Abstract
Background:
Never smokers comprise a substantial proportion of patients with COPD. Their characteristics and possible risk factors in this population are not yet well defined.
Methods:
We analyzed data from 14 countries that participated in the international, population-based Burden of Obstructive Lung Disease (BOLD) study. Participants were aged ≥ 40 years and completed postbronchodilator spirometry testing plus questionnaires about respiratory symptoms, health status, and exposure to COPD risk factors. A diagnosis of COPD was based on the postbronchodilator FEV1/FVC ratio, according to current GOLD (Global Initiative for Obstructive Lung Disease) guidelines. In addition to this, the lower limit of normal (LLN) was evaluated as an alternative threshold for the FEV1/FVC ratio.
Results:
Among 4,291 never smokers, 6.6% met criteria for mild (GOLD stage I) COPD, and 5.6% met criteria for moderate to very severe (GOLD stage II+) COPD. Although never smokers were less likely to have COPD and had less severe COPD than ever smokers, never smokers nonetheless comprised 23.3% (240/1,031) of those classified with GOLD stage II+ COPD. This proportion was similar, 20.5% (171/832), even when the LLN was used as a threshold for the FEV1/FVC ratio. Predictors of COPD in never smokers include age, education, occupational exposure, childhood respiratory diseases, and BMI alterations.
Conclusion:
This multicenter international study confirms previous evidence that never smokers comprise a substantial proportion of individuals with COPD. Our data suggest that, in addition to increased age, a prior diagnosis of asthma and, among women, lower education levels are associated with an increased risk for COPD among never smokers.
COPD is an important and increasing cause of morbidity and mortality worldwide. COPD is projected to rank third among all causes of death by 2020,1 yet its impact is underestimated by health and government officials. The term “COPD” has little public recognition, and a clear connection has not been made to most of its diverse risk factors.
Although cigarette smoke is widely acknowledged as the single most important risk factor for COPD, it is now recognized that never smokers may account for between one-fourth and one-third of all COPD cases.2‐5 A recent review of existing data supports the notion that the burden of nonsmoking COPD is much higher than supposed in both developing and developed countries.6
In the Obstructive Lung Disease in North Sweden (OLIN) study, Lundback et al7 found that smokers accounted for only 45% of COPD cases among adults aged 46-77 years. Thus, other causative factors must be responsible for the remaining COPD burden, and identification of these factors will be helpful for understanding the disease in never smokers. We analyzed data from 14 countries from the international Burden of Obstructive Lung Disease (BOLD) study8 to describe characteristics of COPD in never smokers and to identify possible risk factors in this population.
Materials and Methods
Study Design and Participants
The design and rationale for the BOLD initiative and preliminary prevalence data have been published.8,9 Population-based sampling plans were used for the recruitment of participants for all study sites. As of April 2008, 14 sites had completed data collection and are included in this analysis: Guangzhou (China), Adana (Turkey), Salzburg (Austria), Cape Town (South Africa), Reykjavik (Iceland), Hannover (Germany), Krakow (Poland), Bergen (Norway), Vancouver, British Columbia (Canada), Lexington, Kentucky (United States), Manila (Philippines), Sydney, New South Wales (Australia), London (England), and Uppsala (Sweden). Sampling designs and participant response and cooperation rates for each site have previously been described.8
Each participating site aimed to recruit a population-based sample of at least 600 adults (300 men and 300 women) who were not institutionalized, were aged ≥ 40 years, and were living in a well defined administrative area in which the total population exceeded 150,000. Approval was obtained from each local ethics committee, and written informed consent was obtained from each participant.
The study recorded questionnaire data on respiratory symptoms, health status, and exposure to risk factors for COPD. All participants included in this analysis performed prebronchodilator and postbronchodilator spirometry.
Spirometry Testing
Spirometry was performed according to American Thoracic Society (ATS) criteria10 by trained and certified technicians using the ndd EasyOne spirometer (ndd Medical Technologies; Zurich, Switzerland) with participants in a seated position. Separate measurements were made before and at least 15 min after two puffs of salbutamol (200 μg) administered with a metered dose inhaler with Volumatic spacer (GlaxoSmithKline; Uxbridge, England). Spirometry data were sent electronically to the Pulmonary Function Quality Control Center in Salt Lake City, Utah, where each spirogram was reviewed and graded using ATS guidelines.10
Studies were considered satisfactory if they met ATS acceptability and reproducibility criteria: at least three trials, with two acceptable and reproducible tests for both the FEV1 and FVC. Acceptable trials were defined as those free from artifact, sudden stops, and back-extrapolated volumes greater than 5.0% of FVC. Trials were considered reproducible if the difference between the largest and second-largest values was < 200 mL. Study technicians were continuously monitored. If a technician’s quality score dropped below a preset level, he/she had to stop testing and be retrained and recertified.
Questionnaire Data
Questionnaire data were obtained by face-to-face interviews conducted by trained and certified staff in the participant’s native language. The questionnaire was translated from English into the study-site language and then back-translated to assure accuracy. A core questionnaire, based on standardized instruments,9 was completed for all participants and included information on respiratory symptoms, risk factors for COPD, health status, comorbidities, respiratory diagnoses, and limitation of activity. Data on education were used as surrogates for socioeconomic status.
Definitions
We defined irreversible airway obstruction as a postbronchodilator FEV1/FVC < 0.7 in accordance with the GOLD (Global Initiative for Obstructive Lung Disease) guidelines and used FEV1 to further stage the disease: FEV1 < 80% predicted served as the threshold for GOLD stage II COPD, and an FEV1 < 50% predicted served as the threshold for GOLD stage III or higher. We used the NHANES (National Health and Nutrition Examination Survey) III reference equations for white men and women to calculate predicted values.11 Following the traditional practice of considering irreversible airway obstruction to be COPD, the COPD diagnosis was strictly based on the postbronchodilator lung function criteria without requiring documented exposure to a known causative agent. Unobstructed airways were defined as a postbronchodilator FEV1/FVC ratio ≥ 0.7.12
In addition, the lower limit of normal (LLN) threshold was evaluated as an alternative to the fixed ratio threshold for defining COPD. The LLN is defined as the lower fifth percentile for predicted FEV1/FVC (ie, predicted FEV1/FVC − 1.645 × SD) based on the NHANES III reference equations.11 We further defined doctor-diagnosed chronic obstructive airway disease as a self-reported physician’s diagnosis of chronic bronchitis, emphysema, or COPD.
An ever smoker (current or former) was defined as a person who had smoked > 20 packs of cigarettes in a lifetime or > 1 cigarette/d for a year. Exposure to passive cigarette smoke was defined as an affirmative answer to whether anyone (other than the participant) had smoked a cigarette, pipe, or cigar in the participant’s home during the past 2 weeks.
To assess occupational exposure, participants were asked whether they had worked ≥ 3 months in occupations known or suspected to be associated with the risk of COPD and, if so, the number of years spent in each occupation. Occupational exposures were grouped into three categories: (1) organic dust (through farming; flour-, feed-, or grain-milling; cotton- or jute-processing; forestry- or wood-milling; and fish-processing); (2) inorganic dust (through asbestos; aluminum, coal, or hard-rock mining; tunneling, foundry, or steel-milling; and sandblasting); (3) irritant gases, fumes, or vapors (through welding, fire fighting, chemical or plastic manufacturing, public transportation, and dry-cleaning chemicals).
Four measures of biomass exposure were based on self-reported responses indicating whether participants had experienced at least 6 months’ use of indoor fire for (1) cooking using coal or coke; (2) cooking using wood, crop residues, or dung; (3) heating using coal or coke; and (4) heating using wood, crop residues, or dung. Participants also reported the number of years of exposure for each category.
Additional measures evaluated included BMI (kg/m2); total number of years of education; self-reported hospitalization for breathing problems prior to the age of 10 years; self-reported respiratory symptoms for cough, phlegm, wheezing, and dyspnea; and self-reported physician-diagnosed asthma, COPD, chronic bronchitis, emphysema, TB, heart disease, hypertension, diabetes, or stroke.
Health status measures included two indicators of participants who (1) responded “excellent” or “very good” (vs “good,” “fair,” or “poor”) when asked to rate their general health, and (2) responded “none of the time” or “a little of the time” (vs “some,” “most,” or “all” of the time) when asked how much of the time they experienced limitations in work or other activities “as a result of your physical health.”
Statistical Analysis
Our analysis includes BOLD participants who completed the primary study questionnaire and had acceptable postbronchodilator spirometry measures. Bivariate comparisons were performed using the Wilcoxon rank sum test to compare continuous measures across groups and χ2 tests to compare categorical measures. Logistic regression models were fitted separately for never smoker men and women to evaluate associations with GOLD stage II or higher relative to (ie, FEV1/FVC ≥ 0.7) never smokers with unobstructed airways. Covariates included in the model were specified a priori. Because of the uncertain clinical relevance of GOLD stage I COPD, data for this group are presented separately from those for GOLD stages II+ (moderate to very severe airway obstruction), and most analyses focus on comparing the latter group to the unobstructed-airways group. Models included age category (40-49, 50-59, 60-69, 70-79, and 80+ years); years of school; BMI category (< 18.5, 18.5-24.9, 25-29.9, 30-34.9, ≥ 35 kg/m2); passive-smoking exposure (yes/no [y/n]); hospitalization for breathing problems as a child (y/n); self-reported physician-diagnosed conditions (y/n) including asthma, TB, cardiovascular disease, or diabetes; and indicators (y/n) for ≥ 10 years of exposure to cooking biomass, heating biomass, occupational organic dusts, occupational inorganic dusts, and occupational gases, vapors, or fumes. The model specified robust variance estimators for clustered data to account for correlation of observations within site (or of groups within site, as defined by the site sampling plan).13 Estimated ORs, 95% CIs, and P values are reported. A P value of 0.05 was considered statistically significant. No adjustments for multiple comparisons were made. Analyses were performed using SAS, version 9 (SAS Institute Inc; Cary, North Carolina) and Stata, version 9.2 (Stata Corp, College Station, Texas).
Results
A total of 10,000 subjects completed questionnaires, had acceptable postbronchodilator spirometry data, and had information on smoking status. Of this group, 4,291 (42.9%) were never smokers. Women made up 65.9% of never smokers and 42.2% of ever smokers. Among 5,709 ever smokers, 2,497 (43.7%) were current and 3,212 (56.3%) were former smokers. Characteristics of the study population are summarized in Table 1.
Table 1.
—Population Characteristics for Never Smokers and Ever Smokers
| Characteristic | Never Smokers (n = 4,291) | Ever Smokers (n = 5,709) |
| Men, No. (%) | 1,464 (34.1) | 3,302 (57.8) |
| Age, mean (SE), y | 56.2 (11.7) | 56.3 (11.2) |
| Age category,a No. (%) | ||
| 40-49 | 536 (36.6) | 1,100 (33.3) |
| 50-59 | 387 (26.4) | 1,004 (30.4) |
| 60-69 | 322 (22.0) | 709 (21.5) |
| 70-79 | 170 (11.6) | 399 (12.1) |
| 80+ | 49 (3.4) | 90 (2.7) |
| BMI, mean (SE)b kg/m2 | 27.3 (4.3) | 26.7 (4.6) |
| Education, mean (SE),b y | 12.2 (4.4) | 10.6 (4.1) |
| Smoking, > 20 pack-years | … | 1,764 (53.5) |
| Lung functionb | ||
| FEV1, mean (SD) | 3.46 (0.81) | 3.09 (0.87) |
| FVC, mean (SD) | 4.47 (0.97) | 4.17 (1.0) |
| FEV1/FVC, mean (SD) | 77.38 (6.78) | 73.72 (10.06) |
| FEV1/FVC ≥ 0.7 | 1,273 (87.0) | 2,421 (73.3) |
| FEV1/FVC < 0.7 and FEV1 ≥ 80% predicted (GOLD stage I) | 121 (8.3) | 383 (11.6) |
| FEV1/FVC < 0.7 and FEV1 < 80% predicted (GOLD stage II+) | 70 (4.8) | 498 (15.1) |
| FEV1/FVC < LLN (NHANES fifth percentile) | 87 (5.9) | 557 (16.9) |
| FEV1/FVC < LLN (NHANES fifth percentile) and FEV1 < 80% predicted | 41 (2.8) | 393 (11.9) |
| Women, No. (%) | 2,827 (65.9) | 2,404 (42.2) |
| Age, mean (SE),b y | 57.6 (12.0) | 55.3 (11.0) |
| Age category, No. (%)b | ||
| 40-49 | 876 (31.0) | 868 (36.1) |
| 50-59 | 805 (28.5) | 772 (32.1) |
| 60-69 | 653 (23.1) | 488 (20.3) |
| 70-79 | 366 (13.0) | 199 (8.3) |
| 80+ | 127 (4.5) | 80 (3.2) |
| BMI, mean (SE)a kg/m2 | 27.5 (5.9) | 27.2 (6.1) |
| Education, mean (SE),b y | 10.3 (7.3) | 11.0 (5.2) |
| Smoking, > 20 pack-years | … | 889 (36.9) |
| Lung functionb | ||
| FEV1, mean (SD) | 2.27 (0.61) | 2.36 (0.65) |
| FVC, mean (SD) | 2.90 (0.73) | 3.11 (0.76) |
| FEV1/FVC, mean (SD) | 78.30 (7.35) | 75.50 (9.52) |
| FEV1/FVC ≥ 0.7 | 2,495 (88.3) | 1,922 (79.9) |
| FEV1/FVC < 0.7 and FEV1 ≥ 80% predicted (GOLD stage I) | 162 (5.7) | 192 (8.0) |
| FEV1/FVC < 0.7 and FEV1 < 80% predicted (GOLD stage II+) | 170 (6.0) | 293 (12.2) |
| FEV1/FVC < LLN (NHANES fifth percentile) | 215 (7.6) | 423 (17.6) |
| FEV1/FVC < LLN (NHANES fifth percentile) and FEV1 < 80% predicted | 130 (4.6) | 267 (11.1) |
GOLD = Global Initiative for Chronic Obstructive Lung Disease; GOLD stage II+ = GOLD stages II, III, and IV; LLN = lower limit of normal; NHANES = National Health and Nutrition Examination Survey.
.001 ≤ P < 0.05 for χ test (for categorical data) or Wilcoxon rank sum test (for continuous data), comparing never smokers to ever smokers.
P < 0.001 for χ test (for categorical data) or Wilcoxon rank sum test (for continuous data), comparing never smokers to ever smokers.
Overall among never smokers, 12.2% (523/4,291) fulfilled the criteria for GOLD stage I or higher, 6.6% (283/4,291) met the criteria for mild COPD (GOLD stage I), and 5.6% (240/4,291) met the criteria for moderate to very severe disease (GOLD stage II+). Never smokers made up 27.7% (523/1,889) of all COPD cases: 33.0% (283/858) of all GOLD stage I cases and 23.3% (240/1,031) of all GOLD stage II+ cases. Prevalence of GOLD stage II+ COPD (FEV1/FVC < 0.7 and FEV1 < 80% predicted) in never smokers (n = 4,291) by site, sex, and age group is shown in Table 2.
Table 2.
—Prevalence of GOLD Stage II+ COPD (FEV1/FVC < 0.7 and FEV1 < 80% Predicted) in Never Smokers (n = 4,291) by Site, Sex, and Age Group
| Site (Country) | Sex | 40-49 y | 50-59 y | 60-69 y | 70-79 y | 80+ y | All |
| Guangzhou (China) | Female | 2.1 | 1.6 | 7.8 | 15.4 | … | 4.0 |
| Male | 0.0 | 0.0 | 16.7 | 0.0 | 50.0 | 4.6 | |
| All | 1.8 | 1.3 | 8.8 | 10.0 | 50.0 | 4.1 | |
| Adana (Turkey) | Female | 1.9 | 3.5 | 14.5 | 10.3 | 0.0 | 5.9 |
| Male | 0.0 | 5.0 | 9.1 | 9.1 | 0.0 | 5.3 | |
| All | 1.6 | 3.8 | 13.1 | 10.0 | 0.0 | 5.8 | |
| Salzburg (Austria) | Female | 2.7 | 4.7 | 2.9 | 17.5 | 27.8 | 6.6 |
| Male | 0.0 | 4.4 | 5.3 | 14.3 | 0.0 | 4.4 | |
| All | 1.3 | 4.5 | 3.9 | 16.0 | 17.2 | 5.6 | |
| Cape Town (South Africa) | Female | 1.2 | 6.5 | 14.0 | 23.1 | 20.0 | 8.5 |
| Male | 4.2 | 0.0 | 0.0 | 0.0 | … | 2.0 | |
| All | 1.9 | 5.4 | 11.5 | 20.0 | 20.0 | 7.3 | |
| Reykjavik (Iceland) | Female | 0.0 | 0.0 | 15.4 | 11.5 | 36.4 | 7.9 |
| Male | 0.0 | 0.0 | 12.1 | 20.0 | 16.7 | 5.2 | |
| All | 0.0 | 0.0 | 13.6 | 14.6 | 29.4 | 6.4 | |
| Hannover (Germany) | Female | 0.0 | 2.5 | 2.0 | 5.3 | 12.5 | 3.0 |
| Male | 0.0 | 13.6 | 2.4 | 7.1 | … | 4.7 | |
| All | 0.0 | 6.5 | 2.2 | 5.8 | 12.5 | 3.7 | |
| Krakow (Poland) | Female | 2.8 | 5.9 | 16.3 | 15.4 | 25.0 | 10.9 |
| Male | 0.0 | 10.0 | 0.0 | 0.0 | 50.0 | 3.6 | |
| All | 1.5 | 6.8 | 14.0 | 12.9 | 30.0 | 8.9 | |
| Bergen (Norway) | Female | 0.0 | 0.0 | 2.9 | 0.0 | 22.7 | 4.3 |
| Male | 6.3 | 3.7 | 8.0 | 11.1 | 18.2 | 7.7 | |
| All | 3.7 | 1.7 | 5.1 | 2.6 | 21.2 | 5.7 | |
| Vancouver, British Columbia (Canada) | Female | 2.4 | 1.2 | 7.7 | 6.5 | 0.0 | 3.2 |
| Male | 0.0 | 5.9 | 11.8 | 8.3 | 0.0 | 4.1 | |
| All | 1.4 | 2.9 | 8.9 | 7.0 | 0.0 | 3.5 | |
| Lexington, Kentucky (United States) | Female | 0.0 | 0.0 | 12.1 | 18.8 | 0.0 | 5.0 |
| Male | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| All | 0.0 | 0.0 | 8.9 | 12.5 | 0.0 | 3.5 | |
| Manila (Philippines) | Female | 3.3 | 4.1 | 12.5 | 20.0 | 36.4 | 7.0 |
| Male | 8.1 | 6.3 | 0.0 | 33.3 | 50.0 | 9.5 | |
| All | 4.3 | 4.4 | 11.5 | 21.7 | 38.5 | 7.4 | |
| Sydney, New South Wales (Australia) | Female | 7.1 | 7.5 | 14.8 | 16.1 | 0.0 | 10.1 |
| Male | 2.6 | 3.3 | 0.0 | 4.6 | 50.0 | 4.2 | |
| All | 4.9 | 5.7 | 8.0 | 11.3 | 16.7 | 7.5 | |
| London (England) | Female | 2.5 | 0.0 | 4.1 | 9.5 | 33.3 | 5.2 |
| Male | 2.4 | 0.0 | 8.0 | 30.0 | 0.0 | 5.7 | |
| All | 2.5 | 0.0 | 5.4 | 16.1 | 23.1 | 5.4 | |
| Uppsala (Sweden) | Female | 0.0 | 2.8 | 0.0 | 5.3 | 33.3 | 2.4 |
| Male | 2.9 | 3.2 | 0.0 | 20.0 | 0.0 | 4.7 | |
| All | 1.5 | 3.0 | 0.0 | 11.8 | 14.3 | 3.4 |
Values are presented as %. See Table 1 for expansion of abbreviation.
When the LLN was used as a threshold for the FEV1/FVC ratio instead of the fixed ratio of 0.7, prevalence of COPD was lower in both never smokers and ever smokers. The prevalence of moderate to very severe airways obstruction (GOLD stage II+) decreased by 29% (5.6% vs 4.0%) in never smokers and by 17% (13.9% vs 11.6%) in ever smokers. When the LLN was used to define airways obstruction, the proportion of never smokers among all COPD cases (FEV1/FVC < LLN) and among moderate to severe COPD cases (FEV1/FVC < LLN and FEV1 < 80% predicted) were 23.6% (302/1,282) and 20.5% (171/832), respectively.
Among those with moderate to very severe airway obstruction (ie, GOLD stage II+), never smokers were significantly older than smokers (66.1 years vs 62.7 years, P < .001; data not shown) and were more likely to be women (70.8% vs 37.0%, P < .001; data not shown). The prevalence of reported doctor-diagnosed chronic airway disease was far lower than that determined by spirometry in both smokers and never smokers. Only 18.8% of never smokers with moderate to severe irreversible airway obstruction reported a previous physician’s diagnosis of COPD, emphysema, or chronic bronchitis, compared with 26.0% of ever smokers. Thus, 81.2% of never smokers with moderate to severe airway obstruction were undiagnosed.
COPD Prevalence by Age and Sex
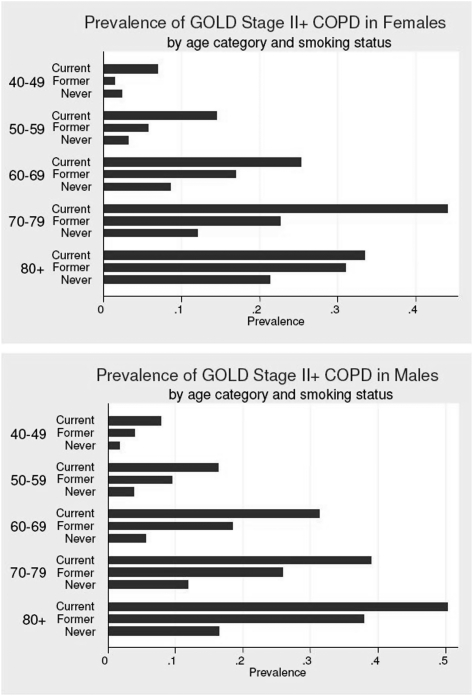
Prevalences of GOLD stage II and GOLD stage III+ by age, sex, and smoking status are shown in Figure 1. As expected, the prevalence of moderate to severe COPD increased with age in both ever and never smokers (P < .001). Among ever smokers, COPD prevalence was generally higher in men than women (17.1% vs 13.2%, P < .001); however, among never smokers the distributions of COPD by age were similar between men and women (5.2% vs 6.2%, P = .142).
Figure 1.
Prevalence of GOLD stage II+ COPD among participants by smoking status, sex, and age (y). GOLD = Global Initiative for Obstructive Lung Disease; GOLD stage II+ = GOLD stages II, III, and IV.
Among those with COPD GOLD stage II+ COPD, the proportion of moderate airway obstruction (GOLD stage II) was similar in never smokers and ever smokers (40.0% vs 43.8%, P = .134). However, severe (GOLD stage III) and very severe (GOLD stage IV) airway obstruction was significantly lower in never smokers (5.9% vs 14.1%, P < .001) (Fig 1).
Clinical Profile of COPD in Never Smokers
Never smokers with moderate to severe airway obstruction tended to be older, had less education, and reported roughly double the frequency of respiratory symptoms (cough, phlegm, wheeze, dyspnea) compared with never smokers with unobstructed airways. The former group also had higher frequencies of self-reported physician-diagnosed asthma, heart disease, TB, and hypertension and were more likely to have been hospitalized for breathing problems as a child and to have left a job due to breathing problems (Table 3). In addition, never smokers with moderate to severe airway obstruction reported more frequent exposure to indoor open fire with coal or coke for cooking (26.9% vs 19.7%), with 22.0% vs 15.3% reporting at least 10 years of exposure, and exposure to organic dusts in the workplace (30.4% vs 23.0%), with 19.3% vs 10.1% reporting at least 10 years of exposure (P < .05, all measures) (Table 2). The clinical profile of never smokers with mild airway obstruction (GOLD stage I) was generally similar to the profile of unaffected never smokers (Table 3).
Table 3.
—Characteristics, Respiratory Symptoms, Physician Diagnoses, and Risk Factors for Airway Obstruction Among Never Smokers (n = 4,291)
| Variable | FEV1/FVC ≥ 0.7 | GOLD stage I | GOLD stage II+ |
| Subjects, No. | 3,768 | 283 | 240 |
| Age, mean (SE), y | 55.7 (11.1) | 66.7 (12.5) | 66.1 (12.0)a |
| Women | 2,495 (66.2) | 162 (57.2) | 170 (70.8) |
| Education < 12 y | 966 (25.6) | 77 (27.2) | 91 (37.9)a |
| BMI, kg/m2, mean (SE) | 27.6 (5.5) | 27.1 (4.2) | 27.4 (6.0) |
| Respiratory symptoms | |||
| Cough | 650 (17.3) | 67 (23.7) | 78 (32.5)a |
| Phlegm | 631 (16.8) | 55 (19.4) | 82 (34.2)a |
| Wheezing | 571 (15.2) | 64 (22.6) | 91 (37.9)a |
| Dyspnea on exertion | 823 (21.8) | 58 (20.5) | 97 (40.4)a |
| Any of the above symptoms | 1,675 (44.5) | 140 (49.5) | 174 (72.5)a |
| Physician diagnosis, ever | |||
| Asthma | 371 (9.9) | 50 (17.7) | 73 (30.4)a |
| COPD | 18 (0.5) | 1 (0.4) | 9 (3.8)a |
| Chronic bronchitis | 105 (2.8) | 14 (5.0) | 33 (13.8)a |
| Emphysema | 27 (0.7) | 10 (3.5) | 15 (6.3)a |
| Any of the above diagnoses | 451 (12.0) | 63 (22.3) | 98 (40.8)a |
| TB | 108 (2.9) | 11 (3.9) | 17 (7.1)a |
| Heart disease | 422 (11.2) | 60 (21.2) | 51 (21.3)a |
| Hypertension | 1,198 (31.8) | 108 (38.2) | 119 (49.6)a |
| Diabetes | 283 (7.5) | 18 (6.4) | 24 (10.0) |
| Stroke | 89 (2.4) | 13 (4.6) | 7 (2.9) |
| Hospitalized for breathing problems prior to the age of 10 y | 111 (3.0) | 5 (1.8) | 15 (6.3)b |
| Worked in any dusty job > 1 y | 1,180 (31.3) | 87 (30.7) | 76 (31.7) |
| Ever had to leave a job due to breathing problems | 83 (2.2) | 5 (1.8) | 13 (5.5)b |
| Exposure to | |||
| Passive smoking at home | 792 (21.0) | 47 (16.6) | 53 (22.1) |
| Indoor open fire with coal/coke for cookingc | 566 (19.7) | 41 (20.4) | 50 (26.9)b |
| ≥ 10 y exposure, cooking with coal/coke | 439 (15.3) | 31 (15.4) | 41 (22.0)b |
| Indoor open fire with coal/coke for heatingc | 532 (18.5) | 51 (25.4) | 39 (21.0) |
| ≥ 10 y exposure, heating with coal/coke | 327 (11.4) | 40 (19.9) | 27 (14.5) |
| Indoor open fire with wood/crop/dung for cookingc | 818 (28.4) | 42 (20.9) | 57 (30.7) |
| ≥ 10 y exposure, cooking with wood/crop/dung | 564 (19.6) | 28 (13.9) | 43 (23.1) |
| Indoor open fire with wood/crop/dung for heatingc | 563 (19.6) | 37 (18.4) | 38 (20.4) |
| ≥ 10 y exposure, heating with wood/crop/dung | 436 (15.1) | 32 (15.9) | 30 (16.1) |
| Biologic/organic dusts at the workplace | 867 (23.0) | 80 (28.3) | 73 (30.4)b |
| ≥ 10 y exposure, biologic/organic dusts | 378 (10.1) | 39 (13.8) | 46 (19.3)a |
| Inorganic dusts at the workplace | 217 (5.8) | 28 (9.9) | 11 (4.6) |
| ≥ 10 y exposure, inorganic dusts | 62 (1.7) | 11 (3.9) | 3 (1.3) |
| Irritant gases, fumes, or vapors at the workplace | 326 (8.7) | 25 (8.8) | 15 (6.3) |
| ≥ 10+ y exposure, gases, fumes or vapors | 141 (3.8) | 10 (3.6) | 12 (5.0) |
Values presented as No. (%) unless otherwise noted.
P < .001 for χ test (for categorical data) or Wilcoxon rank sum test (for continuous data) comparing the unobstructed-airways group (FEV1/FVC ≥ 0.7) with the GOLD stage II+ group.
.001 ≤ P < 0.05 for χ test comparing the unobstructed-airways group (FEV1/FVC ≥ 0.7) with the COPD II+ group.
Three sites, Hannover (Germany), Uppsala (Sweden), and Bergen (Norway), did not collect information on biomass exposures, thus n = 2,879, 201, and 186 for the FEV1/FVC ≥ 0.7, GOLD stage I, and GOLD stage II+ groups, respectively.
Factors Associated With COPD in Never Smokers
Complete data were available on 2,578 women (159 with GOLD stage II+) and 1,311 men (67 with GOLD stage II+) and were used in logistic regression models. Among men and women never smokers, our analyses showed a strong association between increasing age and increasing odds of GOLD stage II+ COPD (Table 4). A strong association was also noted in both men and women for self-reported, physician-diagnosed asthma.
Table 4.
—Independent Predictors of GOLD Stage II+ COPD in Never Smokers: Multivariate Logistic Model
| Women ( n = 2,578) |
Men (n = 1,311) |
|||||
| Variable | OR | P Value | 95% CI | OR | P Value | 95% CI |
| Age, y | ||||||
| 40-49 | Reference | Reference | ||||
| 50-59 | 1.37 | .311 | 0.75-2.52 | 2.71 | .023 | 1.14-6.38 |
| 60-69 | 4.31 | < .001 | 2.50-7.44 | 4.88 | < .001 | 2.03-11.70 |
| 70-79 | 6.15 | < .001 | 3.31-11.44 | 10.85 | < .001 | 4.32-27.22 |
| 80+ | 12.97 | < .001 | 6.56-25.65 | 29.02 | < .001 | 8.56-98.39 |
| Education, y | ||||||
| 1-y increase | 0.94 | .005 | 0.90-0.98 | 0.99 | .700 | 0.93-1.05 |
| ≥ 10 y exposure in high-risk occupation | ||||||
| Organic dust | 1.96 | .007 | 1.20-3.20 | 2.18 | .054 | 0.99-4.80 |
| Inorganic dusta | … | … | … | 0.72 | .617 | 0.19-2.65 |
| Gases/vapors | 0.65 | .506 | 0.18-2.32 | 1.54 | .270 | 0.71-3.34 |
| Biomass fuel | ||||||
| ≥ 10 y cooking | 1.03 | .899 | 0.67-1.57 | 1.39 | .514 | 0.52-3.75 |
| ≥ 10 y heating | 0.81 | .386 | 0.50-1.31 | 0.55 | .255 | 0.20-1.53 |
| Passive smoking | ||||||
| Exposed | 1.53 | .064 | 0.98-2.41 | 0.97 | .954 | 0.40-2.40 |
| Childhood hospitalization | ||||||
| Yes | 2.21 | .087 | 0.89-5.47 | 2.82 | .065 | 0.94-8.51 |
| Comorbidities, diagnosis | ||||||
| HD/HT/DM | 1.13 | .499 | 0.79-1.63 | 1.28 | .433 | 0.69-2.36 |
| Asthma | 4.62 | < .001 | 3.04-7.02 | 4.12 | < .001 | 2.06-8.26 |
| TB | 1.47 | .323 | 0.69-3.12 | 1.65 | .464 | 0.43-6.34 |
| BMI, kg/m2 | ||||||
| BMI < 20 | 2.56 | .002 | 1.40-4.71 | 13.39 | < .001 | 3.67-48.84 |
| ≥ 20 BMI < 25 | Reference | Reference | ||||
| ≥ 25 BMI < 30 | 0.74 | .182 | 0.48-1.15 | 1.28 | .518 | 0.61-2.67 |
| ≥ 30 BMI < 35 | 0.65 | .130 | 0.37-1.14 | 2.19 | .066 | 0.95-5.05 |
| BMI ≥ 35 | 0.62 | .147 | 0.33-1.18 | 3.19 | .030 | 1.11-9.11 |
DM = diabetes mellitus; HD = heart disease; HT = hypertension.
This term was excluded from the model for women as there were only 11 women with the exposure, none of whom had GOLD stage II+ COPD, making the OR unestimable.
OR estimates for hospitalization related to breathing problems as a child were fairly similar in men and women (2.82 and 2.21, respectively); however, neither quite reached statistical significance. The OR estimates for exposure to organic dust were also similar for men (OR = 2.18, P = .054) and women (OR = 1.96, P = .007) but reached statistical significance only in women.
Among women, each additional year of education decreased odds of GOLD stage II+ COPD by about 6% (OR = 0.94, P = .005), and the OR for exposure to passive smoking was 1.53, which did not reach statistical significance (P = .064). In men, the ORs for both these measures were close to 1.00 (P > .69). Among both men and women, BMI < 20 kg/m2 was associated with increased odds of GOLD stage II+ compared with those in the normal range (20-25 kg/m2), and the OR estimate for men was about five times that for women (13.39 vs 2.56).
When these models were adjusted for location, the results were generally similar, although among women the relationship of passive smoking with GOLD stage II+ was attenuated (OR = 1.28, P = .292), whereas the associations for the two highest BMI categories were strengthened (OR = 0.56, P = .044 for BMI 30-35 kg/m2 and OR = 0.51, P = .051 for BMI > 35 kg/m2). Compared with the Hannover site (the site with the lowest overall prevalence of GOLD stage II+ among those studied), never-smoker women from the following sites were most likely to experience increased risk for GOLD stage II+: Cape Town (OR = 4.63, P = .005), Salzburg (OR = 2.75, P = .057), Krakow (OR = 5.70, P = .010), Sydney, Australia (OR = 4.50, P = .010) and Manila (OR = 3.85, P = .010). When location was included in the model for men, the association of GOLD stage II+ with childhood hospitalization for breathing problems was strengthened (OR = 4.55, P = .005), as were associations for those with BMI between 30 and 35 kg/m2 (OR = 2.72, P = .037) and BMI > 35 kg/m2 (OR = 5.28, P = .004) compared with those in the normal range. The largest estimated site OR, relative to Hannover, was for Manila (OR = 3.27, P = .061).
Results of these multivariate analyses did not change markedly for most measures when LLN was used as a threshold for the FEV1/FVC ratio instead of the fixed ratio (Table 5). However, a clear difference was seen in the age association in women. OR estimates for the three age groups 60+ years were smaller in the LLN regression and only remained statistically significant for the oldest age group (80+ years). The OR estimates for organic dust increased from 1.96 (P = .007) to 2.60 (P < .001) in women but were not changed markedly in men. The effect seen for childhood hospitalization was slightly stronger in women in the LLN model (OR 2.21, P = .087 vs OR 2.42, P = .043) and substantially weaker in men (OR 2.82, P = .065 vs OR 1.30, P = .711). The prevalence of the latter two measures were low; hence substantial fluctuations in their OR estimates are coherent.
Table 5.
—Independent Predictors of GOLD Stage II+ COPD (LLN) in Never Smokers (Multivariate Logistic Model)
| Women (n = 2,578) |
Men (n = 1,311) |
|||||
| Variable | OR | P Value | 95% CI | OR | P Value | 95% CI |
| Age, y | ||||||
| 40-49 | Reference | Reference | ||||
| 50-59 | 0.66 | .151 | 0.38-1.16 | 2.63 | .050 | 1.00-6.97 |
| 60-69 | 1.60 | .076 | 0.96-2.69 | 3.57 | .022 | 1.21-10.57 |
| 70-79 | 1.43 | .276 | 0.75-2.74 | 11.47 | < .001 | 3.93-33.52 |
| 80+ | 3.24 | .001 | 1.57-6.71 | 4.20 | .185 | 0.50-35.17 |
| Education, y | ||||||
| 1 y increase | 0.93 | .004 | 0.89-0.98 | 1.03 | .340 | 0.96-1.12 |
| ≥ 10 y exposure in high-risk occupation | ||||||
| Organic dust | 2.60 | < .001 | 1.56-4.35 | 2.60 | .074 | 0.91-7.46 |
| Inorganic dusta | … | … | … | 0.47 | .453 | 0.07-3.30 |
| Gases/vapors | 0.44 | .369 | 0.07-2.64 | 1.44 | .501 | 0.50-4.16 |
| Biomass fuel | ||||||
| ≥ 10 y cooking | 0.81 | .386 | 0.50-1.30 | 0.85 | .838 | 0.19-3.86 |
| ≥ 10 y heating | 0.81 | .448 | 0.48-1.39 | 0.41 | .225 | 0.10-1.73 |
| Passive smoking | ||||||
| Exposed | 1.04 | .873 | 0.65-1.66 | 1.13 | .826 | 0.39-3.30 |
| Childhood hospitalization | ||||||
| Yes | 2.42 | .043 | 1.03-5.74 | 1.30 | .711 | 0.32-5.21 |
| Comorbidities, diagnosis | ||||||
| HD/HT/DM | 1.24 | .279 | 0.84-1.81 | 0.74 | .478 | 0.32-1.69 |
| Asthma | 4.60 | < .001 | 3.01-7.02 | 5.00 | < .001 | 2.29-10.95 |
| TB | 1.29 | .584 | 0.52-3.23 | 3.09 | .177 | 0.60-15.95 |
| BMI kg/m2 | ||||||
| BMI < 20 | 2.06 | .042 | 1.03-4.15 | 6.85 | .024 | 1.29-36.38 |
| ≥ 20 BMI < 25 | Reference | Reference | ||||
| ≥ 25 BMI < 30 | 0.76 | .253 | 0.48-1.21 | 1.44 | .438 | 0.57-3.62 |
| ≥ 30 BMI < 35 | 0.51 | .033 | 0.28-0.95 | 2.15 | .175 | 0.71-6.53 |
| BMI ≥ 35 | 0.75 | .356 | 0.41-1.38 | 5.71 | .004 | 1.76-19 |
Discussion
There were three main findings in this study: (1) This multicenter, international study confirms previous evidence that never smokers are a substantial proportion of individuals with COPD and that they are usually not diagnosed with the disease. (2) More than two-thirds of never smokers with moderate to severe airway obstruction are women. (3) Predictors of COPD in never smokers include age, education, occupational exposure, childhood respiratory diseases, and BMI alterations.
This analysis of trial-wide BOLD data shows that 28% of irreversible airways obstruction—about 33% of mild airway obstruction (GOLD stage I) and about 23% of moderate to very severe airway obstruction (GOLD stage II+)—occurs in never smokers aged 40 to 98 years. This finding is consistent with an analysis of (prebronchodilator) NHANES III data in US adults (aged 18 to 80 years), which showed that about one-fourth of COPD cases occurred in subjects with no smoking history.3 A study in an older population in China has shown that 38.6% of subjects with COPD had never smoked.14 COPD does not develop suddenly, but rather exposure to risk factors over a considerable period of time. Thus, prevalence of COPD increases with age in both smokers and never smokers.12
In our sample, more than two-thirds of never smokers with moderate to severe airway obstruction were women. These results agree with results from a population-based study in Spain15 and recently published results from the Swiss Study on Air Pollution and Lung Disease in Adults (SAPALDIA) cohort study.5 Studies have suggested that women are more susceptible to the effects of tobacco smoke,16‐18 and this susceptibility may also apply to other harmful exposures. Moreover, the presence of chronic airway obstruction in never smokers raises the question of whether there is an autoimmune component to COPD pathogenesis.19,20 As most autoimmune diseases occur more frequently in women than men, the autoimmune hypothesis is worth considering as a contributor to the predominance of females among never smokers with COPD.
In our study population, 81.2% of never smokers with moderate to severe airway obstruction were previously undiagnosed. This clearly reflects the lack of recognition and underdiagnosis of obstructive lung disease among never smokers, possibly as a result of little knowledge on this condition and poor understanding and appreciation of risk factors other than smoking.
Risk Factors for COPD in Never Smokers
There is evidence that a substantial proportion of COPD, up to 20%, can be attributed to occupational exposures.21 The ATS concluded that occupational exposures account for 10% to 20% of both symptoms and functional impairment consistent with COPD.22 In an analysis of NHANES III data, the fraction of COPD in never smokers that is attributable to work has been estimated to be 31%.23 In our data, never smokers with moderate to severe COPD reported exposure to organic dusts in the workplace more often than did never smokers with unobstructed airways (30.4% vs 23%). There is some evidence that the airway response to organic dust inhalation is primarily mediated by nonallergic inflammatory mechanisms.24,25 Grain dust, for example, can cause recruitment of neutrophils to the proximal and distal airways.26,27 The results of our study suggest an increased risk of COPD in women after occupational exposure to organic dusts for ≥ 10 years. Similar effects were not seen for inorganic dusts and gases, vapors, and fumes; however, the numbers of participants with these exposures was relatively small in this never-smoker population.
Biomass fuels used by women for cooking and heating have been shown to cause COPD in nonsmoking women.28‐32 In our data, exposure to indoor open fire (with coal or coke) for cooking was bivariately associated with COPD in never smokers, for whom clinical characteristics, impairment in quality of life, and increase in mortality are similar to tobacco smokers.33 However, we did not find statistically significant associations of at least 10 years of reported exposure to heating or cooking biomass in the logistic regressions.
There is evidence that exposure to environmental tobacco smoke is associated with COPD34‐36 and affects women more often than men.37,38 In our study, we did not observe an increased risk of GOLD stage II+ COPD associated with exposure to passive smoking. However, the BOLD questionnaire only assessed current exposure to passive smoking at home within the last 2 weeks and did not consider earlier exposures to passive smoking or to passive smoking in the workplace.
Low socioeconomic status,39 low level of education,40 and severe childhood respiratory infections41,42 have been shown to be associated with a higher prevalence of COPD. In our analysis, more years of education was associated with lower odds of spirometrically determined COPD among female never smokers. Likewise, severe childhood respiratory infections or other breathing problems (leading to hospitalization) and COPD were associated in never smokers.
Pulmonary TB is a frequent cause of chronic pulmonary function impairment, particularly airflow obstruction,43,44 and constitutes an important differential diagnosis to COPD in high-prevalence areas, especially in the absence of other typical risk factors for COPD.12 Our data show that a significantly higher prevalence of reported TB was present among never smokers with airway obstruction than never smokers without obstruction. However, it did not appear as a significant independent predictor in the multivariable logistic model, possibly because of the widely differing prevalences of TB in the BOLD study sites.
COPD can be associated with a progressive loss of skeletal muscle mass; low BMI is an independent predictor of the risk of death.45 In our study, increased odds of GOLD stage were seen in ≥ 10 men and women from the never-smoker population with low BMI (< 20 kg/m2). However, the OR estimate for men was about five times higher than for women (13.37 vs 2.57). In contrast, a high BMI (> 35 kg/m2) was associated with increased odds of GOLD stage II+ only in men. Overall, the association between BMI alterations and the presence of COPD was much more pronounced in men than women from the never-smoker group. It has been shown that a diet rich in meat and refined grains may increase the risk of COPD, whereas a diet with higher intakes of vegetables, fruits, and fish may reduce this risk.46,47 Given the cross-sectional nature of our data, we cannot discriminate whether low BMI precedes COPD or is rather a consequence of the disease.
Limitations
Reference equations for spirometric variables are not available for many parts of the world. Even if country-specific or race-specific reference equations were available for all sites, their use might have drawbacks, such as masking true differences related to exposure between countries or between racial groups within countries. BOLD uses the widely accepted prediction equations derived from the third US NHANES,11 which may not ideally fit for all studied populations. In addition to this, “self-stated race” has been shown to result in more misclassifications of the severity of impairment of lung function.48
The use of a fixed threshold to define airways obstruction is associated with some extent of misclassification.49 The age- and sex-specific LLN can be used as an alternative threshold for the FEV1/FVC ratio. As summarized in a review,6 previous studies have almost exclusively used the fixed ratio to define COPD in never smokers. However, we performed a sensitivity analysis to evaluate whether use of the LLN would have markedly changed the results of this study. When the LLN was used instead of the fixed ratio, the proportion of never smokers among all cases of moderate to severe COPD was almost unchanged. Except for differences in the age association in women, logistic model results were generally similar regarding characteristics and predictors of GOLD stage II+ COPD in never smokers when the LLN was used instead of the fixed ratio.
The results need to be interpreted with caution because diagnosis of COPD and its severity are based on one lung function measurement (including several postbronchodilator maneuvers). It is known that the obstruction may be more severe or absent after another measurement.
The required sample size of at least 300 men and 300 women in every site allowed more sites the opportunity to participate in the study and provided adequate power for estimating COPD prevalence, but it limits our ability to draw conclusions regarding potential risk factors with low to moderate prevalence, particularly in our never-smoker population.
Some analyses were limited because of the design of the questionnaires, which were intended to be comprehensive and easy to administer, but in some cases did not allow for optimally detailed data collection (eg, for exposures to passive smoke, biomass, and high-risk occupations). Furthermore, our analyses based on ≥ 10 years of exposure may be subject to potential survival bias; susceptible persons might have terminated or decreased their exposure to harmful substances that caused symptoms before a harmful threshold of exposure was reached. However, in this population, we did not have sufficient data to evaluate finer exposure intervals. In addition, many of our measures are based on self-reporting, which can be subject to inaccurate recall.
Conclusions
A substantial proportion of patients with COPD have not had significant exposures to tobacco smoke. Therefore, increased awareness and understanding of other factors that may cause this disease are needed. Our data suggest that, in addition to increased age, a prior diagnosis of asthma and, among women, lower education levels are associated with an increased risk for COPD among never smokers. Exposure to organic dusts in the workplace and history of severe childhood respiratory tract infections may also be important factors. Symptomatic never smokers should be included in clinical surveillance and screening efforts for COPD.
Supplementary Material
Acknowledgments
Author contributions: Dr Lamprecht is independent of any commercial funder, had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Lamprecht: contributed to data analysis and writing the manuscript.
Dr McBurnie: contributed to data analysis and writing the manuscript.
Dr Vollmer: contributed to data analysis and writing the manuscript.
Dr Gudmundsson: contributed to writing and revising the manuscript.
Dr Welte: contributed to writing and revising the manuscript.
Dr Nizankowska-Mogilnicka: contributed to writing and revising the manuscript.
Dr Studnicka: contributed to writing and revising the manuscript.
Dr Bateman: contributed to writing and revising the manuscript.
Dr Anto: contributed to writing and revising the manuscript.
Dr Burney: contributed to writing and revising the manuscript.
Dr Mannino: contributed to writing and revising the manuscript.
Dr Buist: contributed to writing and revising the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr McBurnie received funding for the BOLD study operations center and/or other research from unrestricted educational grants from GlaxoSmithKline, Pfizer, Boehringer Ingelheim, AstraZeneca, ALTANA, Novartis, Merck, Chiesi, Schering Plough, and Sepracor. Dr Vollmer has served on ad hoc advisory boards for Merck and Co; has participated in COPD workshops funded by Merck and GlaxoSmithKline; and is director of the Burden of Obstructive Lung Disease (BOLD) Operations Center, for which funding includes unrestricted educational grants to the Kaiser Permanente Center for Health Research from Boehringer Ingelheim, Pfizer, ALTANA, GlaxoSmithKline, AstraZeneca, Novartis, Chiesi, and Merck. Dr Anto has received an educational grant from AstraZeneca, Spain. Dr Mannino has received research funding from GlaxoSmithKline, Pfizer, and Novartis. He has served as a speaker, advisor, or consultant for AstraZeneca, MAP, Dey, Seprecor, Boehringer-Ingelheim, GlaxoSmithKline, Pfizer, and Novartis. He has also served as an expert witness in active and passive smoking cases. Dr Buist is a member of Advisory Boards for AstraZeneca, GlaxoSmithKline, Merck, and Novartis and has received unrestricted educational grants to the BOLD Operations Center from Chiesi, Boehringer Ingelheim, Pfizer, Novartis, GlaxoSmithKline, Sepracor, and Merck. Drs Lamprecht, Gudmundsson, Welte, Nizankowska-Mogilnicka, Studnicka, Bateman, and Burney have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors of the study (ALTANA, Aventis, AstraZeneca, Boehringer-Ingleheim, Chiesi, GlaxoSmithKline, Merck, Novartis, Pfizer Inc, Schering-Plough, Sunovion Pharmaceuticals Inc, or the University of Kentucky) had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Other contributions: We thank Georg Harnoncourt of the ndd Corporation and Paul Enright for their assistance with spirometry training and quality control during the study.
Additional information: The e-Appendix can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/139/4/752/suppl/DC1.
Abbreviations
- ATS
American Thoracic Society
- BOLD
Burden of Obstructive Lung Disease
- GOLD
Global Initiative for Obstructive Lung Disease
- GOLD stage II+
GOLD stages II, III, and IV
- LLN
lower limit of normal
- NHANES
National Health and Nutrition Examination Survey
- y/n
yes/no
A complete list of the members of the BOLD Collaborative Research Group is available in e-Appendix 1.
For editorial comment see page 735
Funding/Support: The BOLD initiative was funded in part by unrestricted educational grants to the operations center from ALTANA, Aventis, AstraZeneca, Boehringer-Ingleheim, Chiesi, GlaxoSmithKline, Merck, Novartis, Pfizer Inc, Schering-Plough, Sunovion Pharmaceuticals Inc, and the University of Kentucky.
References
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Celli BR, Halbert RJ, Nordyke RJ, Schau B. Airway obstruction in never smokers: results from the Third National Health and Nutrition Examination Survey. Am J Med. 2005;118(12):1364–1372. doi: 10.1016/j.amjmed.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 3.Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest. 2005;128(3):1239–1244. doi: 10.1378/chest.128.3.1239. [DOI] [PubMed] [Google Scholar]
- 4.Lamprecht B, Schirnhofer L, Kaiser B, Buist S, Studnicka M. Non-reversible airway obstruction in never smokers: results from the Austrian BOLD study. Respir Med. 2008;102(12):1833–1838. doi: 10.1016/j.rmed.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Bridevaux PO, Probst-Hensch NM, Schindler C, et al. Prevalence of airflow obstruction in smokers and never smokers in Switzerland. Eur Respir J. 2010;36(6):1259–1269. doi: 10.1183/09031936.00004110. [DOI] [PubMed] [Google Scholar]
- 6.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 7.Lundbäck B, Lindberg A, Lindström M, et al. Obstructive Lung Disease in Northern Sweden Studies Not 15 but 50% of smokers develop COPD?—Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2003;97(2):115–122. doi: 10.1053/rmed.2003.1446. [DOI] [PubMed] [Google Scholar]
- 8.Buist AS, McBurnie MA, Vollmer WM, et al. BOLD Collaborative Research Group International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 9.Buist AS, Vollmer WM, Sullivan SD, et al. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD. 2005;2(2):277–283. [PubMed] [Google Scholar]
- 10.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 11.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 12.Rabe KF, Hurd S, Anzueto A, et al. Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 13.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56(2):645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Wang C, Yao W, et al. COPD in Chinese nonsmokers. Eur Respir J. 2009;33(3):509–518. doi: 10.1183/09031936.00084408. [DOI] [PubMed] [Google Scholar]
- 15.Miravitlles M, Ferrer M, Pont A, et al. Characteristics of a population of COPD patients identified from a population-based study. Focus on previous diagnosis and never smokers. Respir Med. 2005;99(8):985–995. doi: 10.1016/j.rmed.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Xu X, Weiss ST, Rijcken B, Schouten JP. Smoking, changes in smoking habits, and rate of decline in FEV1: new insight into gender differences. Eur Respir J. 1994;7(6):1056–1061. [PubMed] [Google Scholar]
- 17.Silverman EK, Weiss ST, Drazen JM, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(6):2152–2158. doi: 10.1164/ajrccm.162.6.2003112. [DOI] [PubMed] [Google Scholar]
- 18.Dransfield MT, Davis JJ, Gerald LB, Bailey WC. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Respir Med. 2006;100(6):1110–1116. doi: 10.1016/j.rmed.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Curtis JL, Freeman CM, Hogg JC. The immunopathogenesis of chronic obstructive pulmonary disease: insights from recent research. Proc Am Thorac Soc. 2007;4(7):512–521. doi: 10.1513/pats.200701-002FM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(23):2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 21.Trupin L, Earnest G, San Pedro M, et al. The occupational burden of chronic obstructive pulmonary disease. Eur Respir J. 2003;22(3):462–469. doi: 10.1183/09031936.03.00094203. [DOI] [PubMed] [Google Scholar]
- 22.Balmes J, Becklake M, Blanc P, et al. Environmental and Occupational Health Assembly, American Thoracic Society American Thoracic Society Statement: Occupational contribution to the burden of airway disease. Am J Respir Crit Care Med. 2003;167(5):787–797. doi: 10.1164/rccm.167.5.787. [DOI] [PubMed] [Google Scholar]
- 23.Hnizdo E, Sullivan PA, Bang KM, Wagner G. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: a study of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;156(8):738–746. doi: 10.1093/aje/kwf105. [DOI] [PubMed] [Google Scholar]
- 24.Tabona M, Chan-Yeung M, Enarson DA, MacLean L, Dorken E, Schulzer M. Host factors affecting longitudinal decline in lung spirometry among grain elevator workers. Chest. 1984;85(6):782–786. doi: 10.1378/chest.85.6.782. [DOI] [PubMed] [Google Scholar]
- 25.doPico GA, Flaherty D, Bhansali P, Chavaje N. Grain fever syndrome induced by inhalation of airborne grain dust. J Allergy Clin Immunol. 1982;69(5):435–443. doi: 10.1016/0091-6749(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 26.Von Essen SG, Robbins RA, Thompson AB, Ertl RF, Linder J, Rennard S. Mechanisms of neutrophil recruitment to the lung by grain dust exposure. Am Rev Respir Dis. 1988;138(4):921–927. doi: 10.1164/ajrccm/138.4.921. [DOI] [PubMed] [Google Scholar]
- 27.Jagielo PJ, Thorne PS, Watt JL, Frees KL, Quinn TJ, Schwartz DA. Grain dust and endotoxin inhalation challenges produce similar inflammatory responses in normal subjects. Chest. 1996;110(1):263–270. doi: 10.1378/chest.110.1.263. [DOI] [PubMed] [Google Scholar]
- 28.Smith KR. Inaugural article: national burden of disease in India from indoor air pollution. Proc Natl Acad Sci U S A. 2000;97(24):13286–13293. doi: 10.1073/pnas.97.24.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan-Yeung M, Aït-Khaled N, White N, Ip MS, Tan WC. The burden and impact of COPD in Asia and Africa. Int J Tuberc Lung Dis. 2004;8(1):2–14. [PubMed] [Google Scholar]
- 30.Ezzati M. Indoor air pollution and health in developing countries. Lancet. 2005;366(9480):104–106. doi: 10.1016/S0140-6736(05)66845-6. [DOI] [PubMed] [Google Scholar]
- 31.Oroczo-Levi M, Garcia-Aymerich J, Villar J, Ramírez-Sarmiento A, Antó JM, Gea J. Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(3):542–546. doi: 10.1183/09031936.06.00052705. [DOI] [PubMed] [Google Scholar]
- 32.Ekici A, Ekici M, Kurtipek E, et al. Obstructive airway diseases in women exposed to biomass smoke. Environ Res. 2005;99(1):93–98. doi: 10.1016/j.envres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Ramírez-Venegas A, Sansores RH, Pérez-Padilla R, et al. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am J Respir Crit Care Med. 2006;173(4):393–397. doi: 10.1164/rccm.200504-568OC. [DOI] [PubMed] [Google Scholar]
- 34.Yin P, Jiang CQ, Cheng KK, et al. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. Lancet. 2007;370(9589):751–757. doi: 10.1016/S0140-6736(07)61378-6. [DOI] [PubMed] [Google Scholar]
- 35.Eisner MD, Balmes J, Katz PP, Trupin L, Yelin EH, Blanc PD. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health. 2005;4(1):7–14. doi: 10.1186/1476-069X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simoni M, Baldacci S, Puntoni R, et al. Respiratory symptoms/diseases and environmental tobacco smoke (ETS) in never smoker Italian women. Respir Med. 2007;101(3):531–538. doi: 10.1016/j.rmed.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Iribarren C, Friedman GD, Klatsky AL, Eisner MD. Exposure to environmental tobacco smoke: association with personal characteristics and self reported health conditions. J Epidemiol Community Health. 2001;55(10):721–728. doi: 10.1136/jech.55.10.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larsson ML, Loit HM, Meren M, et al. Passive smoking and respiratory symptoms in the FinEsS Study. Eur Respir J. 2003;21(4):672–676. doi: 10.1183/09031936.03.00033702. [DOI] [PubMed] [Google Scholar]
- 39.Prescott E, Lange P, Vestbo J. Socioeconomic status, lung function and admission to hospital for COPD: results from the Copenhagen City Heart Study. Eur Respir J. 1999;13(5):1109–1114. doi: 10.1034/j.1399-3003.1999.13e28.x. [DOI] [PubMed] [Google Scholar]
- 40.Nizankowska-Mogilnicka E, Mejza F, Buist AS, et al. Prevalence of COPD and tobacco smoking in Malopolska region—results from the BOLD study in Poland. Pol Arch Med Wewn. 2007;117(9):402–410. [PubMed] [Google Scholar]
- 41.Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303(6804):671–675. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaheen SO, Barker DJ, Shiell AW, Crocker FJ, Wield GA, Holgate ST. The relationship between pneumonia in early childhood and impaired lung function in late adult life. Am J Respir Crit Care Med. 1994;149(3 Pt 1):616–619. doi: 10.1164/ajrccm.149.3.8118627. [DOI] [PubMed] [Google Scholar]
- 43.Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000;55(1):32–38. doi: 10.1136/thorax.55.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishna K, Bond S, Artvinli M. Pulmonary function in treated tuberculosis; a long-term follow-up. Am Rev Respir Dis. 1977;115(4):402–404. [Google Scholar]
- 45.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 46.Varraso R, Fung TT, Hu FB, Willett W, Camargo CA. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax. 2007;62(9):786–791. doi: 10.1136/thx.2006.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKeever TM, Lewis SA, Cassano PA, et al. Patterns of dietary intake and relation to respiratory disease, forced expiratory volume in 1 s, and decline in 5-y forced expiratory volume. Am J Clin Nutr. 2010;92(2):408–415. doi: 10.3945/ajcn.2009.29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar R, Seibold MA, Aldrich MC, et al. Genetic ancestry in lung-function predictions. N Engl J Med. 2010;363(4):321–330. doi: 10.1056/NEJMoa0907897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardie JA, Buist AS, Vollmer WM, Ellingsen I, Bakke PS, Mørkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never smokers. Eur Respir J. 2002;20(5):1117–1122. doi: 10.1183/09031936.02.00023202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.