Abstract
Background:
Secondhand smoke exposure (SHSe) poses health risks to children living with smokers. Most interventions to protect children from SHSe have coached adult smokers. This trial determined whether coaching and cotinine feedback provided to preteens can reduce their SHSe.
Methods:
Two hundred one predominantly low-income families with a resident smoker and a child aged 8 to 13 years who was exposed to two or more cigarettes per day or had a urine cotinine concentration ≥ 2.0 ng/mL were randomized to control or SHSe reduction coaching groups. During eight in-home sessions over 5 months, coaches presented to the child graphic charts of cotinine assay results as performance feedback and provided differential praise and incentives for cotinine reductions. Generalized estimating equations were used to determine the differential change in SHSe over time by group.
Results:
For the baseline to posttest period, the coaching group had a greater decrease in both urine cotinine concentration (P = .039) and reported child SHSe in the number of cigarettes exposed per day (child report, P = .003; parent report, P = .078). For posttest to month 12 follow-up, no group or group by time differences were obtained, and both groups returned toward baseline.
Conclusions:
Coaching preteens can reduce their SHSe, although reductions may not be sustained without ongoing counseling, feedback, and incentives. Unlike interventions that coach adults to reduce child SHSe, programs that increase child avoidance of SHSe have the potential to reduce SHSe in all settings in which the child is exposed, without requiring a change in adult smoking behavior.
The US Surgeon General concluded that secondhand smoke exposure (SHSe) harms the health of children, and there is no risk-free level of exposure.1,2 SHSe puts children at risk for diseases such as respiratory and middle ear infections, child asthma, and decreased lung function.3‐6 SHSe also leads to children’s behavioral problems7 and may contribute to early smoking initiation.8 National prevalence of youth SHSe varied from about 64% to about 50% between 1999 and 2008, with about 32 million youth exposed in 2007 to 2008.9 Among lower-income populations child exposure rates may be even higher.10 SHSe is a global epidemic.11
Tobacco smoke may pose a longer-term risk to children’s health via contamination of indoor surfaces and house dust (termed thirdhand smoke exposure12). Volatile toxic compounds from smoke deposit onto surfaces in the home or car and off-gas into the air for months.13,14 Nicotine in tobacco smoke residue combines with other agents through oxidative processes to produce recognized carcinogens.15 Reducing indoor cigarette smoking can reduce thirdhand smoke exposure.
Most interventions have focused on smoking cessation or changing smoking patterns of caregivers.16‐18 However, they were not consistent in reducing children’s SHSe. Of 36 trials to reduce child SHSe in the Cochrane Review,19 all targeted adults, but only 11 resulted in significant decreases in child exposure.
The behavior of exposed individuals is an understudied area of SHSe reduction.20 In previous trials for families with children with asthma,21‐23 we noted that some children who attended parent coaching sessions reported that they left the room when a smoker lit a cigarette, or they asked an adult not to smoke in the house. These observations raised the question of whether children could be coached to protect themselves from SHSe. Baseline data collected in the current project indicated that preteens who avoided smokers had lower SHSe.24 The purpose of the current trial, Project Sirocco, was to determine whether coaching, feedback and incentives provided to high-risk, ethnically diverse preteens residing in families with a smoker could reduce SHSe in their homes and cars.
Materials and Methods
Inclusion Criteria
Families were eligible for the baseline interview if they had a child from 8 to 13 years of age who lived in a home with a least one smoker, and they were eligible to continue in the trial if the child had reported exposure to an average of at least two cigarettes per day in the previous week or had a urine cotinine concentration of at least 2.0 ng/mL. Families were excluded if they planned to move out of the county within the next 12 months, if the child did not live in the home at least 4 days per week, or if the child reported tobacco use in the past 30 days or > 10 cigarettes smoked in their lifetime.
Recruitment/Cohort Retention
Screening and Baselines:
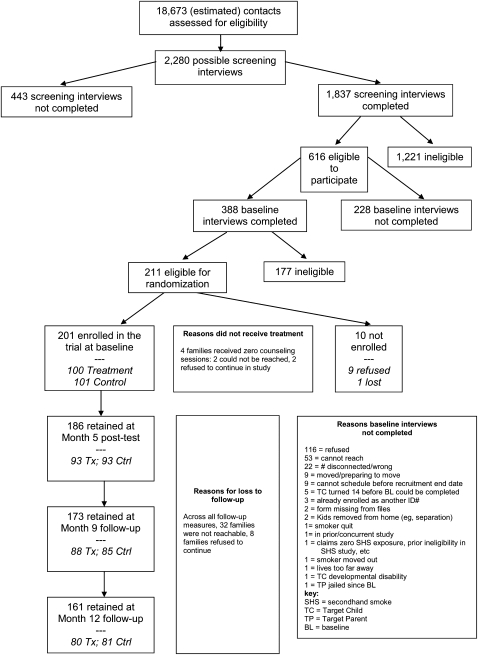
Families were recruited from multiple agencies and direct contact. Sources included the Supplemental Nutrition Program for Women, Infants, and Children, other social service providers, public libraries, YMCAs, local stores (eg, Walmart, Food 4 Less, “99-cent” stores), swap meets, internet, local publications, and community health fairs. Figure 1 details the recruitment process. A total of 18,673 contacts were made. Interested families who reported a child 8 to 13 years of age living in the home were contacted by telephone for screening. A total of 1,837 telephone screenings, spanning June 2004 through March 2007, identified 616 potentially eligible families. Of these, 388 families completed a baseline interview in their home, and 211 families were eligible for the trial. Nine families refused and one was lost, leaving 201 enrolled.
Figure 1.
Participant flow. BL = baseline; Ctrl = control; SHS = secondhand smoke; TC = target child; TP = target parent; Tx = treatment.
In each household, one target child (TC) and one target parent (TP) completed interviews. Most TPs (82.6%) were biologic mothers; 9.0% were biologic fathers. If more than one child qualified, the one with the most recent birthday was selected. Parents signed informed consent and children signed assent forms. Follow-up data collection was completed January 2008, with 80% of participants completing all measures.
Incentives:
Participants were compensated for completing interviews ($10 to $30 per measure for the TC and $20 to $50 per measure for the TP, the amount escalating with each measure to improve cohort maintenance). Additional incentives related to cohort maintenance were available to all families ($25 raffles for returning contact information postcards) and to families assigned to the intervention group (see “Intervention” section). Birthday cards with $5 gift cards were sent to the TP and TC.
Timeline and Group Assignment
Four interviews were conducted: baseline, posttest (month 5), and follow-ups at months 9 and 12. Following the baseline measures, families were assigned at random to coaching or control conditions. After the first 47 families had been enrolled, it was determined that some TCs had low urine cotinine levels (< 2.0 ng/mL) despite qualifying based on reported exposure. To balance conditions for cotinine, and protect power, randomization for subsequent families was stratified by baseline cotinine level (< 2.0 ng/mL vs ≥ 2.0 ng/mL). Forty-eight families were randomized within the low stratum and 106 families within the high stratum.
Randomization was designed and implemented by the measurement coordinator. A random assignment list for all participants was generated in advance of starting recruitment. Random number tables were used to select for coaching or control condition based on even or odd number, respectively. To ensure balanced groups, assignments were made in pairs. That is, the first assignment was based on the random number table, and the second assignment was the alternate group. Predictability was minimized because consecutively enrolled families were unlikely to be randomized consecutively. Enrolled families were assigned to one of several different interview staff, and varying lengths of time were required for families to complete baseline measures.
Procedures
Study procedures were approved by the San Diego State University Institutional Review Board, approval/protocol number 1456. Following completion of baseline measures, trained research assistants delivered 10 min of health education about exercise, diet, and safety, without reference to tobacco. The primary recipient was the TC, but all members of the household were invited.
Following receipt of cotinine results and cotinine-stratified randomization, research assistants (not interviewers) notified families if they qualified for the study; the first coaching session was scheduled for families assigned to intervention. Families assigned to the control group were unaware of specific coaching procedures and continued in the study with measurement visits and cohort retention phone calls only.
Intervention:
Individualized coaching (analogous to athletic coaching) and cotinine feedback for SHSe reduction were guided by previous research21‐23 and the Behavioral Ecological Model,25,26 with emphasis on reinforcing change in adolescents’ avoidance of SHSe. TCs in the coaching group were told that the goal was to help them reduce/eliminate their SHSe. Coaching was provided one-on-one to TCs during eight in-home 30- to 60-min sessions over 5 months, with a brief phone follow-up “booster” in between the in-home sessions. Topics addressed in each session are illustrated in Table 1.
Table 1.
—Project Sirocco Coaching Session Topics
| Session | Topics |
| 1 | SHSe and Health. What is secondhand smoke? Why should we care if other people are smoking around us? Do you know any health problems that can result from being around tobacco smoke? |
| 2 | Tobacco Products. Cigars, cigarillos, pipes, cigarettes, cloves, menthols, hookahs, chewing tobacco, and snuff. There are many kinds of tobacco products that release harmful smoke into the air. |
| 3 | Where’s the Smoke? Tips on communicating with smokers: let them know you care about them, let them talk and listen to what they say, be patient and know that you may not come to an agreement regarding changing smoke exposure the first time you talk with them. |
| 4 | Smoking as Problems: Beaches and Cars. Why do you think that people smoking at the beach could be a problem? Why do you think that people smoking in a car could be a problem? |
| 5 | Tobacco Games, Jokes, and Riddles |
| 6 | Smoking Outside California. Do you think that laws about smoking are the same or different in other states besides California? Do you think smoking habits are different in other countries? |
| 7 | Tobacco True or False Test (Quiz) |
| 8 | Generalization of Problem Solving Methods/Skills Learned During the Coaching Sessions. Review various ways to handle big problems that may initially seem out of a child’s control. |
| Phone sessions | Between each of the eight in-home sessions (above), which occurred every 2 weeks, coaches completed a phone session with the child. No new topics were introduced during the phone sessions. The focus of phone sessions was to check on progress on the child’s customized short-term SHSe reduction goal. |
SHSe = secondhand smoke exposure.
Coaching methods included contingency contracts that specified negotiated long-term goals for target behaviors to be attained, role-plays, and liberal reinforcement consisting of points (exchangeable for small prizes) for meeting behavioral objectives and gift cards for achieving cotinine reductions. In behavior-shaping procedures, coaches provided differential praise and points for increased or novel behaviors leading to SHSe avoidance. At each session, the coach and TC set short-term (2-week) goals for behavior change, which would lead to attainment of the long-term goals. These behavioral objectives could include the TC reducing exposure in one specific location or time (eg, excusing himself or herself from a specific exposure situation, asking a smoker to smoke outside while TC’s favorite television show is on). After achieving success with these goals, the location, time, and so forth, would be expanded in steps that were as large as realistically attainable. At any given session the TC may have had multiple behavioral goals. Reinforcement (praise and points) for meeting behavioral objectives was based on the TC’s report. If the TC reported engaging in novel methods of avoidance, these too were reinforced.
Additionally, cotinine assay results from previous weeks were presented to the TC in graphic form using a line chart with 10 levels ranging from “very dangerous” to “fantastic” on the y-axis. Reduction to a lower level, or maintenance at minimal exposure, was reinforced with praise and $5 or $10 gift cards relative to the cotinine reduction. Actual cotinine values were never presented to the TC; this allowed the coach latitude to shape reduction in level based on the TC’s baseline instead of absolute level.
Coaches were undergraduate and graduate students with majors in counseling, public health, social work, and psychology. Coaching sessions were audio recorded for quality control feedback to coaches in weekly case review meetings. Supervisors provided suggestions to tailor coaching to individuals and situations.
Measures
Biomarker:
Cotinine level is a reliable and valid tobacco-specific biomarker of SHSe among nonsmokers and served as the primary outcome variable.27 At each measurement visit, TCs collected urine samples using a sterile collection cup following instructions for a clean catch. Samples were shaken for 20 min and pipetted to vials prior to delivery to the San Diego State University Chemistry Laboratory; two vials were frozen for quality control. Assays followed Clinical Laboratory Improvement Act guidelines (Centers for Disease Control and Prevention), and results were confirmed by statistical and quality control evaluations. Assay validity was confirmed by an in-house proficiency-testing program using National Institute for Standards and Technology cotinine in urine reference material #8444 (National Institute for Standards and Technology; Gaithersburg, Maryland), and by blank urine samples spiked with known amounts of cotinine perchlorate. Samples were analyzed for cotinine using isotope-dilution liquid chromatography-tandem mass spectrometry with a limit of detection of approximately 20 parts per trillion (0.02 ng/mL) and limit of quantitation (LOQ) of 100 parts per trillion (0.10 ng/mL). The 2.7% of values falling below the LOQ were recoded to the midpoint between the LOQ and limit of detection (0.06 ng/mL).
In past studies, we found some cotinine values outside the range that might reasonably be attributed to SHSe. There were two such values—3,912.5 and 164.5 ng/mL, each confirmed by blind laboratory analysis of split-half urine samples—among the 4,392 samples for the 201 children in our longitudinal subset. We Windsorized these values, replacing them with the next highest value, 76.8 ng/mL. For approximately 14% of the urine samples, split-half reliability samples resulted in Pearson r = 0.996. For outcome analyses, all split-half values were averaged to produce the best estimate at each assessment.
Reports:
At each measurement visit the TP and TC completed separate interviews, averaging about one-half hour in length. Questions included demographics; family health care; tobacco use and SHSe levels; opinions and policies; and social and ecological determinants of smoking, SHSe, and other health risks. Measurements were recorded for quality control. Recordings were compared with hard copies and corrections made and feedback provided to staff. Most errors were corrected, resulting in > 99.9% correct across measures. Interviewers were blind to group assignment and investigators other than the measurement coordinator were blind to group-specific results until all data were collected. All participants were informed to not discuss any other involvement with project staff (eg, coaching sessions) during interviews.
The TC’s recent SHSe level was measured using a timeline follow-back (TLFB) method. The TLFB has been previously validated for a variety of behaviors.28‐31 The TP and TC were separately asked to recall the number of cigarettes to which the TC was exposed on each of the 7 days prior to the interview. To prompt accurate recall, exposure was assessed for specific periods (morning, afternoon, evening), in specific locations (home, car, other), and by a specific smoker (TP, other parent, other person). Studies suggest that reported measures of SHSe yield valid results.32, 33 This study used both parent and child reports of exposure in order to enable comparison with past studies that used only parent reports and to use the child reports as the more proximal “observer” of his/her own behavior/environment. Both measures were predictive of cotinine.34
Reported exposure, which has been shown to be reliable and positively associated with cotinine level, served as the secondary outcome measure. Two additional outcome variables were used in validation analyses. TCs reported whether they left the room in the most recent instance of SHSe, as an exploratory index of avoidance practices. TCs also reported whether there was a complete ban on smoking in the home. This too was exploratory because we reasoned that preteens would not have the authority to establish smoking bans in their homes.
Statistical Analyses
General Statistical Approaches:
For between-groups comparisons at baseline (ie, enrolled vs excluded individuals, adequacy of randomization), selected variables were tested using Pearson χ2 tests for categorical variables, and t tests or Mann-Whitney U tests for quantitative variables. Longitudinal analyses were based on intention to treat. We used log transformation of dependent variables to control for nonnormal distributions and heterogeneous error variances and report geometric means and medians.
Validation of Dependent Variables:
Convergent validity of cotinine and reported exposure was assessed by their intercorrelation and their correlation with variables (presence of home smoking ban and whether TC left the area the last time exposed) hypothesized to covary. Zero-order correlations were computed between variable pairs using data from all four interviews, baseline to month 12, controlling for within-subjects repeated measures.
Repeated Measures Analyses:
To examine differential change in exposure outcomes over time, we used generalized estimating equations (GEEs), with group, time (linear, quadratic, and cubic components), and all group by time interactions as the independent variables.35 Unlike analysis of variance, GEE retains cases having partially missing data and does not require repeated measures to be equally spaced in time. We investigated effects across repeated measures in two time periods: (1) intervention phase: baseline (n = 201) to the month 5 posttest (n = 186); and (2) maintenance phase: posttest to the month 12 follow-up (n = 161).
Time was coded as the number of days from baseline that each subsequent measure occurred, and was mean-centered to reduce collinearity among linear, quadratic, and cubic time terms. No cases were excluded from analysis, but data points for measures occurring more than 2 months early or late were dropped. Outcome variables were regressed on independent variables specifying a Gaussian distribution, an exchangeable correlation structure, and a robust estimate of variance. For each model, we removed independent variables lacking explanatory power one at a time, beginning with the highest-order terms, until a parsimonious model was reached. Analyses were performed using SPSS, version 15.0 (SPSS, Inc; Chicago, Illinois) and Stata, version 10.1 for Windows (Stata Corp; College Station, Texas).
Results
Enrolled TCs consisted of more girls (54.7%) than boys, averaged 10.4 (SD = 1.6) years of age, and were predominantly (> 60%) Hispanics and non-Hispanic blacks. About one-half of TPs (51.2%) were single parents, fewer than one-half (43.8%) were employed, and about one-quarter (25.4%) had not completed high school. Median categorical annual family income was $20,000 to $30,000.
Enrolled vs Excluded Subsets
Of 388 families completing baseline measures, individuals not enrolled in the remainder of the trial due to ineligibility, refusals, and so forth (n = 187) differed little from those enrolled (n = 201). Of 33 comparisons tested, the two subsets differed on four variables. The enrolled subset had more single parents, fewer Hispanics, more non-Hispanic whites, and greater parental prompting for smoking assistance (asking the TC to empty an ashtray or bring a cigarette). As expected, the enrolled subset was significantly higher on all four outcome variables, indicating successful implementation of the exposure criteria for enrollment in the study.
Coaching vs Control Groups
Using the same 33 variables and the four outcomes, we tested the enrolled subset for differences by experimental condition; none was significant (all P > .10) (Table 2). Randomization to experimental condition based on cotinine strata resulted in groups closely balanced on strata: 70.0% of those in the coaching group vs 71.3% of those in the control group were in the high stratum.
Table 2.
—Participant Characteristics and Baseline SHSe-Related Outcomes
| Characteristic | Control Group (n = 101) | Coaching Groupa (n = 100) |
| TC female | 56.4 | 53.0 |
| TC age, y | ||
| 8-9 | 32.6 | 30.0 |
| 10-11 | 41.6 | 39.0 |
| 12-13 | 25.8 | 31.0 |
| TC race/ethnicity | ||
| Hispanic | 36.6 | 39.0 |
| Non-Hispanic black | 29.7 | 24.0 |
| Non-Hispanic white | 18.8 | 24.0 |
| Otherb | 14.9 | 13.0 |
| TC has experimented with cigarettes | 9.9 | 6.0 |
| TC is susceptible to trying cigarettes | 24.0 | 30.3 |
| TC’s friend(s) use nicotine products | 14.9 | 17.0 |
| TC strongly believes tobacco smoke is harmful | 78.0 | 81.0 |
| TC says parents always know what TC is doing | 54.0 | 54.0 |
| Mean exposure to antismoking messages, 0-8 scale | 2.9 | 2.8 |
| Mean No. of friends who dislike smokers, 0-2 scale | 1.2 | 1.0 |
| Mean neighborhood smoking density, 0-2 scale | 1.1 | 1.1 |
| TP education, y | ||
| < 12 | 28.7 | 22.0 |
| 12 | 24.8 | 34.0 |
| > 12 | 46.5 | 44.0 |
| TP is a single parent | 56.4 | 46.0 |
| TP is the biologic mother of TC | 85.1 | 80.0 |
| TP is employed | 46.5 | 41.0 |
N = 201 subjects enrolled longitudinal sample. Data presented as % unless otherwise noted. TC = target child; TP = target parent.
Pearson χ2 tests for categorical variables and t tests or Mann-Whitney U tests for quantitative variables showed no statistically significant group differences (all P > 0.10).
Includes Native American, Asian, Pacific Islander, mixed, unspecified.
Intervention Delivery
Of youth in the coaching condition, four individuals completed zero sessions, and 75% completed all eight sessions. Only 41% of all coaching sessions were considered “timely” (completed within 14 days of the urine collection). Because phone sessions were boosters to maintain continuity while cotinine assays were still in process, data were not maintained on their completion rate.
Convergent Validity of Outcome Variables
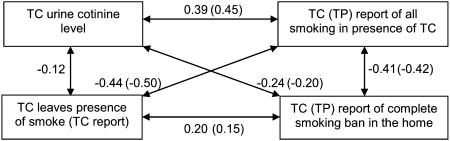
Figure 2 shows the correlations for key dependent variables expected to be related. Each pair was associated in the hypothesized direction and statistically significant.
Figure 2.
Validity correlations among biologic and reported measures related to secondhand smoke exposure (all P < .001). Correlations for TP reported exposure and bans are given in parentheses. See Figure 1 legend for expansion of abbreviations.
Intervention Phase Effects
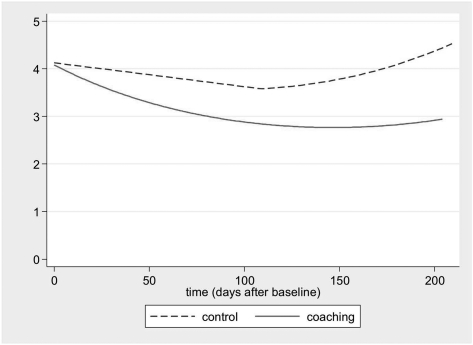
In the baseline to posttest period, the decrease in cotinine level in the coached group was more than twofold that in the control group (Table 3), yielding a two-tail significant group by linear time interaction effect (P = .039) and a quadratic main effect for time (P = .037) (Fig 3). For the TLFB report by TC of his or her SHSe, both groups declined substantially, with a greater decrease in the experimental group than in the control group, yielding a group by linear time effect (P = .003). For the TLFB report by TP of child SHSe, the interaction effect was similar (P = .078).
Table 3.
—SHSe-Related Outcomes, at Baseline and Posttest, by Experimental Condition
| Baseline |
Posttest |
|||||
| Group | Variable | Geometric Mean | 95% CI | Geometric Mean | 95% CI | Change,a % |
| Control | Urine cotinine (ng/mL) | 4.10 | (3.29, 5.08) | 3.68 | (2.85, 4.69) | −10.4 |
| Coaching | Urine cotinine (ng/mL) | 4.14 | (3.27, 5.18) | 3.13 | (2.48, 3.90) | −24.4 |
| Control | Child TLFB report, cigarettes/d | 2.21 | (1.93, 2.52) | 1.98 | (1.70, 2.32) | −10.2 |
| Coaching | Child TLFB report, cigarettes/d | 2.45 | (2.12, 2.84) | 1.73 | (1.55, 1.94) | −29.3 |
| Control | Parent TLFB report, cigarettes/d | 3.37 | (2.62, 4.28) | 1.67 | (1.24, 2.17) | −50.5 |
| Coaching | Parent TLFB report, cigarettes/d | 3.42 | (2.78, 4.17) | 1.39 | (1.02, 1.84) | −59.2 |
The geometric mean of raw values (and 95% CI) is given as an indicator of central tendency for these variables, which, due to skew, were log transformed for analyses. TLFB = timeline follow-back.
Percent change in the geometric mean, from baseline to posttest.
Figure 3.
Change in cotinine (ng/mL) from baseline to posttest by experimental condition. Plots are of fitted values modeled by the generalized estimating equations procedure.
Maintenance Phase Effects
For the posttest to month 12 follow-up period, there were no significant group, time, or group by time interaction effects for either urine cotinine or reported SHSe. This indicates a lack of maintenance of intervention effects.
Discussion
No previous studies have targeted preteens as the primary recipients of coaching to avoid SHSe. This trial was designed to test whether coaching and cotinine feedback provided to preteens—without direct coaching of smokers or caretakers—could reduce preteens’ SHSe in homes and cars. Our objective marker of total nicotine exposure (cotinine assay) showed significant and greater reduction among coached youth than the control group. This was substantiated by significant differential exposure reported by preteens and near-significant differential exposure reported by parents.
Although reactivity to measures may have been responsible for some decline in the dependent measures in both conditions, a number of features raise confidence in the reliability of the observed outcomes. The selection procedures obtained youth similar for those who qualified compared with those who did not, and random assignment resulted in balanced groups. Loss to follow-up was minimal and use of GEE modeling conserves information in the presence of missing data. Reported measures were remarkably free of data-collection error, and cotinine assays were extremely precise. Coaching procedures were closely monitored. Corrective feedback ensured higher-quality coaching than might have been possible otherwise.
The fidelity of the trial and the consistency of results across dependent variables and across previous trials directed to parents make us confident that these results are generalizable to similar populations. Despite likely generalizability and overarching fidelity,36,37 suboptimal cotinine feedback and less-than-ideal coaching procedures may have contributed to the observed return toward baseline level of SHSe for youth in the experimental condition after completion of the coaching phase.
Because of delays in receipt of laboratory results for cotinine assays, the majority of our cotinine feedback deliveries were not within our criterion of 14 days. Feedback latency may have limited our ability to reinforce the correct behavior, since the child’s behavior could have changed substantially during the intervening time since the last urine collection. Behavioral science has demonstrated that consequences delivered promptly after behavior occurs have maximum reinforcing impact.38 Investigations using more immediate feedback than now possible with urine cotinine assays or air nicotine dosimeters are warranted. Current pilot studies with in-home particle monitors (N. E. Klepeis, PhD; S. C. Hughes, PhD; R. D. Edwards, PhD, MPH; M. F. Hovell, PhD, MPH; T. Allen, PhD; M. Johnson, PhD, et al, unpublished data, July 11, 2011) promise a feasible solution to the problem of delayed reinforcement by providing real-time exposure feedback to the whole family.
We also observed substantial qualitative differences within and across coaching staff members. Although most demonstrated face valid coaching skills, we do not now have a basis for determining the specific procedures most responsible for changing youth behavior. Component analyses of coaching procedures are required to identify the features of coaching that are most responsible for changing a youth’s behavior. With identification of functional components, it should be possible to invent scoring systems to provide more precise feedback and ensure higher-quality coaching. It also should be possible to quantify the fidelity of coaching interventions in future trials.
Moreover, although immediate feedback, praise, and incentives from a coach may reinforce a child’s avoidance of SHSe, they may not be sufficient to sustain behavior change after they are discontinued. Theoretically, engineering ongoing social reinforcement for such avoidance from the child’s peers and family members could contribute to maintenance of SHSe reduction.39 Future studies should test preteen coaching procedures with and without concurrent advice to parents to support the preteen’s effort to avoid SHSe.
Significant intervention results encourage us to recommend follow-on trials to build on the findings of our study and address its limitations. Clinical providers, especially those delivering medical, dental, and optometric care, might offer feedback and brief versions of coaching to adult smokers, preteens, or both to determine the most efficient version of an intervention to protect children from SHSe that might be efficacious in clinical care. Our previous study of similar brief coaching to prevent smoking initiation resulted in significant protection of preteens40 and our ongoing orthodontia trial is testing avoidance of SHSe and promotion of healthy diet and activity (M. F. Hovell, PhD, MPH, unpublished data, July 11, 2011).
We believe the results of this study set the stage for the research needed to develop practical pediatric and pulmonary clinical interventions that may be efficacious when delivered within an existing clinical system. Recent evidence suggests that brief interventions with children with asthma could reduce asthma morbidity.41 The ultimate establishment of such programs in diverse clinical services offers the possibility that most patients would obtain clinical advice repeatedly in most years, so that the cumulative effect might be more powerful than when tested in just one specialty (eg, pediatrics). This might contribute substantially to a larger culture change that prohibits SHSe in private homes.42
Acknowledgments
Author contributions: Dr Hovell bears responsibility for the design, conduct, and any adverse effects of the study and for all features of the manuscript. Dr Wahlgren had full access to the data at all times and was responsible for the integrity of data analyses.
Dr Hovell: contributed to supervising all aspects of the study, analyses, and manuscript preparation; designing and conducting the study; and providing extensive edits and feedback throughout the writing process.
Mr Wahlgren: contributed to designing and conducting the study, analyzing data, and providing extensive edits and feedback throughout the writing process.
Mr Liles: contributed to conducting poststudy data management and analyses and providing extensive edits and feedback throughout the writing process.
Ms Jones: contributed to designing and conducting the study and providing extensive edits and feedback throughout the writing process.
Dr Hughes: contributed to designing and conducting the study and providing extensive edits and feedback throughout the writing process.
Dr Matt: contributed to formulating the data analysis plan, supervising analyses, and providing extensive edits and feedback throughout the writing process.
Dr Ji: contributed to running preliminary data analyses and providing extensive edits and feedback throughout the writing process.
Dr Lessov-Schlaggar: contributed to providing extensive edits and feedback throughout the writing process.
Dr Swan: contributed to providing extensive edits and feedback throughout the writing process.
Dr Chatfield: contributed to performing cotinine assays and providing extensive edits and feedback throughout the writing process.
Ms Ding: contributed to providing extensive edits and feedback throughout the writing process.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We thank the members of the Data and Safety Monitoring Board, David Rocke, PhD (chair), Marion Forgatch, PhD, and Jordan Smoller, MD, ScD, as well as the numerous research assistants who made the success of this trial possible. This research was performed at the Center for Behavioral Epidemiology and Community Health, San Diego, CA.
Abbreviations
- GEE
generalized estimating equation
- LOQ
limit of quantitation
- SHSe
secondhand smoke exposure
- TC
target child
- TLFB
timeline follow-back
- TP
target parent
Footnotes
Funding/Support: This research was supported by the National Institutes of Health [Grants HL066307 (M. F. H.), DA018019 (G. E. S.), DA027046 (C. N. L. S.)] and by discretionary funds from the Center for Behavioral Epidemiology and Community Health (M. F. H., Director).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.United States Department of Health and Human Services . The Health Consequence of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2006. [PubMed] [Google Scholar]
- 2.United States Department of Health and Human Services . Children and Secondhand Smoke Exposure–Excerpts From the Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2007. [Google Scholar]
- 3.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113(suppl 4):1007–1015. [PubMed] [Google Scholar]
- 4.Li JS, Peat JK, Xuan W, Berry G. Meta-analysis on the association between environmental tobacco smoke (ETS) exposure and the prevalence of lower respiratory tract infection in early childhood. Pediatr Pulmonol. 1999;27(1):5–13. doi: 10.1002/(sici)1099-0496(199901)27:1<5::aid-ppul3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Lieu JE, Feinstein AR. Effect of gestational and passive smoke exposure on ear infections in children. Arch Pediatr Adolesc Med. 2002;156(2):147–154. doi: 10.1001/archpedi.156.2.147. [DOI] [PubMed] [Google Scholar]
- 6.Larsson ML, Frisk M, Hallström J, Kiviloog J, Lundbäck B. Environmental tobacco smoke exposure during childhood is associated with increased prevalence of asthma in adults. Chest. 2001;120(3):711–717. doi: 10.1378/chest.120.3.711. [DOI] [PubMed] [Google Scholar]
- 7.Williams GM, O’Callaghan M, Najman JM, et al. Maternal cigarette smoking and child psychiatric morbidity: a longitudinal study. Pediatrics. 1998;102(1):e11. doi: 10.1542/peds.102.1.e11. [DOI] [PubMed] [Google Scholar]
- 8.Becklake MR, Ghezzo H, Ernst P. Childhood predictors of smoking in adolescence: a follow-up study of Montreal schoolchildren. CMAJ. 2005;173(4):377–379. doi: 10.1503/cmaj.1041428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Web site Morbidity and Mortality Weekly Report (MMWR). September 7 2010. Vital signs: nonsmokers’ exposure to secondhand smoke–United States, 1999-2008. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm59e0907a2.htm. Accessed June 23, 2011. [PubMed]
- 10.Cornelius MD, Goldschmidt L, Dempsey DA. Environmental tobacco smoke exposure in low-income 6-year-olds: parent report and urine cotinine measures. Nicotine Tob Res. 2003;5(3):333–339. doi: 10.1080/1462220031000094141. [DOI] [PubMed] [Google Scholar]
- 11.Öberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377(9760):139–146. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- 12.Matt GE, Romero R, Ma DS, et al. Tobacco use and asking prices of used cars: prevalence, costs, and new opportunities for changing smoking behavior. Tob Induc Dis. 2008;4:2. doi: 10.1186/1617-9625-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matt GE, Quintana PJ, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control. 2004;13(1):29–37. doi: 10.1136/tc.2003.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer BC, Hodgson AT, Guevarra KS, Hawley EL, Nazaroff WW. Gas-phase organics in environmental tobacco smoke. 1. Effects of smoking rate, ventilation, and furnishing level on emission factors. Environ Sci Technol. 2002;36(5):846–853. doi: 10.1021/es011058w. [DOI] [PubMed] [Google Scholar]
- 15.Sleiman M, Gundel LA, Pankow JF, Jacob P, III, Singer BC, Destaillats H. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc Natl Acad Sci U S A. 2010;107(15):6576–6581. doi: 10.1073/pnas.0912820107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics. 2001;108(1):18–24. doi: 10.1542/peds.108.1.18. [DOI] [PubMed] [Google Scholar]
- 17.Gehrman CA, Hovell MF. Protecting children from environmental tobacco smoke (ETS) exposure: a critical review. Nicotine Tob Res. 2003;5(3):289–301. doi: 10.1080/1462220031000094231. [DOI] [PubMed] [Google Scholar]
- 18.Klerman LV. Protecting children: reducing their environmental tobacco smoke exposure. Nicotine Tob Res. 2004;6(suppl 2):S239–S253. doi: 10.1080/14622200410001669213. [DOI] [PubMed] [Google Scholar]
- 19.Roseby R, Waters E, Polnay A, Campbell R, Webster P, Spencer N. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane Database Syst Rev. 2003;(3):CD001746. doi: 10.1002/14651858.CD001746. [DOI] [PubMed] [Google Scholar]
- 20.Tyc VL, Hovell MF, Winickoff J. Reducing secondhand smoke exposure among children and adolescents: emerging issues for intervening with medically at-risk youth. J Pediatr Psychol. 2008;33(2):145–155. doi: 10.1093/jpepsy/jsm135. [DOI] [PubMed] [Google Scholar]
- 21.Hovell MF, Meltzer SB, Zakarian JM, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: a controlled trial. Chest. 1994;106(2):440–446. doi: 10.1378/chest.106.2.440. [published correction appears in Chest. 1995;107(5):1480] [DOI] [PubMed] [Google Scholar]
- 22.Wahlgren DR, Hovell MF, Meltzer SB, Hofstetter CR, Zakarian JM. Reduction of environmental tobacco smoke exposure in asthmatic children. A 2-year follow-up. Chest. 1997;111(1):81–88. doi: 10.1378/chest.111.1.81. [DOI] [PubMed] [Google Scholar]
- 23.Hovell MF, Meltzer SB, Wahlgren DR, et al. Asthma management and environmental tobacco smoke exposure reduction in Latino children: a controlled trial. Pediatrics. 2002;110(5):946–956. doi: 10.1542/peds.110.5.946. [DOI] [PubMed] [Google Scholar]
- 24.Ding D, Wahlgren DR, Liles S, Jones JA, Hughes SC, Hovell MF. Secondhand smoke avoidance by preteens living with smokers: to leave or stay? Addict Behav. 2010;35(11):989–994. doi: 10.1016/j.addbeh.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hovell MF, Wahlgren DR, Gehrman CA. The behavioral ecological model: integrating public health and behavioral science. In: DiClemente RJ, Crosby RA, Kegler M, editors. New and Emerging Models and Theories in Health Promotion and Health Education. San Francisco, CA: Jossey-Bass Inc; 2002. pp. 347–385. [Google Scholar]
- 26.Hovell MF, Wahlgren DR, Adams MA. The logical and empirical basis for the behavioral ecological model. In: DiClemente RJ, Crosby RA, Kegler M, editors. Emerging Theories and Models in Health Promotion Research and Practice. Strategies for Enhancing Public Health. 2nd ed. San Francisco: Jossey-Bass Publishers; 2009. pp. 415–449. [Google Scholar]
- 27.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 28.Carey KB. Reliability and validity of time-line follow-back interview among psychiatric outpatients: A preliminary report. Psychol Addict Behav. 1997;11(1):26–33. [Google Scholar]
- 29.Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68(1):134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- 30.Sacks JA, Drake RE, Williams VF, Banks SM, Herrell JM. Utility of the time-line follow-back to assess substance use among homeless adults. J Nerv Ment Dis. 2003;191(3):145–153. doi: 10.1097/01.NMD.0000054930.03048.64. [DOI] [PubMed] [Google Scholar]
- 31.Lam W, Fals-Stewart W, Kelley ML. The timeline followback interview to assess children’s exposure to partner violence: reliability and validity. J Fam Violence. 2009;24(2):133–143. [Google Scholar]
- 32.Matt GE, Bernert JT, Hovell MF. Measuring secondhand smoke exposure in children: an ecological measurement approach. J Pediatr Psychol. 2008;33(2):156–175. doi: 10.1093/jpepsy/jsm123. [DOI] [PubMed] [Google Scholar]
- 33.Matt GE, Hovell MF, Zakarian JM, Bernert JT, Pirkle JL, Hammond SK. Measuring secondhand smoke exposure in babies: the reliability and validity of mother reports in a sample of low-income families. Health Psychol. 2000;19(3):232–241. doi: 10.1037//0278-6133.19.3.232. [DOI] [PubMed] [Google Scholar]
- 34.Johnson-Kozlow M, Wahlgren DR, Hovell MF, et al. Adolescents validly report their exposure to secondhand smoke. J Clin Epidemiol. 2010;63(8):914–919. doi: 10.1016/j.jclinepi.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford, England: Clarendon Press; 1995. [Google Scholar]
- 36.Johnson-Kozlow M, Hovell MF, Rovniak LS, Sirikulvadhana L, Wahlgren DR, Zakarian JM. Fidelity issues in secondhand smoking interventions for children. Nicotine Tob Res. 2008;10(12):1677–1690. doi: 10.1080/14622200802443429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rovniak LS, Hovell MF, Wojcik JR, Winett RA, Martinez-Donate AP. Enhancing theoretical fidelity: an e-mail-based walking program demonstration. Am J Health Promot. 2005;20(2):85–95. doi: 10.4278/0890-1171-20.2.85. [DOI] [PubMed] [Google Scholar]
- 38.Catania AC. Learning. 4th ed. Upper Saddle River, NJ: Prentice Hall; 1998. [Google Scholar]
- 39.Stokes TF, Osnes PG. An operant pursuit of generalization. Behav Ther. 1989;20(3):337–355. doi: 10.1016/j.beth.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Wahlgren DR, Hovell MF, Slymen DJ, Conway TL, Hofstetter CR, Jones JA. Predictors of tobacco use initiation in adolescents: a two-year prospective study and theoretical discussion. Tob Control. 1997;6(2):95–103. doi: 10.1136/tc.6.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerald LB, Gerald JK, Gibson L, Patel K, Zhang S, McClure LA. Changes in environmental tobacco smoke exposure and asthma morbidity among urban school children. Chest. 2009;135(4):911–916. doi: 10.1378/chest.08-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hovell MF, Hughes SC. The behavioral ecology of secondhand smoke exposure: A pathway to complete tobacco control. Nicotine Tob Res. 2009;11(11):1254–1264. doi: 10.1093/ntr/ntp133. [DOI] [PMC free article] [PubMed] [Google Scholar]