Abstract
Background:
Chronic bronchitis (CB) in patients with COPD is associated with an accelerated lung function decline and an increased risk of respiratory infections. Despite its clinical significance, the chronic bronchitic phenotype in COPD remains poorly defined.
Methods:
We analyzed data from subjects enrolled in the Genetic Epidemiology of COPD (COPDGene) Study. A total of 1,061 subjects with GOLD (Global Initiative for Chronic Obstructive Lung Disease) stage II to IV were divided into two groups: CB (CB+) if subjects noted chronic cough and phlegm production for ≥ 3 mo/y for 2 consecutive years, and no CB (CB−) if they did not.
Results:
There were 290 and 771 subjects in the CB+ and CB− groups, respectively. Despite similar lung function, the CB+ group was younger (62.8 ± 8.4 vs 64.6 ± 8.4 years, P = .002), smoked more (57 ± 30 vs 52 ± 25 pack-years, P = .006), and had more current smokers (48% vs 27%, P < .0001). A greater percentage of the CB+ group reported nasal and ocular symptoms, wheezing, and nocturnal awakenings secondary to cough and dyspnea. History of exacerbations was higher in the CB+ group (1.21 ± 1.62 vs 0.63 ± 1.12 per patient, P < .027), and more patients in the CB+ group reported a history of severe exacerbations (26.6% vs 20.0%, P = .024). There was no difference in percent emphysema or percent gas trapping, but the CB+ group had a higher mean percent segmental airway wall area (63.2% ± 2.9% vs 62.6% ± 3.1%, P = .013).
Conclusions:
CB in patients with COPD is associated with worse respiratory symptoms and higher risk of exacerbations. This group may need more directed therapy targeting chronic mucus production and smoking cessation not only to improve symptoms but also to reduce risk, improve quality of life, and improve outcomes.
Trial registry:
ClinicalTrials.gov; No.: NCT00608764; URL: www.clinicaltrials.gov
Chronic cough and sputum production are common, but symptoms vary in patients with COPD.1 They result from goblet cell hyperplasia in both large and small airways,2‐4 and the subsequent mucus hypersecretion worsens airflow obstruction and predisposes to bacterial colonization.5 Mucus overproduction may develop as a consequence of cigarette smoke exposure,6,7 acute and chronic viral infection,8 or inflammatory cell activation of mucin gene transcription9 and is compounded by difficulty in clearing secretions because of poor ciliary function, distal airway occlusion, and ineffective cough.2,9,10
Chronic mucus hypersecretion has been shown in some large epidemiologic studies to be associated with an accelerated lung function decline, increased risk for respiratory infection,11,12 and higher mortality.13‐15 Several studies have demonstrated an increased risk of COPD exacerbation.16‐19 Goblet cell hyperplasia also has prognostic value as it has been associated with an increased risk of mortality and a lack of improvement in lung function after lung reduction surgery.20,21 Despite these clinical and pathologic correlates of chronic bronchitis (CB) to various clinical outcomes, the current literature is limited regarding the clinical and radiographic characteristics of CB in patients with COPD.
We analyzed 1,061 patients with moderate to severe COPD in the first 2,500 subjects enrolled in the Genetic Epidemiology of COPD (COPDGene) Study. We specifically sought to carefully characterize those with CB symptoms and compare them to those without these symptoms. We hypothesized that chronic cough and sputum production in patients with COPD are associated with a greater exacerbation frequency, heightened respiratory symptoms, and worse health-related quality of life compared with those without CB symptoms.
Materials and Methods
Patient Selection
The COPDGene Study is a multicenter observational study to analyze genetic susceptibility for the development of COPD. This study met all criteria for institutional review board approval (Temple IRB #11369). Inclusion and exclusion criteria and protocol have been described previously.22 Briefly, enrollees are blacks or non-Hispanic whites aged 45 to 80 years with at least a 10-pack-year smoking history. Exclusion criteria include pregnancy, history of other lung disease except asthma, prior lobectomy or lung volume reduction, active cancer undergoing treatment, or known or suspected lung cancer.
Subjects were asked whether they had cough, and if they responded yes, they were asked whether they coughed on most days for ≥ 3 consecutive mo/y and for how many years. Similar questions were asked regarding phlegm production. Subjects were placed in the CB+ group if they had chronic cough and phlegm production for ≥ 3 mo/y for at least 2 consecutive years or in the CB− (no CB) group if these criteria were not satisfied.
Clinical Characterization
Dyspnea and health-related quality of life were assessed using the Modified Medical Research Council (MMRC) scale and St. George Respiratory Questionnaire (SGRQ). Upper- and lower-respiratory tract symptoms were collected using a modified form of the American Thoracic Society Diffuse Lung Disease Respiratory Epidemiology questionnaire.23 Medical comorbidities were assessed based on subject self-report. Subjects were asked whether they experienced COPD exacerbations in the past year and to quantify the number of episodes. They also were asked whether they had been to the ED or hospitalized for an exacerbation in the past year. These answers were used to determine exacerbation history and history of severe exacerbations, respectively.
Each subject underwent prebronchodilater and postbronchodilator spirometry using an EasyOne spirometer (Welch-Allyn Switzerland GmbH; Vaud, Switzerland). Predicted values were obtained using National Health and Nutrition Examination Survey III data.24 Six-min walk distance was measured in the standard fashion.25
CT Imaging
Volumetric CT scan acquisitions were obtained at full inspiration (200 mA) and at the end of normal expiration (50 mA). Thin-slice collimation with slice thickness and intervals of < 1 mm was used to enhance spatial resolution. Quantitative image analysis to calculate lung volumes, percent emphysema, and percent gas trapping was performed using VIDA (VIDA Diagnostics, Inc; Coralville, Iowa) and 3DSlicer (available at http://www.slicer.org) software.26 Percent emphysema was defined as the total percentage of both lungs with attenuation values < −950 Hounsfield units on inspiratory images, and percent gas trapping was defined as the total percentage of both lungs with attenuation values < −856 Hounsfield units on expiratory images. These percentages were adjusted for type of CT scanner. Total lung capacity and functional residual capacity were calculated based on inspiratory and expiratory CT images, respectively. Airway disease was quantified in a subset of each group as wall area percent (WA%) [(wall area/total bronchial area) × 100)] and airway wall thickness (AWT).27 The mean WA% and AWT were calculated as the average of the values for six segmental bronchi in each subject. Using 3DSlicer, we also expressed AWT as the square root of the wall area of a theoretical 10-mm diameter airway as previously described.28
Statistical Analysis
Analysis was performed using JMP, version 8.0.1 (SAS Inc; Cary, North Carolina). Values are expressed as mean ± SD, unless stated otherwise. Categorical variables (eg, sex, race, presence of ocular or nasal symptoms) were compared between groups using χ2 test. Continuous variables (eg, age, smoking history, exacerbation history) were evaluated using one-way analysis of variance or two-tailed unpaired t test. Wilcoxon rank sum test was used for nonnormally distributed data. P < .05 was considered statistically significant. Multiple logistic regressions were performed to assess the independent effects of CB, sex, age, current smoking, and total pack-year history of smoking on symptoms.
Results
Participant demographics and medications are summarized in Table 1. Of the 1,061 subjects with COPD analyzed, CB was reported in 27.3% (CB+ group, n = 290; CB− group, n = 771). The percentage of subjects with CB in each GOLD (Global Initiative for Chronic Obstructive Lung Disease) stage was similar (GOLD stage II, 27.3%; GOLD stage III, 28.7%; GOLD stage IV, 25.0%; P = .650). In the entire cohort, the CB+ group was younger (62.8 ± 8.4 vs 64.6 ± 8.4 years, P = .002) and had a greater percentage of whites (86% vs 80%, P = .034) and men (57% vs 50%, P = .027). The CB+ group had a greater pack-year smoking history (57 ± 30 pack-years vs 52 ± 25 pack-years, P = .006) and had more current smokers (48% vs 27%, P < .0001). There was no difference in lung function, 6-min walk distance, height, or BMI between the two groups. The OR for CB from current smoking was 2.56 (95% CI, 1.93-3.39). Comparing current smokers with ex-smokers within the CB+ group, current smokers were younger (59.6 ± 7.6 years vs 65.8 ± 8.1 years, P < .0001), were thinner (BMI, 27.0 ± 5.8 kg/m2 vs 28.9 ± 6.7 kg/m2, P = .0127), and had better lung function (FEV1, 54.3% ± 15.6% vs 43.3% ± 17.8%, P < .0001). The CB+ group reported greater use of short-acting bronchodilators (92.7% vs 82.1%, P = .003) and lesser use of combination inhaled steroid/long-acting β-agonists (50.0% vs 57.6%, P = .047). There were no differences in other respiratory medication usage between groups.
Table 1.
—Patient Characteristics and Medication Usage
| Variable | CB+ (n = 290) | CB− (n = 771) | P Value |
| Demographic | |||
| Age, y | 62.8 ± 8.4 | 64.6 ± 8.4 | .002a |
| Smoking history, pack-y | 57 ± 30 | 52 ± 25 | .006a |
| Current smoker, % | 48 | 27 | < .0001a |
| Sex, % | .027a | ||
| Male | 57 | 50 | |
| Female | 43 | 50 | |
| Race, % | .034a | ||
| White | 86 | 80 | |
| Black | 14 | 20 | |
| FEV1, % predicted | 48.5 ± 17.6 | 48.7 ± 18.4 | .904 |
| FVC, % predicted | 77.7 ± 18.2 | 75.6 ± 17.7 | .106 |
| FEV1/FVC | 0.47 ± 0.12 | 0.48 ± 0.13 | .088 |
| 6MWD, m | 342 ± 8 | 348 ± 5 | .540 |
| Height, cm | 170 ± 10 | 169 ± 10 | .055 |
| BMI, kg/m2 | 28.0 ± 6.3 | 28.0 ± 6.2 | .770 |
| BODE scoreb | 3 (2-5) | 3 (1-5) | .033a |
| Medications, % pts use | |||
| SABD | 92.7 | 82.1 | .003a |
| LABA | 12.2 | 10.8 | .626 |
| LAMA | 52.5 | 57.4 | .218 |
| ICS | 18.6 | 13.8 | .086 |
| OCS | 7.7 | 7.8 | .888 |
| Combo ICS/LABA | 50.0 | 57.6 | .047a |
| Theophylline | 10.1 | 6.9 | .150 |
| Oxygen | 27.0 | 33.4 | .054 |
| Comorbidities, % pts | |||
| Angina | 9.0 | 4.5 | .016a |
| Asthma | 30.0 | 25.1 | .229 |
| Congestive heart failure | 5.2 | 5.2 | .990 |
| Coronary artery disease | 8.3 | 8.0 | .900 |
| Diabetes | 16.2 | 10.3 | .010a |
| Hypertension | 49.3 | 47.0 | .535 |
| Hypercholesterolemia | 44.5 | 39.3 | .141 |
| Stroke | 2.4 | 3.9 | .227 |
| Gastroesophageal reflux | 34.6 | 27.6 | .029a |
| Compression fractures | 9.7 | 5.6 | .027a |
| Hip fracture | 2.8 | 2.7 | .979 |
| Osteoarthritis | 25.9 | 18.3 | .008a |
| Osteoporosis | 14.8 | 14.9 | 1.000 |
| Sleep apnea | 22.4 | 14.4 | .002a |
Data are presented as mean ± SD or as a percentage of the group. 6MWD = 6-min walk distance; BODE = BMI, airway obstruction, dyspnea, exercise capacity; CB = chronic bronchitis; ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist; OCS = oral corticosteroid; pt = patient; SABD = short-acting bronchodilator.
P < .05.
Expressed as median (interquartile range).
Comorbidities also are listed in Table 1. Compared with the CB− group, a greater number of subjects in the CB+ group reported a history of angina (9.0% vs 4.5%, P = .011), diabetes (16.2% vs 10.3%, P = .010), gastroesophageal reflux (34.6% vs 27.6%, P = .029), compression fractures (9.7% vs 5.6%, P = .027), osteoarthritis (25.9% vs 18.3%, P = .008), and sleep apnea (22.4% vs 14.4%, P = .002). There were no differences in asthma, congestive heart failure, coronary artery disease, myocardial infarction, hypertension, hypercholesterolemia, stroke, and osteoporosis. When the incidence of angina was controlled for other cardiac risk factors (hypertension, pack-year smoking history, hypercholesterolemia, and diabetes), the presence of CB was still associated with an increased incidence of angina (OR, 1.92; P = .02).
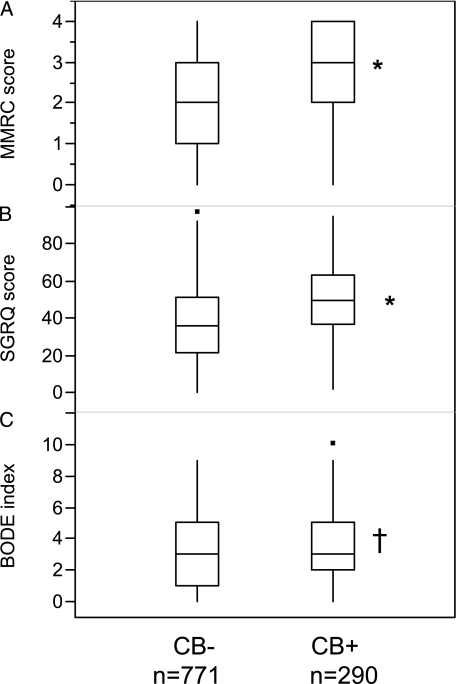
Table 2 summarizes symptoms, quality of life, and exacerbation history. The subjects in the CB+ group were more symptomatic than those in the CB− group. MMRC, SGRQ, and BODE (BMI, airflow obstruction, dyspnea, exercise capacity) index scores were greater in the CB+ group (MMRC, 2.55 ± 1.31 vs 2.11 ± 1.41, P < .0001; SGRQ, 49.9 ± 19.7 vs 36.6 ± 20.0, P < .0001; BODE index, 3.35 ± 2.04 vs 3.05 ± 2.11, P = .033) (Fig 1). Upper-respiratory tract symptoms were greater in the CB+ group (eg, nasal congestion, rhinorrhea) (69.3% vs 53.4%, P < .0001) as were allergic ocular symptoms (eg, itchy, watery eyes) (52.8% vs 40.2%, P < .0001). There was also a greater history of wheezing (86.5% vs 67.6%, P < .0001), nocturnal awakenings secondary to cough (45.9% vs 19.1%, P < .0001), and nocturnal awakenings secondary to dyspnea or chest tightness (39.3% vs 24.4%, P < .0001) in the CB+ group.
Table 2.
—Symptoms, Quality of Life, Exacerbation History, and Radiology
| Variable | CB+ | CB− | P Value |
| Symptoms and quality of life | |||
| MMRC dyspnea scorea | 3 (2-4) | 2 (1-3) | < .0001b |
| SGRQ, total | 49.9 ± 19.7 | 36.6 ± 20.0 | < .0001b |
| SGRQ, respiratory | 62.5 ± 19.0 | 38.0 ± 22.4 | < .0001b |
| Nasal symptoms, % | 69.3 | 53.4 | < .0001b |
| Ocular symptoms, % | 52.8 | 40.2 | < .0001b |
| Wheezing, % | 86.5 | 67.6 | < .0001b |
| Awakened by cough, % | 45.9 | 19.1 | < .0001b |
| Awakened by dyspnea, % | 39.3 | 24.4 | < .0001b |
| Exacerbations in the previous year | |||
| Total exacerbations, No./pt | 1.21 ± 1.62 | 0.63 ± 1.12 | .027b |
| History of severe exacerbations, % | 26.6 | 20.0 | .024b |
| Radiology | |||
| % Emphysemac | 14.2 ± 13.0 | 16.0 ± 13.4 | .212 |
| % Gas trapping | 42.0 ± 20.0 | 42.8 ± 20.3 | .593 |
| Total lung capacity,d L | 6.30 ± 1.50 | 5.88 ± 1.40 | .0004b |
| Functional residual capacity,d L | 4.22 ± 1.19 | 3.92 ± 1.28 | .002b |
| Mean segmental WA%e | 63.2 ± 2.9 | 62.6 ± 3.1 | .013b |
| Mean segmental AWT,e mm | 1.60 ± 0.21 | 1.60 ± 0.22 | .700 |
| Pi10,d mm | 3.800 ± 0.129 | 3.798 ± 0.126 | .816 |
Data are presented as mean ± SD or as a percentage of the group. AWT = airway wall thickness; MMRC = Modified Medical Research Council; Pi10 = 10-mm diameter airway; SGRQ = St. George Respiratory Questionnaire; WA% = wall area percent. See Table 1 legend for expansion of other abbreviations.
Expressed as median (interquartile range).
P < .05.
Percent emphysema was adjusted for type of CT scanner at different institutions.
Total lung capacity and functional residual capacity were adjusted for height, age, sex, and race in multivariate analysis.
Airway data performed in a subset of patients (CB+, n = 242; CB− n = 620).
Figure 1.
Dyspnea, quality of life, and BODE index in each group. A, MMRC scores. B, SGRQ scores. C, BODE index scores. MMRC scores, SGRQ scores, and BODE index scores in the CB+ group were significantly higher than in the CB− group. Data are presented as median (interquartile range). *P < .0001. †P = .033. BODE = BMI, airway obstruction, dyspnea, exercise capacity; CB = chronic bronchitis; MMRC = Modified Medical Research Council; SGRQ = St. George Respiratory Questionnaire.
In the entire cohort, current smoking was not associated with respiratory symptoms or SGRQ scores in multivariate analysis. Subjects who reported the use of supplemental oxygen had higher MMRC scores (2.94 ± 1.02 vs 1.90 ± 1.43, P < .0001) and more nasal symptoms (62% vs 56%, P = .044) than those who did not use oxygen but not other symptoms. When the presence of CB was factored into multivariate analysis, oxygen use did not have a statistically significant impact on any respiratory symptoms. The use of oxygen was associated with higher SGRQ scores (49.1 ± 17.1 vs 35.9 ± 21.3, P < .0001), which was an independent association on multivariate analysis. In multivariate analysis with multiple logistic regression, CB was independently associated with increased respiratory symptoms when adjusting for age, current smoking, pack-year history of smoking, sex, and race (Table 3).
Table 3.
—Independent Effects of CB on Respiratory Symptoms
| Variable | Prevalence Ratio | 95% CI |
| Nasal symptoms | 1.55 | 1.28-1.87 |
| Ocular symptoms | 1.32 | 1.17-1.52 |
| Wheezing | 2.36 | 1.72-3.23 |
| Awakened by cough | 1.30 | 1.18-1.43 |
| Awakened by dyspnea | 1.19 | 1.07-1.31 |
Prevalence ratios of CB on respiratory symptoms in multivariate analysis, adjusting for age, current smoking, pack-year history of smoking, sex, and race. See Table 1 for expansion of abbreviation.
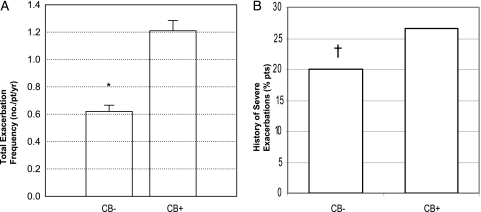
The CB+ group had a greater history of exacerbations in the previous year (1.21 ± 1.62 vs 0.63 ± 1.12 per patient, P = .027), and more patients in the CB+ group reported a history of severe exacerbations in the previous year (26.6% vs 20.0%, P = .024) (Fig 2). The increased exacerbation history in the CB+ group was statistically significant in multivariate analysis controlling for age, current smoking, pack-year history of smoking, sex, and race (all exacerbations prevalence ratio, 1.60; 95% CI, 1.41-1.82; severe exacerbations prevalence ratio, 1.39; 95% CI, 1.24-1.56). Within each GOLD stage, the CB+ group had a significantly greater exacerbation history. The difference in total exacerbation history was greatest in the subgroup with GOLD stage IV disease (GOLD stage II, 0.83 ± 1.24 vs 0.42 ± 0.82 per patient; GOLD stage III, 1.44 ± 1.81 vs 0.78 ± 1.30 per patient; GOLD stage IV, 1.89 ± 1.91 vs 0.93 ± 1.21 per patient). Current smokers in the CB+ group had fewer exacerbations than ex-smokers (0.9 ± 1.6 vs 1.5 ± 1.6 per patient, P = .0031).
Figure 2.
Exacerbation rate in each group. A, Total exacerbation rate. B, History of severe exacerbations. Both total exacerbation rate (data are presented as mean ± SE) and history of severe exacerbations were significantly greater in the CB+ group than in the CB− group. *P < .0001. †P = .0238. pt = patient. See Figure 1 legend for expansion of other abbreviation.
Radiographic measurements are summarized in Table 2. Percent gas trapping (CB+, 42.0% ± 20.0%; CB−, 42.8% ± 20.3%; P = .593) and percent emphysema (CB+, 14.2% ± 13.0%; CB−, 16.0% ± 13.4%, P = .212) were similar in each group. Total lung capacity (6.30 ± 1.50 L vs 5.88 ± 1.40 L, P = .0001) and functional residual capacity (4.22 ± 1.19 L vs 3.92 ± 1.28 L, P = .002) were greater in the CB+ group. When lung volumes were adjusted for age, height, race, and sex in multivariate analysis, the differences in total lung capacity between groups remained statistically significant (point estimate, 1.08; 95% CI, 1.05-1.12). Functional residual capacity, however, only tended to be different between groups after these adjustments (point estimate, 1.07; 95% CI, 0.99-2.29). There were 242 subjects in the CB+ group and 620 in the CB− group with available data on radiographic airway measurements. The CB+ group had a higher mean WA% (63.2% ± 2.9% vs 62.6% ± 3.1%, P = .013), but there was no difference in mean AWT (1.60 ± 0.21 mm vs 1.60 ± 0.22 mm, P = .700) or 10-mm diameter airway (3.800 ± 0.129 mm vs 3.798 ± 0.126 mm, P = .816).
Discussion
In this large, cross-sectional, multicenter study, we describe with great precision the clinical phenotype of patients with COPD and CB. With similar lung function, we found subjects with CB to be younger, have a greater smoking history, and have a greater likelihood of current smoking history than subjects without CB. Moreover, those with CB had higher SGRQ scores, a greater degree of breathlessness, and more upper-airway symptoms. These differences in health-related quality of life and dyspnea are equal to or greater than the minimal clinically important difference thresholds for COPD.29 BODE scores were higher in the CB+ group most likely because of the higher MMRC scores. Finally, there was a higher exacerbation history in the CB+ group, and more subjects in the CB+ group reported severe exacerbations that required hospitalization or urgent care visits. This difference in exacerbation history was most significant in subjects with GOLD stage IV disease.
Exacerbation frequency has been shown to be greater in patients with COPD and CB.16,17,30 Seemungal et al16 found that CB significantly increased the odds of having frequent exacerbations in a group of 70 patients. A cross-sectional analysis of 433 patients also similarly found an increased risk of exacerbation.17 The present study validates these prior findings in a much larger cohort, making the relationship between CB and risk of exacerbation more relevant to the general COPD population. We also demonstrate that the greatest difference in exacerbation history was in the patients with the worst disease severity. Although the assessment of exacerbations lacked validation from medical records and was based on patient reporting of episodes within the year prior to enrollment, the large number of patients in this study adds strength to the conclusion that CB is associated with an increased risk of exacerbation.
Another recent large observational study found a significant, but weak relationship between chronic cough and exacerbation rate.31 In comparison, the link between CB and exacerbations found in the present study is more significant. Although the cohorts had similar lung function and demographics, the aforementioned study enrolled more patients with frequent exacerbations (47% of entire cohort, compared with 39% in the present one), and the incidence of CB in their cohort is unclear. These differences in subject characteristics are most likely to be responsible for the disparity in results.
Of interest, subjects with CB were more likely to be current smokers. We analyzed the effects of current smoking and found no effect on respiratory symptoms or health-related quality of life and found a lower exacerbation history than in ex-smokers. Therefore, the differences seen in symptoms and SGRQ scores between the CB+ and CB− groups cannot be attributed to current smoking alone, and the fewer exacerbations in the current smokers may have been related to better lung function. In addition, there was a trend toward greater use of oxygen in subjects without CB. Although it is established that oxygen supplementation reduces breathlessness in healthy subjects and those with COPD,32‐34 the use of oxygen in the present cohort did not affect respiratory symptoms and actually was associated with higher SGRQ scores, so the lower percentage of respiratory symptoms and lower SGRQ scores in the CB− group is not explained by oxygen use.
Whether the differences in respiratory symptoms between the two groups represent a true relationship with CB is a matter of debate. It is likely that chronic sputum production has significant physiologic effects on airflow, causing greater dyspnea and worse health-related quality of life. Indeed, we have shown that in high-risk patients with severe COPD, chronic sputum production was associated with a lower peak expiratory flow when measured daily for up to 2 years as well as with more breathlessness and more frequent exacerbations.18 Alternatively, it is possible that patients who have complaints of chronic cough and sputum production are simply more likely to describe worse respiratory symptoms to health-care providers without any quantifiable difference in airway inflammation or disease severity. We also did not collect information on use of intranasal steroids or leukotriene antagonists, so it is possible that the CB+ group described more upper-respiratory symptoms as a result of underuse of these medications. Finally, no adjustment was made for multiple comparisons, thereby raising the possibility that by chance alone a few comparisons were spuriously statistically significant. However, the differences in symptoms between the two study groups are large and highly statistically significant (P values often < .0001), and their presence in this large COPD cohort suggests that the differences are indeed real. Furthermore, the greater symptoms coupled with more exacerbations in subjects with CB underscore their clinical significance.
Interestingly, the degree of emphysema and gas trapping were similar in each group, which was not consistent with the study hypothesis. We expected that for a given degree of airflow obstruction, subjects with CB would have a greater proportion of expiratory flow limitation caused by airway disease and, therefore, less emphysema and more gas trapping. Although segmental airway WA% was greater in subjects with CB, there were no statistically significant differences in the other two measures of airway disease. This inconsistency suggests that either WA% is a better barometer of airway inflammation than the other measures or that the detected differences are not true, thereby implying that CB may be related more to large airways disease and not to the small airways. This finding also implies that the phenomenon of CB occurs independently from the presence or degree of emphysema. Alternatively, it is possible that our current means of airway radiographic measurement are not sensitive enough to detect real differences in airway pathology or that radiographically quantifiable differences in morphology do not exist, despite at least qualitative differences in airway inflammation. This issue can be clarified with further analysis of our current radiographic data, advances in technology, and the development of different means of quantifying airway pathology.
The higher lung volumes in the CB+ group were also curious findings. These differences could not be completely explained by the differences in age, sex, and race between the two groups.24,35 However, it should be realized that these values were based on radiographic measurements, and lung volumes measured by plethysmography were not available in this study. At this time, the significance of this finding is uncertain.
Nevertheless, this large cross-sectional analysis of subjects with COPD and a broad spectrum of disease severity shows with greater precision how subjects with COPD and CB are phenotypically different from subjects with COPD without CB. They are younger, more commonly men, more likely to be current smokers, and more symptomatic and have more frequent comorbidities. Eliciting a history of CB can identify a group with worse lower- and upper-airway symptoms, greater risk of exacerbation, and worse prognosis. The clinician also should maintain a heightened suspicion for diabetes, coronary heart disease, osteoporosis, and sleep apnea in this patient population. This group may need more directed therapy targeting chronic mucus production and smoking cessation not only to improve symptoms but also to reduce risk, improve quality of life, and improve outcomes.
Acknowledgments
Author contributions: Dr Kim had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Kim: contributed to the study design, data collection and analysis, and writing of the manuscript.
Dr Han: contributed to the study design, data collection and analysis, and writing of the manuscript.
Ms Vance: contributed to the study design, data collection and analysis, and writing of the manuscript.
Dr Make: contributed to the study design, data collection and analysis, and writing of the manuscript.
Dr Newell: contributed to the study design, data collection and analysis, and writing of the manuscript.
Dr Hokanson: contributed to the study design, data collection and analysis, and writing of the manuscript.
Dr Hersh: contributed to the study design, data collection and analysis, and writing of the manuscript.
Mr Stinson: contributed to the study design, data collection and analysis, and writing of the manuscript.
Dr Silverman: contributed to the study design, data collection and analysis, and writing of the manuscript.
Dr Criner: contributed to the study design, data collection and analysis, and writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Kim has participated in clinical trials sponsored by Boehringer-Ingelheim, GlaxoSmithKline, and Roche Pharmaceuticals. Dr Han has received lecture fees from GlaxoSmithKline, Boehringer-Ingelheim, Pfizer, and CSL Behring; served on advisory boards for CSL Behring and Novartis; and consulted for Novartis and Nycomed. Over the past 3 years, Dr Make has participated in advisory boards, speaker bureaus, consultations, and multicenter clinical trials with funding from the National Heart, Lung, and Blood Institute; Abbott; Astellas; AstraZeneca; Boerhinger-Ingelheim; Dey; Embryon; Forest; GlaxoSmithKline; NABI; Nycomed; Novartis; Pfizer; Respironics; Schering-Plough; Sequal; and Talecris. Dr Newell has received National Institutes of Health grant funding for research into the use of chest CT scanning in assessing emphysema and asthma, has received honoraria from Springer Verlag and WebMD for book contributions, and has freely consulted for VIDA Diagnostics, Inc. Dr Silverman received grant support and consulting fees from GlaxoSmithKline for studies of COPD genetics and honoraria and consulting fees from AstraZeneca. Dr Criner has served on advisory committees for Ortho-Biotech, Schering-Plough, Boehringer-Ingelheim, Actelion, Shire, and Sepracor Pharmaceuticals (all of these sums are < $2,500); has received research grants from Schering-Plough, Boehringer-Ingelheim, Actelion, GlaxoSmithKline, Advanta, Daiichi Asubio, Pfizer, Roche, Sepracor Pharmaceuticals, Emphasys Medical, and Aeris Therapeutics (all research grant monies are deposited and controlled by Temple University). Ms Vance, Drs Hokanson and Hersh, and Mr Stinson have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Abbreviations
- AWT
airway wall thickness
- BODE
BMI, airway obstruction, dyspnea, exercise capacity
- CB
chronic bronchitis
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- MMRC
Modified Medical Research Council
- SGRQ
St. George Respiratory Questionnaire
- WA%
wall area percent
Footnotes
Funding/Support: This study was supported by the National Heart, Lung, and Blood Institute [Grants U01 HL089856 and U01 HL08989].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Fletcher C, Peto R, Tinker C, Speizer FE. The Natural History of Chronic Bronchitis and Emphysema. Oxford, England: Oxford University Press; 1976. [Google Scholar]
- 2.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 3.Innes AL, Woodruff PG, Ferrando RE, et al. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest. 2006;130(4):1102–1108. doi: 10.1378/chest.130.4.1102. [DOI] [PubMed] [Google Scholar]
- 4.Kim V, Kelemen SE, Abuel-Haija M, et al. Small airway mucous metaplasia and inflammation in chronic obstructive pulmonary disease. COPD. 2008;5(6):329–338. doi: 10.1080/15412550802522445. [DOI] [PubMed] [Google Scholar]
- 5.Kim V, Rogers TJ, Criner GJ. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(4):478–485. doi: 10.1513/pats.200802-014ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebert RV, Terracio MJ. The bronchiolar epithelium in cigarette smokers. Observations with the scanning electron microscope. Am Rev Respir Dis. 1975;111(1):4–11. doi: 10.1164/arrd.1975.111.1.4. [DOI] [PubMed] [Google Scholar]
- 7.Deshmukh HS, Case LM, Wesselkamper SC, et al. Metalloproteinases mediate mucin 5AC expression by epidermal growth factor receptor activation. Am J Respir Crit Care Med. 2005;171(4):305–314. doi: 10.1164/rccm.200408-1003OC. [DOI] [PubMed] [Google Scholar]
- 8.Holtzman MJ, Tyner JW, Kim EY, et al. Acute and chronic airway responses to viral infection: implications for asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(2):132–140. doi: 10.1513/pats.200502-015AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax. 2004;59(11):992–996. doi: 10.1136/thx.2003.018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verra F, Escudier E, Lebargy F, Bernaudin JF, De Crémoux H, Bignon J. Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. Am J Respir Crit Care Med. 1995;151(3 pt 1):630–634. doi: 10.1164/ajrccm/151.3_Pt_1.630. [DOI] [PubMed] [Google Scholar]
- 11.Prescott E, Lange P, Vestbo J. Chronic mucus hypersecretion in COPD and death from pulmonary infection. Eur Respir J. 1995;8(8):1333–1338. doi: 10.1183/09031936.95.08081333. [DOI] [PubMed] [Google Scholar]
- 12.Vestbo J, Prescott E, Lange P. Copenhagen City Heart Study Group Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Am J Respir Crit Care Med. 1996;153(5):1530–1535. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- 13.Pelkonen M, Notkola IL, Nissinen A, Tukiainen H, Koskela H. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest. 2006;130(4):1129–1137. doi: 10.1378/chest.130.4.1129. [DOI] [PubMed] [Google Scholar]
- 14.Ekberg-Aronsson M, Pehrsson K, Nilsson JA, Nilsson PM, Löfdahl CG. Mortality in GOLD stages of COPD and its dependence on symptoms of chronic bronchitis. Respir Res. 2005;6:98. doi: 10.1186/1465-9921-6-98. http://respiratory-research.com/content/6/1/98. Accessed May 10, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lange P, Nyboe J, Appleyard M, Jensen G, Schnohr P. Relation of ventilatory impairment and of chronic mucus hypersecretion to mortality from obstructive lung disease and from all causes. Thorax. 1990;45(8):579–585. doi: 10.1136/thx.45.8.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 pt 1):1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 17.Burgel PR, Nesme-Meyer P, Chanez P, et al. Initiatives Bronchopneumopathie Chronique Obstructive Scientific Committee Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135(4):975–982. doi: 10.1378/chest.08-2062. [DOI] [PubMed] [Google Scholar]
- 18.Kim V, Garfield JL, Grabianowski CL, et al. Chronic bronchitic symptoms in severe COPD are associated with increased exacerbation frequency and less emphysema [abstract] Am J Respir Crit Care Med. 2009;179(meeting abstracts):A1521. [Google Scholar]
- 19.Peto R, Speizer FE, Cochrane AL, et al. The relevance in adults of air-flow obstruction, but not of mucus hypersecretion, to mortality from chronic lung disease. Results from 20 years of prospective observation. Am Rev Respir Dis. 1983;128(3):491–500. doi: 10.1164/arrd.1983.128.3.491. [DOI] [PubMed] [Google Scholar]
- 20.Kim V, Criner GJ, Abdallah HY, Gaughan JP, Furukawa S, Solomides CC. Small airway morphometry and improvement in pulmonary function after lung volume reduction surgery. Am J Respir Crit Care Med. 2005;171(1):40–47. doi: 10.1164/rccm.200405-659OC. [DOI] [PubMed] [Google Scholar]
- 21.Hogg JC, Chu FS, Tan WC, et al. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med. 2007;176(5):454–459. doi: 10.1164/rccm.200612-1772OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118(6 pt 2):1–120. [PubMed] [Google Scholar]
- 24.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 25.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 26.Hu S, Hoffman EA, Reinhardt JM. Automatic lung segmentation for accurate quantitation of volumetric X-ray CT images. IEEE Trans Med Imaging. 2001;20(6):490–498. doi: 10.1109/42.929615. [DOI] [PubMed] [Google Scholar]
- 27.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162(3 pt 1):1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 28.Patel BD, Coxson HO, Pillai SG, et al. International COPD Genetics Network Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(5):500–505. doi: 10.1164/rccm.200801-059OC. [DOI] [PubMed] [Google Scholar]
- 29.Gross NJ. Chronic obstructive pulmonary disease outcome measurements: what’s important? What’s useful? Proc Am Thorac Soc. 2005;2(4):267–271. doi: 10.1513/pats.200504-036SR. [DOI] [PubMed] [Google Scholar]
- 30.Foreman MG, DeMeo DL, Hersh CP, Reilly JJ, Silverman EK. Clinical determinants of exacerbations in severe, early-onset COPD. Eur Respir J. 2007;30(6):1124–1130. doi: 10.1183/09031936.00009307. [DOI] [PubMed] [Google Scholar]
- 31.Hurst JR, Vestbo J, Anzueto A, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 32.Chronos N, Adams L, Guz A. Effect of hyperoxia and hypoxia on exercise-induced breathlessness in normal subjects. Clin Sci (Lond) 1988;74(5):531–537. doi: 10.1042/cs0740531. [DOI] [PubMed] [Google Scholar]
- 33.Lane R, Cockcroft A, Adams L, Guz A. Arterial oxygen saturation and breathlessness in patients with chronic obstructive airways disease. Clin Sci (Lond) 1987;72(6):693–698. doi: 10.1042/cs0720693. [DOI] [PubMed] [Google Scholar]
- 34.Swinburn CR, Mould H, Stone TN, Corris PA, Gibson GJ. Symptomatic benefit of supplemental oxygen in hypoxemic patients with chronic lung disease. Am Rev Respir Dis. 1991;143(5 pt 1):913–915. doi: 10.1164/ajrccm/143.5_Pt_1.913. [DOI] [PubMed] [Google Scholar]
- 35.Stocks J, Quanjer PH. Official Statement of the European Respiratory Society Reference values for residual volume, functional residual capacity and total lung capacity. ATS Workshop on Lung Volume Measurements. Eur Respir J. 1995;8(3):492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]