shows cystic change of ablated lesion without evidence of recurrence.
Initial follow-up imaging was performed within three months. Standardized time interval imaging was obtained at three and six months after the RFA procedure and then every six months. Nodular or irregular enhancement seen during the follow-up imaging was considered local tumor progression. Repeat treatment was performed to treat local tumor progression and/or new intrahepatic lesions in three patients who had fewer than five foci of intrahepatic tumor with no evidence of extrahepatic disease, as seen on imaging studies.
Statistical Analysis
The intrahepatic disease-free interval was defined as the interval between the initial treatment and the first appearance of a new lesion in the liver. Overall survival from the time of the initial RFA was also analyzed using the Kaplan-Meier method. MedCalc software (version 10.1.3.0; MedCalc Software, Mariakerke, Belgium) was used for all the statistical analyses.
RESULTS
Technical Effectiveness
Complete ablation of macroscopic tumors, as depicted on the initial follow-up CT images, and the technical effectiveness were confirmed for 22 of 25 (88%) patients after initial treatment. The technical effectiveness for 40 treated lesions was 88% (22 of 25) on a per patient basis and 93% (37 of 40 lesions) on a per tumor basis.
Intrahepatic Tumor Control
The liver CT imaging follow-up time was 19.2 months (range, 2.0-64.9 months; median time, 13.2 months). During the follow-up period, no viable tumors were detected in the liver in seven patients. Four of these seven patients were totally tumor free during the follow-up period, but three of the patients developed distant metastases. Among the 15 patients with intrahepatic tumor recurrence, nine patients also had an extrahepatic metastasis during the follow-up period. Among the 15 patients with intrahepatic tumor recurrence, seven patients had new metastases that were remote from the radiofrequency-treated lesions; two patients had local tumor progression and six patients had both new metastases and local tumor progression.
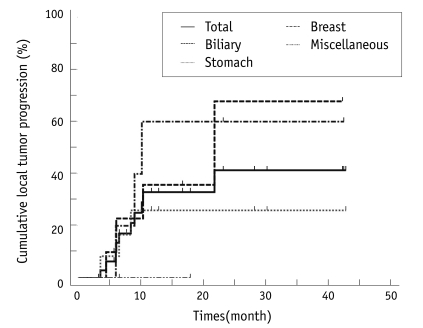
Local tumor Progression
Among the 40 lesions, three metastases were unsuccessfully treated. The patients with the 37 lesions that were effectively treated underwent follow-up. The overall local tumor progression rate was 32% (12 of 37) for all of the totally treated lesions, including 25% (3 of 12) for stomach cancer, 43% (6 of 14) for biliary cancer, 60% (3 of 5) for breast cancer and 0% (0 of 3) for the other tumors (Fig. 2).
Fig. 2.
Rates of local tumor progression after radiofrequency ablation.
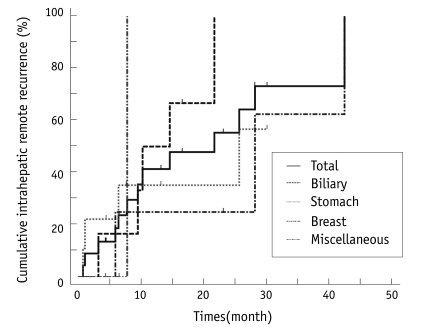
Intrahepatic Recurrence
The intrahepatic recurrence rate was 68% (15 of 22) for the primary treatment session, 66% (6 of 9) for stomach cancer, 83% (5 of 6) for biliary cancer and 75% (3 of 4) for breast cancer. Intrahepatic recurrence developed in the patients with renal cell carcinoma (RCC) liver metastases. The intrahepatic remote recurrence rate was 59% (13 of 22) for all of the treatment sessions, 83% (5 of 6) for biliary cancer, 44% (4 of 9) for stomach cancer and 75% (3 of 4) for breast cancer (Fig. 3).
Fig. 3.
Rates of intrahepatic remote recurrence after radiofrequency ablation.
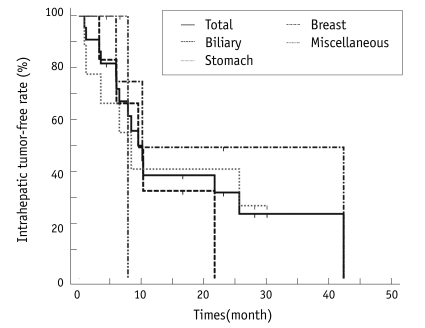
We calculated the time between RFA treatment and the follow-up imaging that showed a new intrahepatic lesion. The median intrahepatic disease-free interval was 10.1 months (Fig. 4). Repeated radiofrequency ablation was performed to treat local disease progression and new lesions for five patients. One biliary cancer patient underwent two curative intent RFA procedures and one palliative intent RFA procedure. One additional RFA session with a curative intent was performed on one breast cancer patient and one biliary cancer patent, respectively, and one additional RFA session with a palliative intent was performed on one stomach cancer patient and one biliary cancer patent, respectively.
Fig. 4.
Intrahepatic tumor-free interval after radiofrequency ablation.
Extrahepatic Tumor Recurrence
The extrahepatic recurrence rate was 55% (12 of 22) for the primary treatment session, 56% (5 of 9) for stomach cancer, 67% (4 of 6) for biliary cancer and 25% (1 of 4) for breast cancer. Extrahepatic recurrence also developed in the RCC and malignant melanoma patients. Seventy five percent of these patients with extrahepatic recurrence combined with intrahepatic metastases. Three of them first presented with extrahepatic metastases. The mean time between RFA treatment and the follow-up imaging showing a new extrahepatic lesion was 13.7 months.
Survival after Radiofrequency Ablation
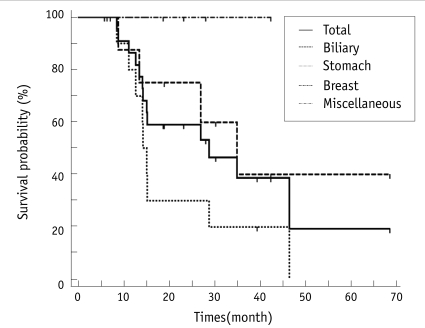
The mean follow-up was 23.3 months (range, 5.9-68.6 months; median time, 18.8 months; n = 25). Thirteen patients died during follow-up. The median overall survival after radiofrequency ablation was 28.8 months. The 1-year, 3-year and 5-year estimated survival rates after radiofrequency ablation were 86%, 39% and 19%, respectively (Fig. 5).
Fig. 5.
Survival rate after radiofrequency ablation.
Complications
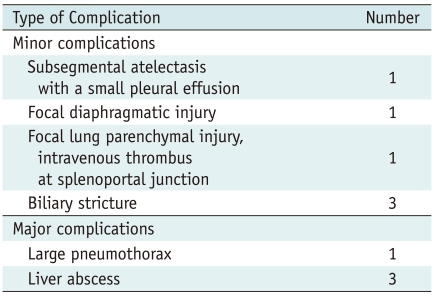
The side effects and complications after RFA are summarized in Table 2. No intraprocedural deaths occurred. Four major complications occurred. One patient who developed a large pneumothorax was treated with a chesttube and three patients who developed liver abscesses were treated with percutaneous drainage. For the 25 patients, the total complication rate was 40% (10 of 25).
Table 2.
Side Effects and Complications
DISCUSSION
Our study provides the therapeutic results of percutaneous RFA for non-colorectal liver metastases, and the estimated survival rate for patients with non-colorectal liver metastases was 86%, 39% and 19% for one year, three years and five years, respectively. These results are not as good as the results of the previous studies on RFA in patients with colorectal liver metastases, which showed the 1, 3 and 5-year estimated survival rates were 87-96%, 42-57% and 24-44%, respectively (7-12).
Although the results of RFA for patients with noncolorectal liver metastases were not as good as that for RFA in patients with colorectal liver metastases, our study results might have some clinical meaning. For example, surgical resection for a gastric liver metastasis is rarely performed, which is different from the treatment of colorectal liver metastases. Following curative resection of gastric cancer, the liver is the most common site of hematogenous metastases and approximately 15% of all recurrences were liver metastasis alone (36). However, the majority of liver metastases are combined with locoregional or peritoneal seeding, and even if a metastasis is confined in the liver, bilobar disease is common and so there is a very limited number of surgical candidates (36, 37).
The reported 5-year survival rate after hepatic resection of selected patients with resectable gastric liver metastases is approximately 30% (38-40). Considering this clinical situation, our estimated survival rate after RFA for one year, three years and five years was 78%, 22% and 0%, respectively, with a median survival of 15.1 months. The local tumor control rate of stomach cancer was excellent. However, as compared with other metastatic tumors, a relatively high rate of intrahepatic remote metastases and extrahepatic metastases was found. This recurrence pattern has also been reported in previous studies regarding surgical resection of gastric liver metastases (36, 41). Although our results are not as good as compared with the surgical results, a complementary role for RFA should be considered when remembering that all our patients were in an inoperable state (21, 22, 42). However, because of the high rate of remote recurrence of stomach cancer, RFA as a sole treatment is ethically unacceptable and systemic therapy should also be considered.
Surgical resection is the only potentially curative treatment for patients with biliary cancer. However, after curative resection, the five-year survival rate for patients with tumor-free margins is 20-40% and the operative mortality is approximately 10%. Even after curative resection, 31-50% of these patients have a recurrence in one year, and about half of the cancers recur in the remaining liver (43-45). The patients who have had prior surgery have a lesser chance of curative resection. In addition, postoperative chemotherapy and radiation therapy were not significantly identified as being efficacious for treating biliary cancer (46, 47). Based on our results, half of the patients had complete tumor resection and half of the patients had palliative surgery and the latter received chemoradiation treatment. Because of bilobar disease or having undergone hepatectomy, the patients underwent RFA for the treatment of recurred cancer. The local tumor progression-free rates on a per lesion-basis were 64% and 32% for one year and two years, respectively, and the median intrahepatic disease free interval was 9.5 months. Considering the recurrence rate after surgery, there might be some role for RFA to control local tumor in patients with unresectable disease.
A small number of breast cancer patients were included in our series. None of the patients with breast cancer died during the follow-up period (range, 5-42 months; mean, 25 months; median, 25 months). New metastases were observed in three (75%) of four patients. It was not possible to compare our study with the previous studies. However, for the intrahepatic tumor control of the previous largest study, 58% of the patients developed tumors after a 4-44 month period (mean, 10 months) (25). In comparison, the estimated 10-month intrahepatic recurrence rate in our study is similar to the rate in a previous study (27). The median intrahepatic disease-free interval determined in our study (10.1 months) is similar with the 13-month median time to recurrence for surgical resection in a previous study (48). However, different patient selection and the prior disease status should be considered and our findings suggest the usefulness of RFA as an alternative procedure in place of surgery. As most of our patients received pre-RFA and/or post-RFA chemotherapy and other adjuvant therapy, the results of our study were, perhaps, partially due to the effects of the previous therapies. Maybe a synergistic effect of chemotherapy and RF ablation played a role in our study.
There are several limitations of our study. First, this was a retrospective study and some heterogeneity existed for the patient characteristics and their tumors. Second, the study population was not sufficiently large to demonstrate the clinical value of RFA. Despite these limitations, our study results are important to provide the preliminary findings to evaluate the short-term efficacy of RFA for treating non-colorectal liver metastases. A randomized controlled prospective trial will be needed to prove the clinical role of RFA. Third, we did not always apply RFA for the treatment of recurred tumors after the initial RFA and we were not able to determine the potential maximum role of RFA to treat non-colorectal liver metastases.
In summary, RFA showed relatively less therapeutic effectiveness for the treatment of non-colorectal origin liver metastases as compared with colorectal liver metastases. However, the therapeutic results of RFA were not disappointing as compared with the therapeutic results of surgical resection for liver metastases from the same patient population with non-colorectal primary cancer. In addition, the low morbidity and mortality rates and the improved selectivity of candidates for RFA suggest the possible broad application of RFA treatment for liver metastases. A prospective randomized trial that will compare systemic treatment alone and systemic treatment with RFA for liver metastases is needed.
Footnotes
This study was supported by a grant from the National R & D program for Cancer Control, Ministry of Health & Welfare Republic of KOREA (No1120310).
References
- 1.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–799. doi: 10.1097/00000658-199906000-00005. discussion 799-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 3.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 5.Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684–692. doi: 10.1007/s00330-006-0461-5. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda S, Itamoto T, Nakahara H, Kohashi T, Ohdan H, Hino H, et al. Clinicopathologic features and prognostic factors of resected solitary small-sized hepatocellular carcinoma. Hepatogastroenterology. 2005;52:1163–1167. [PubMed] [Google Scholar]
- 7.Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 8.Iannitti DA, Dupuy DE, Mayo-Smith WW, Murphy B. Hepatic radiofrequency ablation. Arch Surg. 2002;137:422–426. doi: 10.1001/archsurg.137.4.422. discussion 427. [DOI] [PubMed] [Google Scholar]
- 9.Machi J, Oishi AJ, Sumida K, Sakamoto K, Furumoto NL, Oishi RH, et al. Long-term outcome of radiofrequency ablation for unresectable liver metastases from colorectal cancer: evaluation of prognostic factors and effectiveness in first- and second-line management. Cancer J. 2006;12:318–326. doi: 10.1097/00130404-200607000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Abitabile P, Hartl U, Lange J, Maurer CA. Radiofrequency ablation permits an effective treatment for colorectal liver metastasis. Eur J Surg Oncol. 2007;33:67–71. doi: 10.1016/j.ejso.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Sorensen SM, Mortensen FV, Nielsen DT. Radiofrequency ablation of colorectal liver metastases: long-term survival. Acta Radiol. 2007;48:253–258. doi: 10.1080/02841850601161539. [DOI] [PubMed] [Google Scholar]
- 12.Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19:1206–1213. doi: 10.1007/s00330-008-1258-5. [DOI] [PubMed] [Google Scholar]
- 13.Hur H, Ko YT, Min BS, Kim KS, Choi JS, Sohn SK, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg. 2009;197:728–736. doi: 10.1016/j.amjsurg.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. 2003;90:1240–1243. doi: 10.1002/bjs.4264. [DOI] [PubMed] [Google Scholar]
- 15.Veltri A, Sacchetto P, Tosetti I, Pagano E, Fava C, Gandini G. Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol. 2008;31:948–956. doi: 10.1007/s00270-008-9362-0. [DOI] [PubMed] [Google Scholar]
- 16.Ahn YO. Cancer in Korea: present features. Jpn J Clin Oncol. 2002;32(Suppl):S32–S36. doi: 10.1093/jjco/hye124. [DOI] [PubMed] [Google Scholar]
- 17.Shin HR. Global activity of cancer registries and cancer control and cancer incidence statistics in Korea. J Prev Med Public Health. 2008;41:84–91. [PubMed] [Google Scholar]
- 18.Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806–813. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 19.Kim HO, Hwang SI, Hong HP, Yoo CH. Radiofrequency ablation for metachronous hepatic metastases from gastric cancer. Surg Laparosc Endosc Percutan Tech. 2009;19:208–212. doi: 10.1097/SLE.0b013e3181a033d7. [DOI] [PubMed] [Google Scholar]
- 20.An JY, Kim JY, Choi MG, Noh JH, Choi D, Sohn TS, et al. Radiofrequency ablation for hepatic metastasis from gastric adenocarcinoma. Yonsei Med J. 2008;49:1046–1051. doi: 10.3349/ymj.2008.49.6.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carditello A, Scisca C, Stilo F, Parisi A, Basile M. The possible role of radiofrequency as complementary treatment of locally advanced gastric cancer. Ann Ital Chir. 2005;76:39–41. [PubMed] [Google Scholar]
- 22.Kim JH, Won HJ, Shin YM, Kim PN, Lee SG, Hwang S. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur J Radiol. 2010 doi: 10.1016/j.ejrad.2010.09.019. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Carrafiello G, Lagana D, Cotta E, Mangini M, Fontana F, Bandiera F, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33:835–839. doi: 10.1007/s00270-010-9849-3. [DOI] [PubMed] [Google Scholar]
- 24.Sofocleous CT, Nascimento RG, Gonen M, Theodoulou M, Covey AM, Brody LA, et al. Radiofrequency ablation in the management of liver metastases from breast cancer. AJR Am J Roentgenol. 2007;189:883–889. doi: 10.2214/AJR.07.2198. [DOI] [PubMed] [Google Scholar]
- 25.Livraghi T, Goldberg SN, Solbiati L, Meloni F, Ierace T, Gazelle GS. Percutaneous radio-frequency ablation of liver metastases from breast cancer: initial experience in 24 patients. Radiology. 2001;220:145–149. doi: 10.1148/radiology.220.1.r01jl01145. [DOI] [PubMed] [Google Scholar]
- 26.Lawes D, Chopada A, Gillams A, Lees W, Taylor I. Radiofrequency ablation (RFA) as a cytoreductive strategy for hepatic metastasis from breast cancer. Ann R Coll Surg Engl. 2006;88:639–642. doi: 10.1308/003588406X149129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunabushanam G, Sharma S, Thulkar S, Srivastava DN, Rath GK, Julka PK, et al. Radiofrequency ablation of liver metastases from breast cancer: results in 14 patients. J Vasc Interv Radiol. 2007;18:67–72. doi: 10.1016/j.jvir.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Rath GK, Julka PK, Thulkar S, Sharma DN, Bahl A, Bhatnagar S. Radiofrequency ablation of hepatic metastasis: results of treatment in forty patients. J Cancer Res Ther. 2008;4:14–17. doi: 10.4103/0973-1482.39599. [DOI] [PubMed] [Google Scholar]
- 29.Penka I, Kaplan Z, Sefr R, Sirotek L, Eber Z, Ondrak M. Use of radiofrequency ablation in the treatment of malignant liver lesions. Hepatogastroenterology. 2008;55:562–567. [PubMed] [Google Scholar]
- 30.Mazzaglia PJ, Berber E, Milas M, Siperstein AE. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery. 2007;142:10–19. doi: 10.1016/j.surg.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 31.Fan WJ, Wu PH, Zhang L, Huang JH, Zhang FJ, Gu YK, et al. Radiofrequency ablation as a treatment for hilar cholangiocarcinoma. World J Gastroenterol. 2008;14:4540–4545. doi: 10.3748/wjg.14.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gervais DA, Arellano RS, Mueller PR. Percutaneous radiofrequency ablation of ovarian cancer metastasis to the liver: indications, outcomes, and role in patient management. AJR Am J Roentgenol. 2006;187:746–750. doi: 10.2214/AJR.05.1106. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama T, Egami K, Miyamoto M, Watanabe H, Hasegawa H, Iida S, et al. Percutaneous and laparoscopic approaches of radiofrequency ablation treatment for liver cancer. J Hepatobiliary Pancreat Surg. 2003;10:425–427. doi: 10.1007/s00534-002-0830-7. [DOI] [PubMed] [Google Scholar]
- 34.Kim SW, Rhim H, Park M, Kim H, Kim YS, Choi D, et al. Percutaneous radiofrequency ablation of hepatocellular carcinomas adjacent to the gallbladder with internally cooled electrodes: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009;10:366–376. doi: 10.3348/kjr.2009.10.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang TW, Rhim H, Kim EY, Kim YS, Choi D, Lee WJ, et al. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009;10:34–42. doi: 10.3348/kjr.2009.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 37.Kwok CM, Wu CW, Lo SS, Shen KH, Hsieh MC, Lui WY. Survival of gastric cancer with concomitant liver metastases. Hepatogastroenterology. 2004;51:1527–1530. [PubMed] [Google Scholar]
- 38.Okano K, Maeba T, Ishimura K, Karasawa Y, Goda F, Wakabayashi H, et al. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg. 2002;235:86–91. doi: 10.1097/00000658-200201000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakamoto Y, Ohyama S, Yamamoto J, Yamada K, Seki M, Ohta K, et al. Surgical resection of liver metastases of gastric cancer: an analysis of a 17-year experience with 22 patients. Surgery. 2003;133:507–511. doi: 10.1067/msy.2003.147. [DOI] [PubMed] [Google Scholar]
- 40.Koga R, Yamamoto J, Ohyama S, Saiura A, Seki M, Seto Y, et al. Liver resection for metastatic gastric cancer: experience with 42 patients including eight long-term survivors. Jpn J Clin Oncol. 2007;37:836–842. doi: 10.1093/jjco/hym113. [DOI] [PubMed] [Google Scholar]
- 41.D'Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–816. doi: 10.1097/01.sla.0000143245.28656.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamakado K, Nakatsuka A, Takaki H, Mori Y, Tonouchi H, Kusunoki M, et al. Prospective study of arterial infusion chemotherapy followed by radiofrequency ablation for the treatment of liver metastasis of gastric cancer. J Vasc Interv Radiol. 2005;16:1747–1751. doi: 10.1097/01.RVI.0000188738.84911.3B. [DOI] [PubMed] [Google Scholar]
- 43.Hasegawa S, Ikai I, Fujii H, Hatano E, Shimahara Y. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg. 2007;31:1256–1263. doi: 10.1007/s00268-007-9001-y. [DOI] [PubMed] [Google Scholar]
- 44.Valverde A, Bonhomme N, Farges O, Sauvanet A, Flejou JF, Belghiti J. Resection of intrahepatic cholangiocarcinoma: a Western experience. J Hepatobiliary Pancreat Surg. 1999;6:122–127. doi: 10.1007/s005340050094. [DOI] [PubMed] [Google Scholar]
- 45.El Rassi ZE, Partensky C, Scoazec JY, Henry L, Lombard-Bohas C, Maddern G. Peripheral cholangiocarcinoma: presentation, diagnosis, pathology and management. Eur J Surg Oncol. 1999;25:375–380. doi: 10.1053/ejso.1999.0660. [DOI] [PubMed] [Google Scholar]
- 46.McMasters KM, Tuttle TM, Leach SD, Rich T, Cleary KR, Evans DB, et al. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am J Surg. 1997;174:605–608. doi: 10.1016/s0002-9610(97)00203-1. discussion 608-609. [DOI] [PubMed] [Google Scholar]
- 47.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 48.Vlastos G, Smith DL, Singletary SE, Mirza NQ, Tuttle TM, Popat RJ, et al. Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann Surg Oncol. 2004;11:869–874. doi: 10.1245/ASO.2004.01.007. [DOI] [PubMed] [Google Scholar]