Abstract
Background:
COPD is a heterogeneous disease characterized by airflow obstruction and diagnosed by lung function. CT imaging is emerging as an important, noninvasive tool in phenotyping COPD. However, the use of CT imaging in defining the disease heterogeneity above lung function is not fully known.
Methods:
Seventy-five patients with COPD (58 men, 17 women) were studied with CT imaging and with measures of airway inflammation. Airway physiology and health status were also determined.
Results:
The presence of emphysema (EM), bronchiectasis (BE), and bronchial wall thickening (BWT) was found in 67%, 27%, and 27% of subjects, respectively. The presence of EM was associated with lower lung function (mean difference % FEV1, −20%; 95% CI, −28 to −11; P < .001). There was no difference in airway inflammation, exacerbation frequency, or bacterial load in patients with EM alone or with BE and/or BWT ± EM. The diffusing capacity of the lung for carbon monoxide/alveolar volume ratio was the most sensitive and specific parameter in identifying EM (area under the receiver operator characteristic curve, 0.87; 95% CI, 0.79-0.96). Physiologic cluster analysis identified three clusters, two of which were EM predominant and the third characterized by a heterogeneous combination of EM and BE.
Conclusions:
The application of CT imaging can be useful as a tool in the multidimensional approach to phenotyping patients with COPD.
COPD is a heterogeneous disease characterized by airflow obstruction that is not fully reversible and is associated with a progressive decline in lung function.1 Airway inflammation in COPD is usually neutrophilic,2,3 but eosinophilic airway inflammation has been found at stable state4,5 and during exacerbations.6,7 Pathologic processes in COPD include destruction of lung parenchyma and changes within the large and small airways.8 This evident disease heterogeneity cannot be defined by the FEV1 alone, and alternative methods need to be sought to develop mechanistic, prognostic, and therapeutic applications in the management of COPD.9 CT imaging has emerged as a noninvasive tool in the “phenotyping” of COPD, with measures investigating changes in the airway wall and lumen and within the lung parenchyma,10‐12 as well as assessments of the degree of emphysema (EM) and burden of small airways disease. CT imaging quantitative assessments have shown, inter alia, associations with parameters of airway physiology,13‐15 important COPD outcome measures,15‐18 and systemic inflammatory mediators.19
However, the role of CT imaging in defining COPD phenotypes above and beyond conventional characterization with full lung function (spirometry, static lung volumes, and gas transfer) remains to be fully determined. Furthermore, the validity of CT imaging as a biomarker of airways disease has yet to be established.20,21
We hypothesized that CT imaging provides additional value to clinical and physiologic parameters in the multidimensional phenotyping of COPD. We tested our hypothesis by evaluating whether phenotyping of COPD using factor and cluster analysis with lung function parameters (physiologic clustering) would generate the radiologic COPD disease groups seen in clinical practice (EM, bronchial wall thickening [BWT], and bronchiectasis [BE]).
Materials and Methods
Patients
Patients with a physician diagnosis of COPD as per GOLD (Global Initiative for Chronic Obstructive Lung Disease) criteria1 were recruited consecutively from general respiratory clinics and through local advertising to enter the longitudinal Biomarkers in COPD Exacerbation study. Patients with a diagnosis of asthma, current active pulmonary TB, or any other clinically relevant lung disease were excluded. All patients had obstructive spirometry with a postbronchodilator FEV1/FVC ratio of < 0.7, whereas severity was classified according to the GOLD criteria.1 Patients with COPD who demonstrated bronchodilator reversibility were not excluded. All patients gave written informed consent, and the study was approved by the Leicestershire, Northamptonshire, and Rutland ethics committee (REC 07/H0406/157).
Study Design
Seventy-five patients enrolled in the Biomarkers in COPD Exacerbation study, who had previously undergone a CT scan as part of their clinical management, were studied. The median (interquartile range) interval time between patient characterization and CT scanning was 15 (25) months.
Measurements
All patients had complete demographic data recorded, including age, duration of symptoms, and full smoking and medical history. Full lung function, including reversibility testing with 400 μg inhaled albuterol, was also performed according to the American Thoracic Society/European Respiratory Society consensus guidelines.22‐26 Health status and symptom scores were measured using the St. George Respiratory Questionnaire (University of London; London, England),27 a two-part questionnaire measuring recollection of symptoms and assessment of current activity and impact, in which scores are expressed as a percentage of overall impairment, with zero indicating best possible health status and 100 indicating worst possible health status; the Chronic Respiratory Disease Interviewer-Administered Questionnaire (McMaster University; Hamilton, Ontario, Canada),28 which measures disease-specific quality of life with scores ranging from 1 to 7, with a higher score indicating a better quality of life for the domains of emotion, mastery, dyspnea, and fatigue; and the visual analog scale (VAS) for the domains of cough, breathlessness, sputum production, and sputum purulence, which consists of a 100-mm line for each measured domain, with “no symptoms” at one end and “the worst symptoms ever” at the other.29 Body composition was calculated using BMI.30 Spontaneous or induced sputum was collected and analyzed for bacteria,31 including colony-forming units.32 Quantitative real-time polymerase chain reaction was used to estimate the total bacterial load based on the abundance of 16S ribosomal subunit encoding genes. In brief, bacterial DNA was extracted from homogenized sputum according to the manufacturer’s instructions, using the QIAmp DNA Mini Kit assay (QIAGEN, Ltd; Hilden, Germany). Quantification of the total bacterial load was performed using the SYBR green assay (PE Applied Biosystems; Warrington, England). Sputum samples were then processed to produce cytospins for cell differential.33,34 Venous blood was collected for assessment of peripheral blood differential cell counts and serum C-reactive protein.
CT Imaging
CT imaging was performed using a Sensation 16-slice scanner (Siemens Healthcare; Knoxville, Tennessee). Sequential scanning was performed at maximal inspiration from the apex to the diaphragm at 10-mm increments, with 1-mm collimation, while patients were in the supine position. Image reconstruction was performed by using a high-spatial-frequency algorithm through a 512 × 512 matrix with a small field of view. Scanning time ranged from 30 to 45 s with a 120-kV peak and an effective tube current of 140 mA. Images were reported at a window width and level of 1,600 and −500 Hounsfield units, respectively. CT scan qualitative radiologic reporting for EM, BE, and BWT was performed in accordance with published guidelines.35 BE was present when one or more of the following criteria were fulfilled: (1) an internal diameter of the bronchus greater than that of the adjacent pulmonary artery, (2) a lack of tapering of the bronchial lumen toward the periphery, or (3) visualization of the bronchus within 10 mm of the pleural space. EM was present when focal areas of low attenuation without visible walls were detected, and included the presence of centrilobular, panlobular, and paraseptal EM. BWT was assessed subjectively as described previously.36 Previous studies at our institution have shown that the qualitative description method of EM, BWT, and BE has good interobserver agreement and image reconstruction; scanning and reporting was optimized for analysis of BWT, EM, and BE as previously described.36
Statistical Analysis
Statistical analysis was performed using PRISM, version 4 (GraphPad Software; San Diego, California) and SPSS, version 16 (SPSS Inc; Chicago, Illinois). Parametric and nonparametric data are presented as mean (SEM) and median (interquartile range) unless stated otherwise. Log-transformed data are presented as geometric mean (95% CI). For comparison of unpaired or paired, parametric or nonparametric groups, the Student t test, paired t test, Mann-Whitney test, and Wilcoxon matched pairs test were used, respectively. For comparison of three groups or more for parametric and nonparametric variables, the one-way analysis of variance or Kruskal-Wallis test was used and the χ2 test was used for proportions. Logistic regression analysis was used37 to assess the relationship of the dependant variables of (1) presence of EM, (2) presence of BE, and (3) presence of BWT with explanatory (independent) variables, using the block entry method. Variables entered into the logistic regression model, chosen for clinical relevance, were the following continuous variables: FEV1 % predicted, diffusing capacity of the lung for carbon monoxide (Dlco)/alveolar volume (Va), residual volume (RV) % predicted, total sputum neutrophil count, and percentage sputum eosinophils (both log transformed). Model inspection showed no multicollinearity, goodness of fit was performed using the Hosmer-Lemeshow χ2 test, and the NagelKerke R2 was used to estimate the variance explained by the model. The Wald test was used to assess the significance of the individual dependant variables to the model. Unsupervised multivariate modeling using principal component factor reduction analysis (orthogonal varimax rotation method) was used to explore airway physiologic pattern expression in the patients. Hierarchic cluster analysis was then applied from the identified factors to determine physiologic clusters of COPD. Clinical characteristics for the physiologic clusters were tabulated. One-way analysis of variance, Kruskal Wallis, and the χ2 test were used to compare parametric, nonparametric, and proportions of clinical characteristics between physiologic cluster groups. A P value of < .05 was deemed to be statistically significant.
Results
CT data were available in 75 patients (58 men, 17 women). The most common clinical indication for CT scanning was investigation or exclusion of malignancy (40%), followed by assessment for radiographic evidence of BE (30%) and suitability for lung volume reduction surgery (20%). The clinical characteristics of patients are shown in Table 1.
Table 1.
—Clinical Characteristics of Patients With COPD With Corresponding CT Scan
| Characteristic | Patients With COPD With CT scan Data (n = 75) |
| Male, No. (%) | 58 (77) |
| Age, y, mean (range) | 67 (43-88) |
| Current smoker, No. (%) | 24 (32) |
| Ex-smoker, No. (%) | 50 (67) |
| Pack-y smoked, mean (range) | 48 (10-153) |
| Exacerbation history in previous y | 4.3 (0.3) |
| BMI, kg/m2 | 26.1 (0.6) |
| ICS usage, No. (%) | 68 (91) |
| ICS dosage, μg | 1424 (92) |
| FEV1,a L | 1.22 (0.06) |
| FEV1 % predicteda | 46 (2) |
| Reversibility, % | 3 (1) |
| FEV1/FVC,a % | 47 (2) |
| SGRQ total, units | 55.2 (2.0) |
| CRQ total, units | 4.00 (0.13) |
| VAS total, mm | 147 (9) |
| Sputum total cell count × 106 cells/g, geometric mean (95% CI) | 3.8 (2.8-5.3) |
| Sputum neutrophils, % | 69 (3) |
| Sputum eosinophils, %, geometric mean (95% CI) | 1.3 (0.9-1.9) |
Data are presented as mean (SEM) unless otherwise indicated. CRQ = Chronic Respiratory Disease Interviewer-Administered Questionnaire; ICS = inhaled corticosteroid; SGRQ = St. George Respiratory Questionnaire; VAS = visual analog scale.
Postbronchodilator.
EM, BE, and BWT Are Common Radiologic Observations in COPD, With Significant Overlap
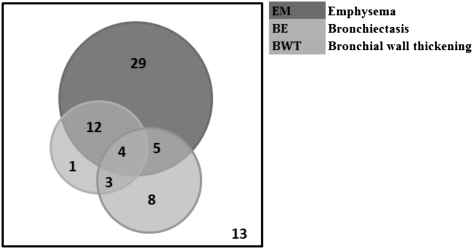
The CT scan description of EM, BE, and BWT was present in 67%, 27%, and 27%, respectively. The number of patients with EM, BE, and BWT, and the associated overlap, are shown in Figure 1. No evidence of EM, BE, or BWT on CT scanning was found in 13 patients in the COPD cohort. Subjects with CT scan evidence of disease had worse lung function, but there was no difference in demographic distribution, health status, or symptom scores (Table 2).
Figure 1.
Nonproportional Venn diagram representing patients with EM, BE, and BWT on CT scanning. BE = bronchiectasis; BWT = bronchial wall thickening; EM = emphysema.
Table 2.
—Clinical Comparisons in Patients With Demonstrable Absence or Presence of CT Scan Evidence of EM, BE, or BWT
| Characteristic | Absence of EM/BE/BWT (n = 13) | Presence of EM/BE/BWT (n = 62) | P Value |
| Male, No. (%) | 8 (62) | 50 (81) | 0.63 |
| Age, y, mean (range) | 68 (49-88) | 67 (43-85) | .90 |
| Pack-y smoked mean (range) | 35 (10-68) | 50 (10-153) | .10 |
| Exacerbation history in previous y | 3.7 (0.9) | 4.4 (0.3) | .43 |
| ICS dosage, μg | 831 (213) | 1548 (95) | < .001 |
| FEV1 % predicteda | 62 (5) | 43 (2) | < .001 |
| FEV1/FVC,a % | 59 (3) | 45 (2) | < .001 |
| SGRQ total, units | 50.3 (5.9) | 56.4 (2.0) | .23 |
| CRQ total, units | 4.21 (0.35) | 3.95 (0.14) | .45 |
| VAS total, mm | 149 (16) | 146 (10) | .92 |
| Sputum total cell count × 106 cells/g, geometric mean (95% CI) | 3.5 (1.9 -6.6) | 3.9 (2.7-5.6) | .82 |
| Sputum neutrophils, % | 66 (5) | 70 (3) | .64 |
| Sputum eosinophils, %, geometric mean (95% CI) | 1.2 (0.5-2.8) | 1.3 (0.9-2.0) | .83 |
Data are presented as mean (SEM) unless otherwise indicated. BE = bronchiectasis; BWT = bronchial wall thickening; EM = emphysema. See Table 1 legend for expansion of other abbreviations.
Postbronchodilator.
Conventional Lung Function Reliably Identifies Patients With Radiologic EM and COPD
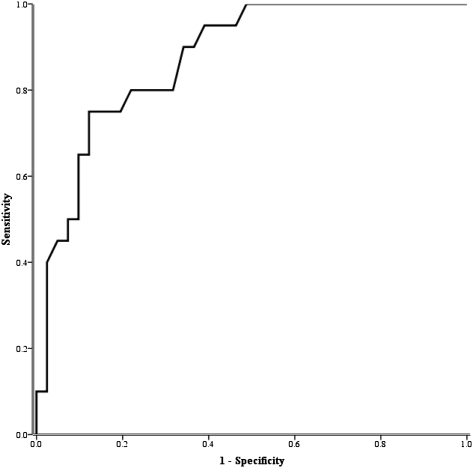
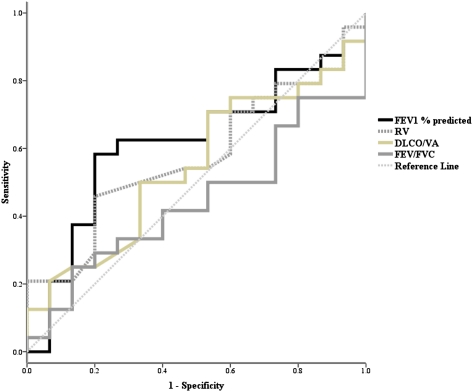
CT scan evidence of EM compared with no evidence of EM was associated with lower lung function (39 vs 61; mean difference, −22%; 95% CI, −30 to −13; P < .001), impaired Dlco (3.8 vs 5.6; mean difference, −1.8; 95% CI, −2.7 to −0.9; P < .001), and increased airway obstruction (FEV1/FVC) (0.43 vs 0.57; mean difference, −0.14; 95% CI, −0.20 to −0.08; P < .001). The receiver operator characteristic curve (area under the curve) for the Dlco/Va to correctly identify the presence of EM was 0.87 (95% CI, 0.79-0.96) (Fig 2). All of the conventional measures of lung function (FEV1, Dlco/Va, RV, and FEV1/FVC) were reliable in distinguishing between EM only and EM plus BE and/or BWT (EM + BE/BWT), with receiver operator characteristic curves of < 0.6 (Fig 3).
Figure 2.
Receiver operator characteristic curve (sensitivity and 1-specificity) of diffusing capacity of the lung for carbon monoxide corrected for alveolar volume for identifying emphysema.
Figure 3.
Receiver operator characteristic curves for distinguishing the presence of emphysema alone vs emphysema plus bronchiectasis and/or bronchial wall thickening. Dlco/Va = diffusing capacity of the lung for carbon monoxide/alveolar volume; RV = residual volume.
In contrast to this, patients with radiologic EM in the presence or absence of BE and/or BWT did not have any significant difference in markers of proximal airway inflammation (sputum total cell count and cellular differential count) or bacterial load (measured by colony-forming units and abundance of 16S ribosomal subunit encoding genes) (Table 3).
Table 3.
—Airway Inflammometry, Physiology, and Health Status in Patients With EM Alone, BE and/or BWT Without EM, and EM With BWT and/or BE
| Characteristic | EM (n = 29) | BE/BWT − EM (n = 12) | EM + BWT/BE (n = 21) | P Value |
| Male, No. (%) | 22 (76) | 9 (75) | 19 (91) | .37 |
| Age, y, mean (range) | 64 (43-83) | 71 (60-84) | 69 (47-85) | .07 |
| Ex-smoker, No. (%) | 17 (59) | 11 (92) | 12 (57) | .09 |
| Pack-y smoked, mean (range) | 48 (18-120) | 47 (10-153) | 55 (10-134) | .68 |
| Exacerbation history in previous y | 4.0 (0.5) | 3.9 (0.9) | 5.1 (0.5) | .32 |
| BMI, kg/m2 | 25.2 (0.9) | 28.2 (1.4) | 24.7 (0.4) | .10 |
| Sputum total cell count × 106 cells/g, geometric mean (95% CI) | 3.2 (2.0-5.2) | 8.4 (4.3-16.5) | 3.6 (1.7-7.7) | .18 |
| Sputum neutrophils, % | 70 (4) | 77 (6) | 65 (6) | .44 |
| Sputum eosinophil, %, geometric mean (95% CI) | 1.6 (0.9-2.8) | 0.8 (0.4-1.7) | 1.3 (0.6-2.7) | .55 |
| Potential pathogenic organism, % | 14 | 25 | 38 | .08 |
| Sputum neutrophil count × 106 cells/g, geometric mean (95% CI) | 2.3 (1.3-4.0) | 6.3 (2.8-14.1) | 1.9 (0.8-4.8) | .18 |
| Total bacterial load (16S), geometric mean (95% CI) | 1.58 (6.67-3.28) | 1.78 (3.57-8.18) | 1.58 (3.27-6.78) | .99 |
| Colony forming units per mL of sputum, geometric mean (95% CI) | 1.46 (6.35-3.06) | 1.26 (1.56-9.16) | 7.85 (3.05-2.06) | .71 |
| FEV1 % predicteda | 41 (3) | 59 (5) | 37 (4) | < .01 |
| FEV1/FVC,a % | 42 (2) | 56 (4) | 44 (2) | .01 |
| Reversibility, % | 4.0 (1.9) | 3.0 (2.9) | −0.4 (3.3) | .44 |
| RV, % | 162 (10) | 115 (10) | 139 (10) | .02 |
| Total lung capacity, % | 124 (10) | 99 (5) | 103 (7) | .13 |
| Dlco, mL CO/min/mm Hg | 4.0 (0.3) | 5.8 (0.6) | 3.6 (0.3) | < .01 |
| Dlco % predicted | 46 (4) | 73 (7) | 32 (4) | <.01 |
| Dlco/Va, mL CO/min/mm Hg/L | 0.8 (0.1) | 1.0 (0.1) | 1.3 (0.4) | .37 |
| Dlco/Va % predicted | 56 (5) | 89 (10) | 49 (4) | < .01 |
| SGRQ total, units | 57.9 (3.1) | 52.3 (3.5) | 56.4 (3.6) | .61 |
| CRQ total, units | 3.79 (0.21) | 4.07 (0.31) | 4.11 (0.26) | .57 |
| VAS total, mm | 163 (13) | 143 (25) | 126 (18) | .24 |
Static Lung Volume and Transfer Factor Are Independent Predictors of EM in COPD, But Not of BE or BWT
The Dlco/Va and RV were confirmed by logistic regression analysis to be independent predictors of EM on CT scanning in patients with COPD. In contrast, the variables entered into the model were not independent predictors of BE or BWT (Table 4).
Table 4.
—Logistic Regression for the Presence of EM, BE, and BWT (Dependent Variables) and the Listed Independent Variables
| EM (R2 = 0.68) |
BE (R2 = 0.14) |
BWT (R2 = 0.14) |
||||||||||
| Multivariate Modeling for Detection of Radiologic Phenotypes | B (SE) | Wald Test | P Value | OR (95% CI) | B (SE) | Wald Test | P Value | OR (95% CI) | B (SE) | Wald Test | P Value | OR (95% CI) |
| FEV1 % predicted | −0.22 (0.03) | 0.69 | .41 | 0.98 (0.93-1.03) | −0.03 (0.02) | 1.62 | .21 | 0.97 (0.93-1.02) | < 0.01 (0.02) | < 0.01 | .97 | 1.00 (0.96-1.04) |
| Dlco/Va % predicted | −0.06 (0.03) | 5.63 | .02 | 0.94 (0.89-0.99) | 0.02 (0.02) | 1.76 | .26 | 1.02 (0.99-1.05) | −0.03 (0.02) | 2.67 | .10 | 0.97 (0.93-1.01) |
| RV % predicted | 0.04 (0.02) | 4.46 | .04 | 1.04 (1.00-1.08) | −0.01 (0.01) | 0.15 | .70 | 1.00 (0.98-1.02) | < 0.01 (0.01) | 0.01 | .91 | 1.00 (0.98-1.02) |
| Total sputum neutrophils × 106 cells/g sputum | −1.71 (0.87) | 3.57 | .05 | 0.18 (0.03-1.00) | 0.49 (0.56) | 0.79 | .38 | 1.63 (0.55-4.84) | −0.49 (0.49) | 0.99 | .32 | 0.60 (0.23-1.61) |
| Sputum eosinophils, % | −0.33 (0.84) | 0.15 | .70 | 0.72 (0.14-3.77) | −0.95 (0.66) | 2.04 | .15 | 0.39 (0.11-1.42) | −0.33 (0.58) | 0.32 | .57 | 0.72 (0.23-2.25) |
Conventional Lung Function Fails to Identify Patients With BE and BWT in COPD
Having confirmed that static lung volumes and transfer factor were predictive of radiologic EM, we sought to identify whether these markers could independently group patients with EM-predominant disease (EM+) or disease without EM (EM−) in the presence or absence of BWT/BE, using unsupervised statistical techniques. Principal component analysis using airway physiologic parameters in all patients with full physiologic testing and CT scan (n = 64) identified three factors with eigen values > 1 (Table 5), highlighting the following dominant physiologic components: (1) RV, (2) Dlco/Va, and (3) lung capacity. Hierarchic cluster analysis determined three cluster groups. Two were EM predominant, discrete only in their degree of air trapping and gas transfer. The third cluster was characterized by a heterogeneous combination of EM and BE, with preservation of gas transfer and lung volumes (Table 6).
Table 5.
—Extracted Factors With Eigenvalues > 1 Using Unsupervised Principal Component Analysis for Airway Physiologic Parameters
| Factors |
|||
| Correlation Matrix | 1 | 2 | 3 |
| FEV1/FVC, % | −0.49 | 0.60 | … |
| FEV1 % predicted | −0.35 | 0.44 | 0.74 |
| VC, % | … | … | 0.94 |
| FRC, % | 0.89 | −0.40 | … |
| RV, % | 0.85 | … | … |
| TLC, % | 0.90 | … | … |
| Dlco, % | … | 0.93 | … |
| Dlco/Va, % | … | 0.95 | … |
FRC = functional residual capacity; TLC = total lung capacity; VC = vital capacity. See Table 3 for expansion of other abbreviations.
Table 6.
—Demographic and Clinical Characteristics of Physiologic Clusters in COPD
| Characteristic | Cluster 1 (EM-Predominant, Moderate Lung Function [n = 30]) | Cluster 2 (Heterogeneous, Lung Function Preserved [n = 20]) | Cluster 3 (EM-Predominant, Severe Lung Function [n = 14]) | P Value |
| Male, No. (%) | 23 (77) | 17 (85) | 12 (86) | .68 |
| Age, y, mean (range) | 68 | 70 | 64 | .23 |
| Pack-y smoked, mean (range) | 63 | 32 | 44 | <.01 |
| Exacerbation history in previous y | 4.3 (0.5) | 3.3 (0.6) | 4.6 (0.6) | .25 |
| BMI, kg/m2 | 24.6 (0.8) | 28.8 (1.2) | 24.9 (1.3) | .01 |
| Sputum total cell count × 106 cells/g, geometric mean (95% CI) | 4.2 (3.3-6.8) | 4.7 (3.6-8.0) | 3.4 (2.1-8.6) | .79 |
| Sputum neutrophils, % | 68 (5) | 68 (5) | 73 (6) | .77 |
| Sputum eosinophils, %, geometric mean (95% CI) | 1.6 (1.2-2.9) | 0.8 (0.6-1.4) | 2.0 (1.3-4.5) | .17 |
| Potential pathogenic organism, % (95% CI) | 41 (23-61) | 11 (2-34) | 20 (5-52) | .09 |
| Absolute neutrophil count × 106 cells/g, geometric mean (95% CI) | 2.8 (2.0-5.0) | 3.0 (2.2-5.8) | 2.3 (1.4-6.6) | .91 |
| Total bacterial load (16S), geometric mean (95% CI) | 2.68 (1.68-6.68) | 1.48 (7.97-4.28) | 6.87 (3.67-2.48) | .30 |
| Colony-forming units, geometric mean (95% CI) | 7.25 (4.55-1.88) | 1.56 (9.45-3.66) | 2.96 (1.76-8.26) | .17 |
| FEV1 % predicteda | 45 (4) | 62 (3) | 27 (3) | <.01 |
| FEV1/FVC,a % | 45 (2) | 58 (3) | 35 (2) | <.01 |
| Reversibility, % | 4 (2) | 5 (3) | −3 (4) | .12 |
| RV, % | 143 (3) | 88 (6) | 194 (11) | <.01 |
| TLC, % | 110 (4) | 85 (5) | 124 (4) | <.01 |
| Dlco/Va % predicted | 63 (4) | 90 (6) | 42 (4) | <.01 |
| SGRQ total, units | 54 (3) | 53 (5) | 57 (4) | .79 |
| CRQ total, units | 3.8 (0.2) | 4.3 (0.3) | 4.3 (0.3) | .30 |
| VAS total, units | 154 (13) | 164 (16) | 102 (18) | .03 |
| VAS dyspnea, mm | 53 (4) | 44 (6) | 38 (8) | .13 |
| VAS cough, mm | 41 (5) | 45 (6) | 25 (8) | .12 |
| VAS sputum production, mm | 35 (4) | 38 (7) | 28 (8) | .53 |
| VAS sputum purulence, mm | 25 (4) | 39 (6) | 11 (3) | <.01 |
| EM, % (95% CI) | 80 (62-91) | 25 (11-47) | 93 (66-100) | <.01 |
| BE, % (95% CI) | 23 (12-41) | 35 (18-57) | 21 (7-48) | .58 |
| BWT, % (95% CI) | 37 (22-55) | 5 (0-25) | 29 (11-55) | .04 |
Repeatability of Airway Physiologic Phenotypes
Forty-seven patients had spirometry performed 12 months after the initial characterization visit. In these patients, the repeatability of the measures of FEV1 and FVC was good, with intraclass coefficients of 0.91 and 0.82, respectively (P <.001). There was no difference in the mean change in lung function for each of the physiologic clusters identified after 12 months (cluster 1 [EM-predominant, moderate lung function], 0.03; 95% CI, −0.11 to 0.17; cluster 2 [heterogeneous, lung function preserved], −0.13; 95% CI, −0.23 to −0.03; and cluster 3 [EM-predominant, severe lung function], 0.00; 95% CI, −0.12 to 0.12; P = .17).
Discussion
In this study, we have shown that there is overlap in radiologic evidence of EM, BE, and BWT in patients with COPD, whereas the presence of BWT alone is sparse. Current guidelines use bedside spirometry and FEV1 to diagnose and guide the severity of COPD.1 Although this tool can be widely applied, it has less accuracy when used to measure small airways dysfunction (airways with internal diameter < 2 mm).9 Using CT imaging, we have shown that, in patients with COPD, there are variation and overlap in the associated pathologic findings. Thus, the use of FEV1 alone to diagnose and guide management in COPD highlights that spirometry cannot define these pathologic processes with clarity. Our finding of BE in those with spirometric classification of COPD is comparable to those observed previously38 and highlights the heterogeneity that exists, which can, in turn, be partly delineated by radiologic characterization.38‐40
In patients with EM on CT scan, we found worsened lung function, airflow obstruction, and larger increases in residual lung volume, compared with patients with radiologic evidence of BE or BWT, although the clinical parameters of airway inflammation, health status, and microbiologic qualitative or quantitative inflammation are indistinguishable between these radiologic phenotypes. This is in keeping with previous physiologic observations15 and evidence of a lack of correlation between sputum neutrophils and EM score in patients with COPD,15,41 and outlines the radiologic complement to phenotyping COPD. The evidence of eosinophilic airway inflammation and CT imaging in patients with COPD has been conflicting.40,42 However, these differences could be accounted for by differences in study design. Interestingly, Miller and colleagues42 found close correlations between eosinophil degradation products and EM score in a group of well-characterized patients with COPD, suggesting that these may contribute to the progression of EM.
In this study, we have shown that FEV1 using regression model analysis was not predictive for the identification of EM, BE, or BWT on CT scan. This further illustrates the reduced accuracy of FEV1 in identifying the key pathologic processes of COPD. On the other hand, CT scan COPD phenotype identification has been associated with key clinical outcomes, including bronchodilator reversibility,39,43 effect of airflow obstruction,11 and severity of COPD exacerbations.44 We also concluded that the use of conventional lung function testing alone was unable to delineate between patients with EM alone and those with EM plus phenotypes.
Using cluster analysis, an unbiased statistical approach investigating airway physiologic pattern expression, we identified three clusters that were largely clinically indistinguishable in the expression of airway inflammation, bacterial load, and quality-of-life scores. These clusters identified two emphysematous groups discrete by the degree and severity of hyperinflation and gas exchange, and a third group that was heterogeneous. Although largely useful, physiology alone could not clearly and robustly identify the pathologic processes seen in COPD. The application of CT imaging provided additional clinically important information as a noninvasive biomarker in COPD.20
One limitation of this study is that we used qualitative and not quantitative classification of EM, BE, and BWT. Although this qualitative description of EM, BE, and BWT introduces bias into a study such as this one, we aimed to reduce this by showing substantial agreement between two observers in using this form of radiologic description.36 Future studies that use similar unbiased statistical approaches combining multiple dimensions of airway inflammation, physiology, and structure determined by quantitative densitometry and geometry are required. The study investigated patients who were concurrently enrolled in a longitudinal “biomarkers in COPD exacerbation” study. This may have introduced selection bias in the physiologic analysis because the indication for CT scan was primarily for clinical purposes, namely malignancy, BE, and lung volume reduction surgery. However, as commonly found in clinical practice, the group with COPD was likely to have measures of lung function with imaging in a selected group only; our study thus highlights that lung function tools alone do not clearly identify important pathologic processes that may subsequently alter management. The time interval between characterization and CT scanning had a median of 15 months and may have affected the stability of the physiologic phenotype. We found in a large cohort of subjects with COPD that parameters of spirometry (namely, FEV1 and FVC) were unchanged 12 months after characterization, and that measures of detailed lung function were also repeatable in this cohort. This further confirms our finding that the use of physiology that is repeatable can identify an EM phenotype, whereas imaging is required to identify BE and BWT in COPD. Whether the presence of radiologic BE and BWT in COPD alters the clinical prognosis, management, and therapeutic response needs to be examined further in larger clinical studies with specific phenotypic strategies of trial design.
Conclusions
In conclusion, COPD is a heterogeneous disease currently diagnosed and classified according to spirometry. The application of radiologic imaging can be a useful tool in further “phenotyping” patients with COPD where clinical markers of airway inflammation and health status are indistinguishable, and where static or dynamic lung function tests provide some, but not all, clarification.
Acknowledgments
Author contributions: All authors approved the final version of the manuscript.
Dr Bafadhel: contributed to the design of the study, data collection, data analysis, and writing of the manuscript, and can vouch for the integrity of the data analysis.
Mr Umar: contributed to the data collection and writing of the manuscript.
Dr Gupta: contributed to the data collection and writing of the manuscript.
Dr Raj: contributed to the data collection and writing of the manuscript.
Mr Vara: contributed to the data collection and writing of the manuscript.
Dr Entwisle: contributed to the data collection and writing of the manuscript.
Dr Pavord: contributed to the design of the study, data analysis, and writing of the manuscript.
Dr Brightling: contributed to the design of the study, data collection, data analysis, and writing of the manuscript, and can vouch for the integrity of the data analysis.
Dr Siddiqui: contributed to the design of the study, data analysis, and writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Pavord has received consultancy fees from AstraZeneca, GlaxoSmithKline, and Novartis. Dr Brightling has received consultancy fees from Medimmune, AstraZeneca, GlaxoSmithKline, and Roche, and has received research grants from AstraZeneca, Medimmune, and GlaxoSmithKline. Dr Siddiqui has received pharmaceutic grant monies and has participated in speaking activities for Merck, Sharp, and Dome. Drs Bafadhel, Gupta, Raj, Entwisle and Messrs Umar and Vara have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the writing or analysis of the manuscript.
Other contributions: The authors thank all the patients who took part in the study and acknowledge the following persons for their assistance in patient characterization, laboratory support, and microbiology facilitation: S. Terry, BSc; S. McKenna, RGN;V. Mistry, BSc; M. Pancholi, BSc; C. Reid, BSc; A. Singapuri, BSc; K. Haldar, MSc; H. Patel, BSc; B. Hargadon, RGN; M. Shelley, RGN; M. Bourne, RGN; and M. R. Barer, PhD.
Abbreviations
- BE
bronchiectasis
- BWT
bronchial wall thickening
- Dlco
diffusing capacity of the lung for carbon monoxide
- EM
emphysema
- RV
residual volume
- Va
alveolar volume
Footnotes
Drs Brightling and Siddiqui were joint senior authors of this article.
Funding/Support: Dr Bafadhel is funded by a grant from the Medical Research Council, and Dr Brightling is funded by a Wellcome Senior Clinical Fellowship [03/91/68] that requires Open Access placement. The research was performed in laboratories partly funded by the European Regional Development Fund [ERDF 05567].
References
- 1.Rabe KF, Hurd S, Anzueto A, et al. Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153(2):530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 3.Ronchi MC, Piragino C, Rosi E, Amendola M, Duranti R, Scano G. Role of sputum differential cell count in detecting airway inflammation in patients with chronic bronchial asthma or COPD. Thorax. 1996;51(10):1000–1004. doi: 10.1136/thx.51.10.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pizzichini E, Pizzichini MM, Gibson P, et al. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1511–1517. doi: 10.1164/ajrccm.158.5.9804028. [DOI] [PubMed] [Google Scholar]
- 5.Brightling CE, McKenna S, Hargadon B, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. 2005;60(3):193–198. doi: 10.1136/thx.2004.032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papi A, Luppi F, Franco F, Fabbri LM. Pathophysiology of exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(3):245–251. doi: 10.1513/pats.200512-125SF. [DOI] [PubMed] [Google Scholar]
- 7.Bathoorn E, Kerstjens H, Postma D, Timens W, MacNee W. Airways inflammation and treatment during acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(2):217–229. doi: 10.2147/copd.s1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeffery PK. Structural and inflammatory changes in COPD: a comparison with asthma. Thorax. 1998;53(2):129–136. doi: 10.1136/thx.53.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive lung disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 11.Patel BD, Coxson HO, Pillai SG, et al. International COPD Genetics Network Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(5):500–505. doi: 10.1164/rccm.200801-059OC. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa M, Nasuhara Y, Onodera Y, et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(12):1309–1315. doi: 10.1164/rccm.200601-037OC. [DOI] [PubMed] [Google Scholar]
- 13.Kinsella M, Müller NL, Abboud RT, Morrison NJ, DyBuncio A. Quantitation of emphysema by computed tomography using a “density mask” program and correlation with pulmonary function tests. Chest. 1990;97(2):315–321. doi: 10.1378/chest.97.2.315. [DOI] [PubMed] [Google Scholar]
- 14.Yuan R, Hogg JC, Paré PD, et al. Prediction of the rate of decline in FEV(1) in smokers using quantitative computed tomography. Thorax. 2009;64(11):944–949. doi: 10.1136/thx.2008.112433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Donnell RA, Peebles C, Ward JA, et al. Relationship between peripheral airway dysfunction, airway obstruction, and neutrophilic inflammation in COPD. Thorax. 2004;59(10):837–842. doi: 10.1136/thx.2003.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han MK, Bartholmai B, Liu LX, et al. Clinical significance of radiologic characterizations in COPD. COPD. 2009;6(6):459–467. doi: 10.3109/15412550903341513. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa E, Nakano Y, Ohara T, et al. Body mass index in male patients with COPD: correlation with low attenuation areas on CT. Thorax. 2009;64(1):20–25. doi: 10.1136/thx.2008.097543. [DOI] [PubMed] [Google Scholar]
- 18.Camp PG, Coxson HO, Levy RD, et al. Sex differences in emphysema and airway disease in smokers. Chest. 2009;136(6):1480–1488. doi: 10.1378/chest.09-0676. [DOI] [PubMed] [Google Scholar]
- 19.Bon JM, Leader JK, Weissfeld JL, et al. The influence of radiographic phenotype and smoking status on peripheral blood biomarker patterns in chronic obstructive pulmonary disease. PLoS ONE. 2009;4(8):e6865. doi: 10.1371/journal.pone.0006865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coxson HO. Quantitative computed tomography assessment of airway wall dimensions: current status and potential applications for phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5(9):940–945. doi: 10.1513/pats.200806-057QC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coxson HO, Lam S. Quantitative assessment of the airway wall using computed tomography and optical coherence tomography. Proc Am Thorac Soc. 2009;6(5):439–443. doi: 10.1513/pats.200904-015AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brusasco V, Crapo R, Viegi G. American Thoracic Society European Respiratory Society Coming together: the ATS/ERS consensus on clinical pulmonary function testing. Eur Respir J. 2005;26(1):1–2. doi: 10.1183/09031936.05.00034205. [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Crapo R, Hankinson J, et al. ATS/ERS Task Force General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 26.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 27.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 28.Guyatt G. Measuring health status in chronic airflow limitation. Eur Respir J. 1988;1(6):560–564. [PubMed] [Google Scholar]
- 29.Brightling CE, Monterio W, Green RH, et al. Induced sputum and other outcome measures in chronic obstructive pulmonary disease: safety and repeatability. Respir Med. 2001;95(12):999–1002. doi: 10.1053/rmed.2001.1195. [DOI] [PubMed] [Google Scholar]
- 30.Bailey KV, Ferro-Luzzi A. Use of body mass index of adults in assessing individual and community nutritional status. Bull World Health Organ. 1995;73(5):673–680. [PMC free article] [PubMed] [Google Scholar]
- 31.Health Protection Agency Investigation of bronchoalveolar lavage, sputum and associated specimens. BSOP 57. 2009:3. [Google Scholar]
- 32.Pye A, Stockley RA, Hill SL. Simple method for quantifying viable bacterial numbers in sputum. J Clin Pathol. 1995;48(8):719–724. doi: 10.1136/jcp.48.8.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pizzichini E, Pizzichini MM, Efthimiadis A, et al. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996;154(2 Pt 1):308–317. doi: 10.1164/ajrccm.154.2.8756799. [DOI] [PubMed] [Google Scholar]
- 34.Pizzichini MM, Popov TA, Efthimiadis A, et al. Spontaneous and induced sputum to measure indices of airway inflammation in asthma. Am J Respir Crit Care Med. 1996;154(4 Pt 1):866–869. doi: 10.1164/ajrccm.154.4.8887576. [DOI] [PubMed] [Google Scholar]
- 35.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 36.Gupta S, Siddiqui S, Haldar P, et al. Qualitative analysis of high-resolution CT scans in severe asthma. Chest. 2009;136(6):1521–1528. doi: 10.1378/chest.09-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moss M, Wellman DA, Cotsonis GA. An appraisal of multivariable logistic models in the pulmonary and critical care literature. Chest. 2003;123(3):923–928. doi: 10.1378/chest.123.3.923. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55(8):635–642. doi: 10.1136/thorax.55.8.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujimoto K, Kitaguchi Y, Kubo K, Honda T. Clinical analysis of chronic obstructive pulmonary disease phenotypes classified using high-resolution computed tomography. Respirology. 2006;11(6):731–740. doi: 10.1111/j.1440-1843.2006.00930.x. [DOI] [PubMed] [Google Scholar]
- 40.Kitaguchi Y, Fujimoto K, Kubo K, Honda T. Characteristics of COPD phenotypes classified according to the findings of HRCT. Respir Med. 2006;100(10):1742–1752. doi: 10.1016/j.rmed.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Simpson JL, Milne DG, Gibson PG. Neutrophilic asthma has different radiographic features to COPD and smokers. Respir Med. 2009;103(6):881–887. doi: 10.1016/j.rmed.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Miller M, Ramsdell J, Friedman PJ, Cho JY, Renvall M, Broide DH. Computed tomographic scan-diagnosed chronic obstructive pulmonary disease-emphysema: eotaxin-1 is associated with bronchodilator response and extent of emphysema. J Allergy Clin Immunol. 2007;120(5):1118–1125. doi: 10.1016/j.jaci.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 43.Kurashima K, Takayanagi N, Sato N, et al. High resolution CT and bronchial reversibility test for diagnosing COPD. Respirology. 2005;10(3):316–322. doi: 10.1111/j.1440-1843.2005.00701.x. [DOI] [PubMed] [Google Scholar]
- 44.Patel IS, Vlahos I, Wilkinson TM, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(4):400–407. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]