Abstract
An increase in intrahepatic triglyceride (IHTG) content is the hallmark of nonalcoholic fatty liver disease (NAFLD) and is strongly associated with insulin resistance and dyslipidemia. Although regular aerobic exercise improves metabolic function, its role in regulating fat accumulation in the liver is incompletely understood, and human data are scarce. Results from exercise training studies in animals highlight a number of potential factors that could possibly mediate the effect of exercise on liver fat, but none of them has been formally tested in man. The effect of exercise on IHTG content strongly depends on the background diet, so that exercise is more effective in reducing IHTG under conditions that favor liver fat accretion (e.g., when animals are fed high-fat diets). Concurrent loss of body weight or visceral fat does not appear to mediate the effect of exercise on IHTG, whereas sex (males versus females), prandial status (fasted versus fed), and duration of training, as well as the time elapsed from the last bout of exercise could all be affecting the observed exercise-induced changes in IHTG content. The potential importance of these factors remains obscure, thus providing a wide array of opportunities for future research on the effects of exercise (and diet) on liver fat accumulation.
1. Introduction
Excessive accumulation of fat in the liver, that is, intrahepatic triglyceride (IHTG), is associated with increased prevalence rates of and risk for dyslipidemia, diabetes, and cardiovascular disease [1–3]. Data from epidemiological as well as metabolic studies indicate that increased IHTG content is accompanied by insulin resistance and dysregulation of lipid metabolism [4–6]. Exercise is known to improve metabolic function [7, 8]; however its effects on IHTG remain elusive [9, 10]. Data from studies in humans are scarce and not entirely consistent [11]. In this paper, the results from a number of animal studies are briefly reviewed in an attempt to highlight putative factors that may modulate the effect of exercise on IHTG content.
2. Exercise Training in Animals
Many studies have evaluated the effect of aerobic exercise training on IHTG content in rodents; their design varies in terms of sex, strain, background diet, training duration, the prandial status, and the time of assessment after the last bout of exercise (Table 1) [12–37]. Results are largely heterogeneous, but a crude analysis of the data suggests that endurance training decreases IHTG (median: −16%, range: −92% to +97%, n = 50 studies; Table 1). Most frequently [14, 16, 18, 20, 26, 31, 35] but not always [15, 17, 34, 37], exercise has been shown to be more effective in reducing liver fat or attenuating its accretion in animals fed high-fat rather than standard, low-fat diets (median decrease: 25% and 14%, resp., Figure 1(a)). This is consistent with data from human studies, in which exercise training appears to be more potent in reducing IHTG in subjects with increased baseline IHTG, for example, subjects with NAFLD, type II diabetes, or the elderly [11].
Table 1.
Effect of aerobic exercise training on liver fat in animals.
| Withdrawal before | Effect of training (EX versus | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | Animals | Intervention | measurements | respective SED group on the same diet) | ||||
| Diet | Exercise | Duration | Food | Exercise | BW/VAT | Liver fat | ||
| Ahrens et al., 1972 [12] | Male Wistar rats; young and mature | HF (ad libitum or pair-fed) with two different carbohydrate sources | SED or EX (1/d, running, 30 min at ∼13 m/min and 0% incline) | 8 wk | 12 h | ? | ↓/? (both diets, feeding patterns, and age groups) | Young: −15% Mature: −35% |
|
| ||||||||
| Barakat et al., 1987 [13] | Female rats; control and alloxan-diabetic | SC? (ad libitum) | SED or EX (1/d, running, 2 h at 20 m/min and 0% incline) | 7 d | 0 h | 24 h | ↔/? (both groups) | Control: −14% (NS) Diabetic: −2% (NS) |
|
| ||||||||
| Cha et al., 1999 [14] | Male Sprague-Dawley rats | SC or HF (ad libitum) | SED or EX (1/d, running, 1.5 h at 150 m/min and 1% incline) | 1 mo | ? | ? | SC: ↓/? HF: ↓/? |
SC: −24% HF: −40% |
|
| ||||||||
| Chapados et al., 2009 [15] | Female Sprague-Dawley rats | SC or HF (ad libitum) | SED or EX (5/wk, running, progressive until 1 h at 26 m/min and 10% incline for the last 4 wk) | 8 wk | 12 h | 48 h | SC: ↔/↓ HF: ↔/↓ |
SC: −16% (NS) HF: −22% (NS) |
|
| ||||||||
| Charbonneau et al., 2005 [16] | Female Sprague-Dawley rats | SC or HF (ad libitum) | SED or EX (6/wk, running, progressive until 1 h at 26 m/min and 10% incline for the last 3 wk) | 6 wk (+2 wk diet lead-in) | 2-3 h | 48 h | SC: ↔/↓ HF: ↔/↓ |
SC: 0% (NS) HF: −14% |
|
| ||||||||
| Fukuda et al., 1991 [17] | Male Wistar rats | SC, HF or HChol (ad libitum) | SED or EX (voluntary running) | 4 wk | 3 h | ? | SC: ↓/? HF: ↓/? HChol: ↓/? |
SC: −1% (NS) HF: +14% (NS) HChol: −30% (NS) |
|
| ||||||||
| Gauthier et al., 2003 [18] | Female Sprague-Dawley rats | SC or HF (ad libitum) | SED or EX (5/wk, running, progressive until 1 h at 26 m/min and 10% incline for the last 4 wk) | 8 wk | 2 h | 48 h | SC: ↔/↓ HF: ↓/↓↓ |
SC: +16% (NS) HF: −29% |
|
| ||||||||
| Gauthier et al., 2004 [19] | Female Sprague-Dawley rats | HF (ad libitum) | SED or EX (5/wk, running, progressive until 1 h at 26 m/min and 10% incline for the last 4 wk) | 8 wk (+8 wk diet lead-in) | 2 h | 48 h | ↔/↓ | −16% (NS) |
|
| ||||||||
| Gollisch et al., 2009 [20] | Female Sprague-Dawley rats | SC or HF (ad libitum) | SED or EX (voluntary running) | 4 wk | 10 h | 24 h | SC: ↔/↓ HF: ↓/↓↓ |
SC: −16% (NS) HF: −31% |
| Hao et al., 2010 [21] | Female Sprague-Dawley rats; sham-operated or OVX with and without E2 | SC (ad libitum) | SED or EX (5/wk, running, 1 h at 18 m/min and 0% incline) | 12 wk | ? | 24 h | Sham: ↔/↔
OVX: ↓/↓ OVX+E2: ↔/↔ |
Sham: −24% OVX: −30% OVX+E2: −5% (NS) |
|
| ||||||||
| Karanth and Jeevaratnam, 2009 [22] | Male Wistar rats | HF rich in SFA or MUFA (ad libitum) with carnitine or not | SED or EX (6/wk, swimming, 1 h) | 6 mo | Overnight | 20 h | ↔/? (both diets) | SFA: −35% MUFA: −25% (average with or without carnitine) |
|
| ||||||||
| Lessard et al., 2007 [23] | Male Sprague-Dawley rats | HF (ad libitum) | SED or EX (5/wk, running, progressive until 1 h at 32 m/min and 15% incline for the last 3 wk) | 4 wk (+4 wk diet lead-in) | 8–12 h | 36–48 h | ↓/↓ | −41% |
|
| ||||||||
| Lira et al., 2008 [24] | Male Wistar rats; control and tumor-bearing | SC (ad libitum) | SED or EX (5/wk, running, progressive until 1 h at 20 m/min and 0% incline for the last 2 wk) | 8 wk | ? | 24 h | ↓/? (both groups) | Control: −30% Tumor: −92% |
|
| ||||||||
| Morifuji et al., 2006 [25] | Male Sprague-Dawley rats | SC with casein or soya as protein source (ad libitum) | SED or EX (6/wk, swimming, 2 h) | 2 wk | Nonfasting | 24 h | ↓/↓ (both groups) | Casein: −21% Soya: −24% |
|
| ||||||||
| Narayan et al., 1975 [26] | Male Holtzman rats | SC or HF (ad libitum) | SED or EX (5/wk, running, progressive until 80–85 min at 23 m/min and 8.5% incline for the last 3 wk) | 6 wk | Nonfasting | 24 h | ?/? | SC: +81% (NS) HF: −51% |
|
| ||||||||
| Petridou et al., 2005 [27] | Male Wistar rats | SC (ad libitum) | SED or EX (voluntary running) | 8 wk | 6 h | 12 h | ?/? | −12% (NS) |
|
| ||||||||
| Pighon et al., 2010 [28] | Female Sprague-Dawley rats | SC (ad libitum) | SED or EX (5/wk, running, progressive until 1 h min at 26 m/min and 10% incline for the last 4 wk) | 6 wk | 3 h | 48 h | ↔/↓ | +1% (NS) |
|
| ||||||||
| Pighon et al., 2010 [28] | Female Sprague-Dawley rats; sham-operated or OVX with and without E2 | SC (ad libitum) | SED or EX (5/wk, running, progressive until 1 h min at 26 m/min and 10% incline for the last 3 wk) | 5 wk | 3 h | 48 h | ↓/↓ (all groups) | Sham: −1% (NS) OVX: −26% OVX+E2: −5% (NS) |
| Rector et al., 2008 [29] | Male OLETF rats (obese and diabetic) | SC (ad libitum) | SED or EX (voluntary running) | 16 wk | 5 h | 48 h | ↓/↓ | −45% |
| Rothfeld et al., 1977 [30] | Male Sprague-Dawley rats | HF (pair-fed) | SED or EX (voluntary running) | 3 wk | ? | ? | ?/? | −14% |
|
| ||||||||
| Straczkowski et al., 2001 [31] | Male Wistar rats | SC for wk 0–3 and SC or HF for wk 4–6 (pair-fed) | SED or EX (6/wk, running, 3 h at 20 m/min and 10% incline) | 6 wk | ? | 48 h | ↓/? (both diets) | SC: +97% HF: −9% (NS) |
|
| ||||||||
| Terao et al., 1987 [32] | Male Wistar rats | HChol (ad libitum) | SED or EX (5/wk, running, progressive until 1 h at 20 m/min and 0% incline for the last 2 wk) | 5 wk (+5 wk SC diet lead-in) | ? | ? | ↓/? | −16% (NS) |
|
| ||||||||
| Tsutsumi et al., 2001 [33] | Male Sprague-Dawley old rats | SC (ad libitum) | SED or EX (1/d, running, 30 min at 15 m/min and 10% incline) | 3 mo | ∼12 h | ∼12 h | ↓/↓ | −41% |
|
| ||||||||
| Vieira et al., 2009 [34] | Male C57BL/6, HF diet-induced obese mice | SC or HF (ad libitum) | SED or EX (5/wk, running, 40 min at 12 m/min and 12% incline) | 6 wk | 12 h | 48 h | SC: ↔/↔
HF: ↓/↓ |
SC: −49% (NS) HF: −11% (NS) |
|
| ||||||||
| Vieira et al., 2009 [34] | Male C57BL/6, HF diet-induced obese mice | SC or HF (ad libitum) | SED or EX (5/wk, running, 40 min at 12 m/min and 12% incline) | 12 wk | 12 h | 48 h | SC: ↔/↓ HF: ↓/↓ |
SC: −72% HF: −48% |
|
| ||||||||
| Vieira et al., 2009 [35] | Male Balb/cByJ mice (with defective fatty acid oxidation) | SC or HF (ad libitum) | SED or EX (5/wk, running, 1 h at 12 m/min and 5% incline) | 12 wk | 12 h | 48 h | SC: ↔/? HF: ↓/? |
SC: −5% (NS) HF: −40% (P = 0.09) |
|
| ||||||||
| Yasari et al., 2006 [36] | Female Sprague-Dawley rats | SC (ad libitum) | SED or EX (5/wk, running, progressive until 1 h at 26 m/min and 10% incline for the last 4 wk) | 8 wk | 3 h | 48 h | ↔/↓ | −9% (NS) |
|
| ||||||||
| Yasari et al., 2010 [37] | Female Sprague-Dawley rats | SC for wk 0–6 and SC or HF for wk 7-8 (ad libitum) | SED or EX (5/wk, running, progressive until 1 h at 26 m/min and 10% incline for the last 4 wk) | 8 wk | 3 h | 36–48 h | ↔/↓ (both diets) | SC: −13% (NS) HF: +7% (NS) |
All changes shown are statistically significant versus control group (SED), unless indicated otherwise (↔ is unchanged; ↓ is reduced; NS is not significant; ? is unknown).
BW: body weight; E2: estradiol; EX: exercised; HChol: high fat and cholesterol; HF: high fat; MUFA: monounsaturated fatty acids; OVX: ovariectomized; SC: standard chow (low fat); SED: sedentary; SFA: saturated fatty acids; VAT: visceral adipose tissue (mesenteric, retroperitoneal, and/or epididymal fat pads).
Figure 1.
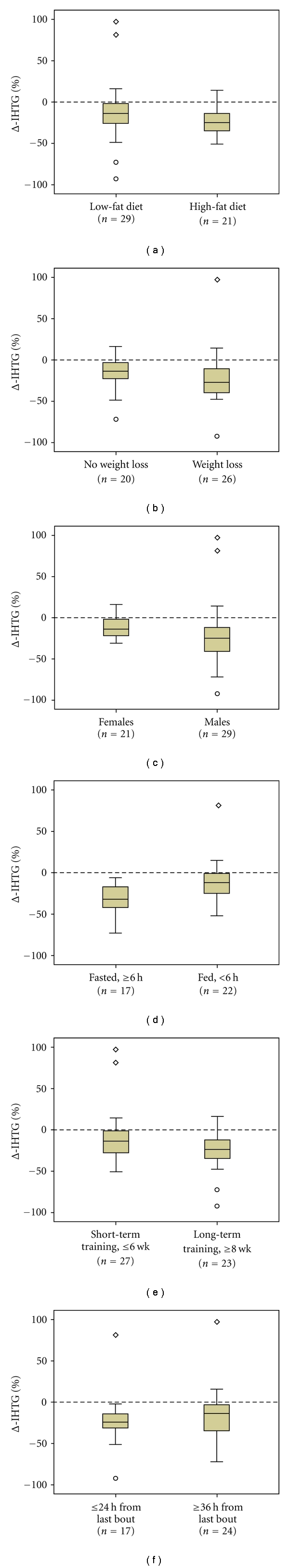
Factors that may affect changes in liver fat in response to exercise training in animals. Exercise-induced changes in intrahepatic triglyceride content (Δ-IHTG) are shown for: (a) animals fed high fat or standard chow (low fat) diets; (b) animals that experienced weight loss (or attenuated weight gain) or not; (c) male or female animals; (d) fasted or fed animals; (e) animals trained for longer or shorter periods of time; (f) animals examined within one day from the last bout of exercise or later during recovery. Box plots have been constructed using average changes in liver fat (% difference relative to sedentary controls) for each group of animals in the studies depicted in Table 1, and illustrate median, first, and third quartiles, minimum and maximum values, as well as potential positive and negative outliers.
The reasons why exercise is more effective in reducing IHTG on high-fat than low-fat diets are not entirely clear but are likely related to the hepatosteatotic effect of high-fat feeding. Fat is mainly stored as microvesicles (<1 μm2) within hepatocytes, whereas the high-fat diet-induced hepatic steatosis occurs via accumulation of macrovesicles (>1 μm2) [18, 38]. Endurance training has been shown to completely prevent the high-fat diet-induced hepatic steatosis [18, 38], that is, the hepatocyte surface area occupied by the lipid vacuoles, solely by reducing the number of lipid vacuoles in all sizes between 1 and 10 μm2 (i.e., macrovesicles), without affecting the number of vacuoles of surface area <1 μm2 (i.e., microvesicles) [18]. Hence exercise may have less of an effect when on low-fat diets, not only because of lower total IHTG content, but also because most of this fat (∼75%) is stored in microvesicles, not macrovesicles. A more pronounced IHTG-depleting effect of exercise has also been observed under other conditions that favor the development of fatty liver, such as overfeeding [34], ovariectomy [28], ethanol ingestion [39], or tumor-bearing [24]. Apart from the fat content of the background diet, the type of dietary carbohydrate [12], protein [25], and fat (i.e., saturated or unsaturated fatty acids) [22], as well as the feeding pattern (ad libitum or paired) [12] do not appear to affect, at least not in a major way, the exercise-induced change in IHTG content.
The collective of available data in animals highlights a number of other putative factors that may modulate the effect of exercise on liver fat; however, none of these factors has been formally tested using rigorous experimental designs. Concurrent weight loss or attenuated weight gain is not likely critical for the exercise-induced depletion of IHTG to manifest, albeit they may lead to greater reductions in liver fat when compared to no weight loss or similar weight gain (median decrease: 27.5% and 14%, resp., Figure 1(b)). However, just like in humans [11], loss of visceral adipose tissue mass with exercise training is not necessarily coupled with a corresponding decrease in liver fat [16, 18, 28, 37]. Likewise, human studies have shown that exercise-induced reductions in IHTG content can occur in the absence of changes in total body fat [40] or even visceral adipose tissue [41].
Exercise may be more effective in reducing IHTG content in males than in females (median decrease: 25% and 14%, resp., Figure 1(c)), in fasted (≥6 h) than in fed animals (median decrease: 31% and 11%, resp., Figure 1(d)), and after longer (≥8 wk) than shorter interventions [34] (median decrease: 24% and 14%, resp.; Figure 1(e)). The time elapsed from the last bout of exercise (≤24 h or ≥36 h) may also mediate the observed changes in IHTG (median decrease: 24% and 13.5%, resp., Figure 1(f)), suggesting that even acute exercise could affect liver fat. However, relevant information is scarce and inconclusive. A single bout of aerobic exercise (30–60 min) did not affect IHTG content, measured immediately after exercise, in female rats [42] but caused a ∼30% decrease in male rats [43] of the same strain, under both standard and high-fat feeding conditions. This is in line with data from exercise training studies in animals raising the possibility that males may be more sensitive to the IHTG-reducing effect of exercise than females (Figure 1(c)), as well as with recent observations in humans [44]. Studies in which male rats were exercised until exhaustion provide conflicting results, some observed a mild [45] or marked [46, 47] increase in hepatic steatosis whereas others found a decrease of 30–60% [48] at the end of exercise.
3. Detraining after Regular Exercise
If regular exercise reduces liver fat, cessation of exercise should lead to an increase in IHTG content. Only a few animal studies have evaluated the effect of detraining on liver fat accumulation, and all have demonstrated that cessation of regular exercise (after 6–16 weeks of training) for a short (2-3 days) or a long (6 weeks) period of time is not associated with any significant changes in IHTG content compared with the trained state (i.e., before discontinuation of exercise) when animals are fed a standard low-fat diet [28, 36, 49, 50]. Furthermore, detraining for 2–7 days does not alter the total number of hepatocyte lipid vacuoles and their size, even though it does activate precursors and processes in the liver known to initiate steatosis (e.g., decreased mitochondrial oxidative capacity, increased expression of de novo lipogenesis proteins, and increased malonyl CoA levels) [49]. Interpretation of detraining data is not straightforward, though. It is possible that this lack of an effect of detraining relates to the lesser potency of exercise in reducing liver fat content in animals fed standard low-fat diets (Figure 1), so that changes after detraining are similarly less pronounced. For instance, two [28, 36] out of three studies that reported no effect of detraining on liver fat also failed to observe a training-induced decrease in IHTG content, suggesting that training and detraining have no effect on liver fat accumulation under low-fat feeding conditions. Whereas one study [49] did observe a training-induced decrease in liver fat in rats fed a low-fat diet but found no changes after detraining, implying that the IHTG-depleting effect of regular exercise is long-lived and is not readily reversed by detraining. Still, compared with sedentary, never-exercised counterparts, detrained animals appear to be relatively protected from mild hepatic steatosis induced by 2 weeks of high-fat feeding [36], but not from the development of frank fatty liver 6 weeks after ovariectomy [28], even though cessation of exercise training in ovariectomized rats resulted in a nearly 40% increase in IHTG content compared with ovariectomized rats who did not stop exercising [28]. It is thus possible that the relevant molecular and biochemical adaptations to exercise are readily reversed when the exercise routine is interrupted (<1 week), however, changes in IHTG manifest later in time and only when strong triggering factors for liver fat accretion coexist, such as high-fat feeding or ovariectomy. Support for this notion is provided by an earlier study, where detraining resulted in a striking increase in IHTG content only when animals were subjected to starvation and refeeding [50].
4. Conclusion
The effect of exercise on IHTG content has recently attracted much scientific interest in light of the apparent detrimental metabolic effects of excessive liver fat accumulation. Although the results from a few studies in human subjects are promising, as exercise appears to reduce IHTG [11], the importance of the factors highlighted herein on the basis of studies in animals has never been evaluated in man. Future studies should at least control for—in order to avoid confounding—or directly investigate the role of these factors in affecting the exercise-induced changes in liver fat content.
Conflict of Interests
The author declares that there is no conflict of interests.
References
- 1.Adams LA, Lymp JF, Sauver JS, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Hsiao PJ, Kuo KK, Shin SJ, et al. Significant correlations between severe fatty liver and risk factors for metabolic syndrome. Journal of Gastroenterology and Hepatology. 2007;22(12):2118–2123. doi: 10.1111/j.1440-1746.2006.04698.x. [DOI] [PubMed] [Google Scholar]
- 3.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191(2):235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speliotes EK, Massaro JM, Hoffmann U, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham heart study. Hepatology. 2010;51(6):1979–1987. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(36):15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magkos F. Basal very low-density lipoprotein metabolism in response to exercise: mechanisms of hypotriacylglycerolemia. Progress in Lipid Research. 2009;48(3-4):171–190. doi: 10.1016/j.plipres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Magkos F, Sidossis LS. Exercise and insulin sensitivity: where do we stand? You’d better run! European Journal of Endocrinology. 2008;4(1):22–25. [Google Scholar]
- 9.Caldwell S, Lazo M. Is exercise an effective treatment for NASH? Knowns and unknowns. Annals of Hepatology. 2009;8(1):S60–S66. [PubMed] [Google Scholar]
- 10.Spassiani NA, Kuk JL. Exercise and the fatty liver. Applied Physiology, Nutrition and Metabolism. 2008;33(4):802–807. doi: 10.1139/H08-059. [DOI] [PubMed] [Google Scholar]
- 11.Magkos F. Exercise and fat accumulation in the human liver. Current Opinion in Lipidology. 2010;21(6):507–517. doi: 10.1097/MOL.0b013e32833ea912. [DOI] [PubMed] [Google Scholar]
- 12.Ahrens RA, Bishop CL, Berdanier CD. Effect of age and dietary carbohydrate source on the responses of rats to forced exercise. Journal of Nutrition. 1972;102(2):241–247. doi: 10.1093/jn/102.2.241. [DOI] [PubMed] [Google Scholar]
- 13.Barakat HA, Carpenter JW, Lennon YA, Hanna WR, O’Brien KF, Dohm GL. The effects of exercise on lipogenic enzyme activity and glyceride synthesis by liver homogenates of diabetic rats. Metabolism. 1987;36(10):983–987. doi: 10.1016/0026-0495(87)90137-5. [DOI] [PubMed] [Google Scholar]
- 14.Cha Y-S, Sohn H-S, Daily JW, III, Oh S-H. Effects of exercise training and/or high fat diet on lipid metabolism and carnitine concentrations in rats. Nutrition Research. 1999;19(6):937–945. [Google Scholar]
- 15.Chapados NA, Seelaender M, Levy E, Lavoie JM. Effects of exercise training on hepatic microsomal triglyceride transfer protein content in rats. Hormone and Metabolic Research. 2009;41(4):287–293. doi: 10.1055/s-0028-1102937. [DOI] [PubMed] [Google Scholar]
- 16.Charbonneau A, Couturier K, Gauthier MS, Lavoie JM. Evidence of hepatic glucagon resistance associated with hepatic steatosis: reversal effect of training. International Journal of Sports Medicine. 2005;26(6):432–441. doi: 10.1055/s-2004-821225. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda N, Tojho M, Hidaka T, Sho H, Sugano M. Reciprocal responses to exercise in hepatic ketogenesis and lipid secretion in the rat. Annals of Nutrition and Metabolism. 1991;35(4):233–241. doi: 10.1159/000177651. [DOI] [PubMed] [Google Scholar]
- 18.Gauthier MS, Couturier K, Latour JG, Lavoie JM. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. Journal of Applied Physiology. 2003;94(6):2127–2134. doi: 10.1152/japplphysiol.01164.2002. [DOI] [PubMed] [Google Scholar]
- 19.Gauthier MS, Couturier K, Charbonneau A, Lavoie JM. ffects of introducing physical training in the course of a 16-week high-fat diet regimen on hepatic steatosis, adipose tissue fat accumulation, and plasma lipid profile. International Journal of Obesity and Related Metabolic Disorders. 2004;28(8):1064–1071. doi: 10.1038/sj.ijo.0802628. [DOI] [PubMed] [Google Scholar]
- 20.Gollisch KS, Brandauer J, Jessen N, et al. Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. American Journal of Physiology—Endocrinology and Metabolism. 2009;297(2):E495–E504. doi: 10.1152/ajpendo.90424.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao L, Wang Y, Duan Y, Bu S. Effects of treadmill exercise training on liver fat accumulation and estrogen receptor alpha expression in intact and ovariectomized rats with or without estrogen replacement treatment. European Journal of Applied Physiology. 2010;109(5):879–886. doi: 10.1007/s00421-010-1426-6. [DOI] [PubMed] [Google Scholar]
- 22.Karanth J, Jeevaratnam K. Effect of dietary lipid, carnitine and exercise on lipid profile in rat blood, liver and muscle. Indian Journal of Experimental Biology. 2009;47(9):748–753. [PubMed] [Google Scholar]
- 23.Lessard SJ, Rivas DA, Chen ZP, et al. Tissue-specific effects of rosiglitazone and exercise in the treatment of lipid-induced insulin resistance. Diabetes. 2007;56(7):1856–1864. doi: 10.2337/db06-1065. [DOI] [PubMed] [Google Scholar]
- 24.Lira FS, Tavares FL, Yamashita AS, et al. Effect of endurance training upon lipid metabolism in the liver of cachectic tumour-bearing rats. Cell Biochemistry and Function. 2008;26(6):701–708. doi: 10.1002/cbf.1495. [DOI] [PubMed] [Google Scholar]
- 25.Morifuji M, Sanbongi C, Sugiura K. Dietary soya protein intake and exercise training have an additive effect on skeletal muscle fatty acid oxidation enzyme activities and mRNA levels in rats. The British Journal of Nutrition. 2006;96(3):469–475. [PubMed] [Google Scholar]
- 26.Narayan KA, McMullen JJ, Butler DP. Effect of exercise on tissue lipids and serum lipoproteins of rats fed two levels of fat. Journal of Nutrition. 1975;105(5):581–587. doi: 10.1093/jn/105.5.581. [DOI] [PubMed] [Google Scholar]
- 27.Petridou A, Nikolaidis MG, Matsakas A, Schulz T, Michna H, Mougios V. Effect of exercise training on the fatty acid composition of lipid classes in rat liver, skeletal muscle, and adipose tissue. European Journal of Applied Physiology. 2005;94(1-2):84–92. doi: 10.1007/s00421-004-1294-z. [DOI] [PubMed] [Google Scholar]
- 28.Pighon A, Barsalani R, Yasari S, Prud’Homme D, Lavoie JM. Does exercise training prior to ovariectomy protect against liver and adipocyte fat accumulation in rats? Climacteric. 2010;13(3):238–248. doi: 10.3109/13697130903009203. [DOI] [PubMed] [Google Scholar]
- 29.Rector RS, Thyfault JP, Morris RT, et al. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. American Journal of Physiology. 2008;294(3):G619–G626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 30.Rothfeld B, Levine A, Varady A. The effect of exercise on lipid deposition in the rat. Biochemical Medicine. 1977;18(2):206–209. doi: 10.1016/0006-2944(77)90091-6. [DOI] [PubMed] [Google Scholar]
- 31.Straczkowski M, Kowalska I, Dzienis-Straczkowska S, Kinalski M, Górski J, Kinalska I. The effect of exercise training on glucose tolerance and skeletal muscle triacylglycerol content in rats fed with a high-fat diet. Diabetes and Metabolism. 2001;27(1):19–23. [PubMed] [Google Scholar]
- 32.Terao T, Fujise T, Nakano S. Effects of long-term exercise and high-cholesterol diet on lipid-lipoprotein metabolism in rats. Tokai Journal of Experimental and Clinical Medicine. 1987;12(4):243–251. [PubMed] [Google Scholar]
- 33.Tsutsumi K, Kusunoki M, Hara T, et al. Exercise improved accumulation of visceral fat and simultaneously impaired endothelium-dependent relaxation in old rats. Biological and Pharmaceutical Bulletin. 2001;24(1):88–91. doi: 10.1248/bpb.24.88. [DOI] [PubMed] [Google Scholar]
- 34.Vieira VJ, Valentine RJ, Wilund KR, Antao N, Baynard T, Woods JA. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. American Journal of Physiology—Endocrinology and Metabolism. 2009;296(5):E1164–E1171. doi: 10.1152/ajpendo.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vieira VJ, Valentine RJ, Wilund KR, Woods JA. Effects of diet and exercise on metabolic disturbances in high-fat diet-fed mice. Cytokine. 2009;46(3):339–345. doi: 10.1016/j.cyto.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yasari S, Paquette A, Charbonneau A, Gauthier MS, Savard R, Lavoie JM. Effects of ingesting a high-fat diet upon exercise-training cessation on fat accretion in the liver and adipose tissue of rats. Applied Physiology, Nutrition and Metabolism. 2006;31(4):367–375. doi: 10.1139/h06-032. [DOI] [PubMed] [Google Scholar]
- 37.Yasari S, Prud’Homme D, Wang D, et al. Exercise training decreases hepatic SCD-1 gene expression and protein content in rats. Molecular and Cellular Biochemistry. 2010;335(1-2):291–299. doi: 10.1007/s11010-009-0279-y. [DOI] [PubMed] [Google Scholar]
- 38.He Y, Zhang H, Fu FH. The effects of swimming exercise on high-fat-diet-induced steatohepatitis. Journal of Sports Medicine and Physical Fitness. 2008;48(2):259–265. [PubMed] [Google Scholar]
- 39.Trudell JR, Lin WQ, Chrystof DA, Kirshenbaum G, Ardies CM. Induction of HSP72 in rat liver by chronic ethanol consumption combined with exercise: association with the prevention of ethanol-induced fatty liver by exercise. Alcoholism: Clinical and Experimental Research. 1995;19(3):753–758. doi: 10.1111/j.1530-0277.1995.tb01578.x. [DOI] [PubMed] [Google Scholar]
- 40.Finucane FM, Sharp SJ, Purslow LR, et al. The effects of aerobic exercise on metabolic risk, insulin sensitivity and intrahepatic lipid in healthy older people from the Hertfordshire Cohort Study: a randomised controlled trial. Diabetologia. 2010;53(4):624–631. doi: 10.1007/s00125-009-1641-z. [DOI] [PubMed] [Google Scholar]
- 41.Bonekamp S, Barone BB, Clark J, Stewart KJ. The effect of an exercise training intervention on hepatic steatosis. Hepatology. 2008;48(supplement 1):p. 806A. [Google Scholar]
- 42.Charbonneau A, Melancon A, Lavoie C, Lavoie JM. Alterations in hepatic glucagon receptor density and in Gsα and Giα2 protein content with diet-induced hepatic steatosis: effects of acute exercise. American Journal of Physiology—Endocrinology and Metabolism. 2005;289(1):E8–E14. doi: 10.1152/ajpendo.00570.2004. [DOI] [PubMed] [Google Scholar]
- 43.Charbonneau A, Unson CG, Lavoie JM. High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise. Journal of Physiology. 2007;579(1):255–267. doi: 10.1113/jphysiol.2006.121954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haufe S, Engeli S, Budziarek P, et al. Cardiorespiratory fitness and insulin sensitivity in overweight or obese subjects may be linked through intrahepatic lipid content. Diabetes. 2010;59(7):1640–1647. doi: 10.2337/db09-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maling HM, Stern DN, Altland PD, Highman B, Brodie BB. The physiologic role of the sympathetic nervous system in exercise. Journal of Pharmacology and Experimental Therapeutics. 1966;154(1):35–45. [PubMed] [Google Scholar]
- 46.Gorski J, Kiryluk T. The post-exercise recovery of triglycerides in rat tissues. European Journal of Applied Physiology and Occupational Physiology. 1980;45(1):33–41. doi: 10.1007/BF00421199. [DOI] [PubMed] [Google Scholar]
- 47.Altland PD, Highman B. Effects of exercise on serum enzyme values and tissues of rats. American Journal of Physiology. 1961;201:393–395. doi: 10.1152/ajplegacy.1961.201.2.393. [DOI] [PubMed] [Google Scholar]
- 48.Straczkowski M, Kowalska I, Górski J, Kinalska I. The effect of a single bout of exhaustive exercise on muscle carbohydrate and lipid metabolism in a rat model of type 2 diabetes mellitus. Acta Diabetologica. 2000;37(1):47–53. doi: 10.1007/s005920070035. [DOI] [PubMed] [Google Scholar]
- 49.Rector SR, Thyfault JP, Laye MJ, et al. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Journal of Physiology. 2008;586(17):4241–4249. doi: 10.1113/jphysiol.2008.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lowney P, Lee VM, Hansen RJ, Stern JS. Effects of exercise, detraining, starvation, and refeeding on lipogenic capacity of Osborne-Mendel rat. American Journal of Physiology. 1988;254(4):R648–R654. doi: 10.1152/ajpregu.1988.254.4.R648. [DOI] [PubMed] [Google Scholar]


