Abstract
There is growing evidence that sonic hedgehog (Shh) signaling regulates ventral neuronal fate in the vertebrate central nervous system through Nkx-class homeodomain proteins. We have examined the patterns of neurogenesis in mice carrying a targeted mutation in Nkx6.1. These mutants show a dorsal-to-ventral switch in the identity of progenitors and in the fate of postmitotic neurons. At many axial levels there is a complete block in the generation of V2 interneurons and motor neurons and a compensatory ventral expansion in the domain of generation of V1 neurons, demonstrating the essential functions of Nkx6.1 in regional patterning and neuronal fate determination.
Keywords: Nkx, spinal cord, motor neurons, neuronal specification, interneurons
During the development of the embryonic central nervous system (CNS) the mechanisms that specify regional identity and neuronal fate are intimately linked (Anderson et al. 1997; Lumsden and Krumlauf 1996; Rubenstein et al. 1998). In the ventral half of the CNS, for example, the secreted factor Sonic hedgehog (Shh) has a fundamental role in controlling both regional pattern and neuronal fate (Tanabe and Jessell 1996; Ericson et al. 1997a; Hammerschmidt et al. 1997). Shh appears to function as a gradient signal. In the spinal cord, five distinct classes of neurons can be generated in vitro in response to two- to threefold changes in the concentration of Shh, and the position at which each neuronal class is generated in vivo is predicted by the concentration required for their induction in vivo (Ericson et al. 1997a,b; Briscoe et al. 2000). Thus, neurons generated in more ventral regions of the neural tube require progressively higher concentrations of Shh for their induction.
The genetic programs activated in neural progenitor cells in response to Shh signaling, however, remain incompletely defined. Emerging evidence suggests that homeobox genes function as critical intermediaries in the neural response to Shh signals (Lumsden and Krumlauf 1996; Tanabe and Jessell 1996; Ericson et al. 1997a,b; Hammerschmidt et al. 1997; Rubenstein et al. 1998). Several homeobox genes are expressed by ventral progenitor cells, and their expression is regulated by Shh. Gain-of-function studies on homeobox gene action in the chick neural tube have provided evidence that homeodomain proteins are critical for the interpretation of graded Shh signaling and that they function to delineate progenitor domains and control neuronal subtype identity (Briscoe et al. 2000). Consistent with these findings, the pattern of generation of neuronal subtypes in the basal telencephalon and in the ventral-most region of the spinal cord is perturbed in mice carrying mutations in certain Shh-regulated homeobox genes (Ericson et al. 1997b; Sussel et al. 1999; Pierani et al., unpubl.).
Members of the Nkx class of homeobox genes are expressed by progenitor cells along the entire rostro-caudal axis of the ventral neural tube, and their expression is dependent on Shh signaling (Rubenstein and Beachy 1998). Mutation in the Nkx2.1 or Nkx2.2 genes leads to defects in ventral neural patterning (Briscoe et al. 1999; Sussel et al. 1999), raising the possibility that Nkx genes play a key role in the control of ventral patterning in the ventral region of the CNS. Genetic studies to assess the role of Nkx genes have, however, focused on only the most ventral region of the neural tube. A recently identified Nkx gene, Nkx6.1, is expressed more widely by most progenitor cells within the ventral neural tube (Pabst et al. 1998; Qiu et al. 1998; Briscoe et al. 1999), suggesting that it may have a prominent role in ventral neural patterning. Here we show that in mouse embryos Nkx6.1 is expressed by ventral progenitors that give rise to motor (MN), V2, and V3 neurons. Mice carrying a null mutation of Nkx6.1 exhibit a ventral-to-dorsal switch in the identity of progenitor cells and a corresponding switch in the identity of the neuronal subtype that emerges from the ventral neural tube. The generation of MN and V2 neurons is markedly reduced, and there is a ventral expansion in the generation of a more dorsal V1 neuronal subtype. Together, these findings indicate that Nkx6.1 has a critical role in the specification of MN and V2 neuron subtype identity and, more generally, that Nkx genes play a role in the interpretation of graded Shh signaling.
Results and Discussion
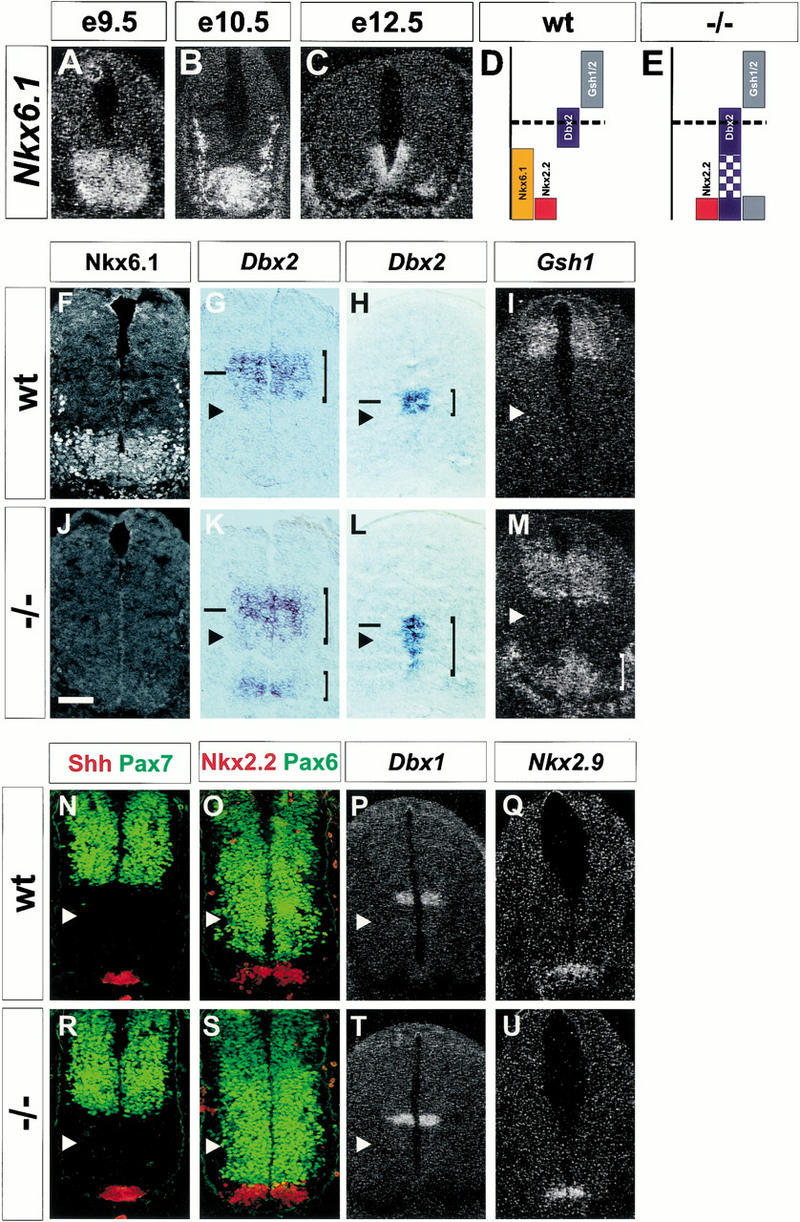
To define the role of Nkx6.1 in neural development, we compared patterns of neurogenesis in the embryonic spinal cord and hindbrain of wild-type mice and mice lacking Nkx6.1 (Sander et al. 1998). In wild-type embryos, neural expression of Nkx6.1 is first detected at spinal cord and caudal hindbrain levels at about embryonic day 8.5 (E8.5; Qiu et al. 1998; data not shown), and by E9.5 the gene is expressed throughout the ventral third of the neural tube (Fig. 1A). The expression of Nkx6.1 persists until at least E12.5 (Fig. 1B,C; data not shown). Nkx6.1 expression was also detected in mesodermal cells flanking the ventral spinal cord (Fig. 1B,C). To define more precisely the domain of expression of Nkx6.1, we compared its expression with that of 10 homeobox genes—Pax3, Pax7, Gsh1, Gsh2, Irx3, Pax6, Dbx1, Dbx1, Dbx2, and Nkx2.9—that have been shown to define discrete progenitor cell domains along the dorsoventral axis of the ventral neural tube (Goulding et al. 1991; Valerius et al. 1995; Ericson et al. 1997a,b; Pierani et al. 1999; Briscoe et al. 2000).
Figure 1.
Selective changes in homeobox gene expression in ventral progenitor cells in Nkx6.1 mutant embryos. Expression of Nkx6.1 in transverse sections of the ventral neural tube at E9.5 (A), E10.5 (B), or E12.5 (C). Summary diagrams showing domains of homeobox gene expression in wild-type mouse embryos (D) and the change in pattern of expression of these genes in Nkx6.1 mutants (E), based on analyses at E10.0–12.5. Expression of Nkx6.1 (F,J), Dbx2 (G,H,K,L), and Gsh1 (I,M) in the caudal neural tube of wild-type (F–I) and Nkx6.1 mutant (J–M) embryos. Horizontal lines, approximate position of dorsoventral boundary of the neural tube; vertical lines, expression levels of Dbx2 and Gsh1. Expression of Shh (N,R), Pax7 (N,R), Nkx2.2 (O,S), Pax6 (P,S), Dbx1 (P,T), and Nkx2.9 (Q,U) in wild-type (N–Q) or Nkx6–1 mutant (R–U) embryos at spinal (N–P,R–T) and caudal hindbrain (Q,U) levels. Arrowheads, approximate position of the dorsal limit of Nkx6.1 expression. Scale bar shown in J = 100 μm (A–C), 50 μm (F–M), or 60 μm (N–U).
This analysis revealed that the dorsal boundary of Nkx6.1 expression is positioned ventral to the boundaries of four genes expressed in by dorsal progenitor cells: Pax3, Pax7, Gsh1, and Gsh2 (Fig. 1I,N; data not shown). Within the ventral neural tube, the dorsal boundary of Nkx6.1 expression is positioned ventral to the domain of Dbx1 expression and close to the ventral boundary of Dbx2 expression (Fig. 1G,H,P). The domain of Pax6 expression extends ventrally into the domain of Nkx6.1 expression (Fig. 1O), whereas the expression of Nkx2.2 and Nkx2.9 overlaps with the ventral-most domain of Nkx6.1 expression (Fig. 1O,Q).
To address the function of Nkx6.1 in neural development, we analyzed progenitor cell identity and the pattern of neuronal differentiation in Nkx6.1 null mutant mice (Sander et al. 1998). We detected a striking change in the profile of expression of three homeobox genes, Dbx2, Gsh1, and Gsh2, in Nkx6.1 mutants. The domains of expression of Dbx2, Gsh1, and Gsh2 each expanded into the ventral neural tube (Fig. 1K–M; data not shown). At E10.5, Dbx2 was expressed at high levels by progenitor cells adjacent to the floor plate, but at this stage ectopic Dbx2 expression was detected only at low levels in regions of the neural tube that generate motor neurons (Fig. 1K). By E12.5, however, the ectopic ventral expression of Dbx2 had become more uniform and now clearly included the region of motor neuron and V2 neuron generation (Fig. 1L). Similarly, in Nkx6.1 mutants, both Gsh1 and Gsh2 were ectopically expressed in a ventral domain of the neural tube and also in adjacent paraxial mesodermal cells (Fig. 1M; data not shown).
The ventral limit of Pax6 expression was unaltered in Nkx6.1 mutants, although the most ventrally located cells within this progenitor domain expressed a higher level of Pax6 protein than those in wild-type embryos (Fig. 1O,S). We detected no change in the patterns of expression of Pax3, Pax7, Dbx1, Irx3, Nkx2.2, or Nkx2.9 in Nkx6.1 mutant embryos (Fig. 1R–U; data not shown). Importantly, the level of Shh expression by floor plate cells was unaltered in Nkx6.1 mutants (Fig. 1N,R). Thus, the loss of Nkx6.1 function deregulates the patterns of expression of a selected subset of homeobox genes in ventral progenitor cells without an obvious effect on Shh levels (Fig. 1D,E). The role of Shh in excluding Dbx2 from the most ventral region of the neural tube (Pierani et al. 1999) appears therefore to be mediated through the induction of Nkx6.1 expression. Consistent with this view, ectopic expression of Nkx6.1 represses Dbx2 expression in chick neural tube (Briscoe et al. 2000). The detection of sites of ectopic Gsh1/2 expression in the paraxial mesoderm as well as the ventral neural tube, both sites of Nkx6.1 expression, suggests that Nkx6.1 has a general role in restricting Gsh1/2 expression. The signals that promote ventral Gsh1/2 expression in Nkx6.1 mutants remain unclear but could involve factors other than Shh that are secreted by the notochord (Hebrok et al. 1998).
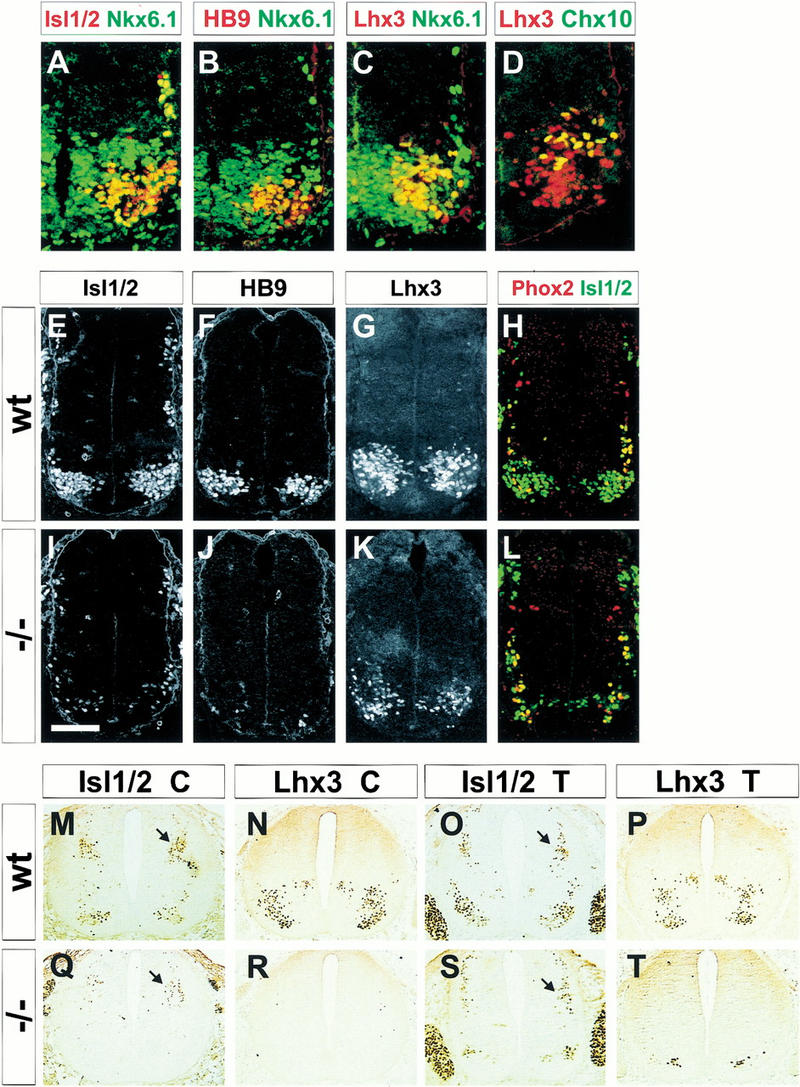
The domain of expression of Nkx6.1 within the ventral neural tube of wild-type embryos encompasses the progenitors of three main neuronal classes: V2, MN, and V3 interneurons (Goulding et al. 1991; Ericson et al. 1997a,b; Qiu et al. 1998; Briscoe et al. 1999, 2000; Pierani et al. 1999; Fig. 2A–D). We examined whether the generation of any of these neuronal classes is impaired in Nkx6.1 mutants, focusing first on the generation of motor neurons. In Nkx6.1 mutant embryos there was a marked reduction in the number of spinal motor neurons, as assessed by expression of the homeodomain proteins Lhx3, Isl1/2, and HB9 (Arber et al. 1999; Tsuchida et al. 1994; Fig. 2E–L) and by expression of the gene encoding the transmitter synthetic enzyme choline acetyltransferase (data not shown). In addition, few if any axons were observed to emerge from the ventral spinal cord (data not shown). The incidence of motor neuron loss, however, varied along the rostrocaudal axis of the spinal cord. Few if any motor neurons were detected at caudal cervical and upper thoracic levels of Nkx6.1 mutants analyzed at E11–E12.5 (Fig. 2M,N,Q,R), whereas motor neuron number was reduced only by 50%–75% at more caudal levels (Fig. 2O,P,S,T; data not shown). At all axial levels, the initial reduction in motor neuron number persisted at both E12.5 and p0 (Fig. 2M–T; data not shown), indicating that the loss of Nkx6.1 activity does not simply delay motor neuron generation. Moreover, we detected no increase in the incidence of TUNEL+ cells in Nkx6.1 mutants (data not shown), providing evidence that the depletion of motor neurons does not result solely from apoptotic death.
Figure 2.
Disruption of motor neuron differentiation in Nkx6.1 mutant embryos. The relationship between the domain of Nkx6.1 expression (A–C, green) by ventral progenitors and the position of generation of motor neurons and V2 interneurons (A–D, red) in the ventral spinal cord of E10.5 wild-type embryos. (A) Isl1/2 motor neurons; (B) HB9 motor neurons; (C) Lhx3 (Lim3) expression (red) by motor neurons, V2 interneurons, and their progenitors is confined to the Nkx6.1 progenitor domain. (D) Chx10 (green) V2 interneurons coexpress Lhx3 (red). Expression of Isl1/2 (E,I), HB9 (F,J), Lhx3 (G,K), and Phox2a/b (H,L) in the ventral spinal cord (E,F,G) and caudal hindbrain (H) of E10.5 wild-type (E–H) and Nkx6.1 mutant (I–L) embryos. Pattern of expression of Isl1/2 and Lhx3 at cervical (M,N,Q,R) and thoracic (O,P,S,T) levels of E12.5 wild-type (M–P) and Nkx6.1 mutant (Q–T) embryos. Arrows, position of Isl1 dorsal D2 interneurons. Scale bar shown in I = 60 μm (A–D), 80 μm (E–L), or 120 μm (M–T).
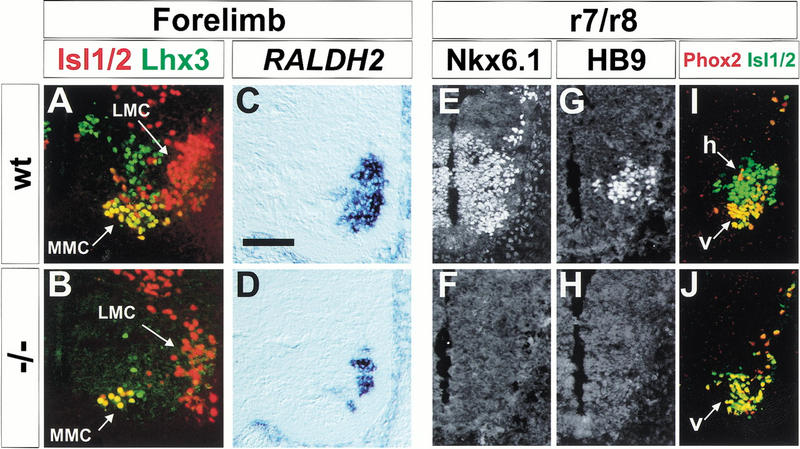
The persistence of some spinal motor neurons in Nkx6.1 mutants raised the possibility that the generation of particular subclasses of motor neurons is selectively impaired. To address this issue, we monitored the expression of markers of distinct subtypes of motor neurons at both spinal and hindbrain levels of Nkx6.1 mutant embryos. At spinal levels, the extent of the reduction in the generation of motor neurons that populate the median (MMC) and lateral (LMC) motor columns was similar in Nkx6.1 mutants as assessed by the number of motor neurons that coexpressed Isl1/2 and Lhx3 (defining MMC neurons; Fig. 3A,B) and by the expression of Raldh2 (defining LMC neurons; Sockanathan and Jessell 1998; Arber et al. 1999; Fig. 3C,D). In addition, the generation of autonomic visceral motor neurons was reduced to an extent similar to that of somatic motor neurons at thoracic levels of the spinal cord of E12.5 embryos (data not shown). Thus, the loss of Nkx6.1 activity depletes the major subclasses of spinal motor neurons to a similar extent.
Figure 3.
Motor neuron subtype differentiation in Nkx6.1 mutant mice. Depletion of both median (MMC) and lateral (LMC) motor column neurons in Nkx6.1 mutant mice. Expression of Isl1/2 (red) and Lhx3 (green) in E12.5 wild-type (A,C) and Nkx6.1 mutant (B,D) mice spinal cord at forelimb levels. (E–J) Motor neuron generation at caudal hindbrain level. (E,F) Nkx6.1 expression in progenitor cells and visceral motor neurons in the caudal hindbrain (rhombomere [r] 7/8) of E10.5–E11 wild-type (E) Nkx6.1 mutant (F) mice. HB9 expression in hypoglossal motor neurons in E10.5–E11 wild-type (G) and Nkx6.1 mutant (H) mice. Coexpression of Isl1 (green) and Phox2a/b (red) in wild-type (I) or Nkx6.1 mutant (J) mice. (h) hypoglossal motor neurons; (v) visceral vagal motor neurons. Scale bar shown in C = 50 μm (A–D) or 70 μm (E–J).
At hindbrain levels, Nkx6.1 is expressed by the progenitors of both somatic and visceral motor neurons (Fig. 3E,F; data not shown). We therefore examined whether the loss of Nkx6.1 might selectively affect subsets of cranial motor neurons. We detected a virtually complete loss in the generation of hypoglossal and abducens somatic motor neurons in Nkx6.1 mutants, as assessed by the absence of dorsally generated HB9+ motor neurons (Fig. 3G,H; data not shown; Arber et al. 1999; Briscoe et al. 1999). In contrast, there was no change in the initial generation of any of the cranial visceral motor neuron populations, assessed by coexpression of Isl1 and Phox2a (Briscoe et al. 1999; Pattyn et al. 1997) within ventrally generated motor neurons (Fig. 3I,J; data not shown). Moreover, at rostral cervical levels, the generation of spinal accessory motor neurons (Ericson et al. 1997a,b) was also preserved in Nkx6.1 mutants (data not shown). Thus, in the hindbrain the loss of Nkx6.1 activity selectively eliminates the generation of somatic motor neurons, while leaving visceral motor neurons intact. Cranial visceral motor neurons, unlike spinal visceral motor neurons, derive from progenitors that express the related Nkx genes Nkx2.2 and Nkx2.9 (Briscoe et al. 1999). The preservation of cranial visceral motor neurons in Nkx6.1 mutant embryos may therefore reflect the dominant activities of Nkx2.2 and Nkx2.9 within these progenitor cells.
We next examined whether the generation of ventral interneurons is affected by the loss of Nkx6.1 activity. V2 and V3 interneurons are defined, respectively, by expression of Chx10 and Sim1 (Arber et al. 1999; Briscoe et al. 1999; Fig. 4A,G). A severe loss of Chx10 V2 neurons was detected in Nkx6.1 mutants at spinal cord levels (Fig. 4B), although at hindbrain levels of Nkx6.1, mutants ∼50% of V2 neurons persisted (data not shown). In contrast, there was no change in the generation of Sim1 V3 interneurons at any axial level of Nkx6.1 mutants (Fig. 4H). Thus, the elimination of Nkx6.1 activity affects the generation of only one of the two major classes of ventral interneurons that derive from the Nkx6.1 progenitor cell domain.
Figure 4.
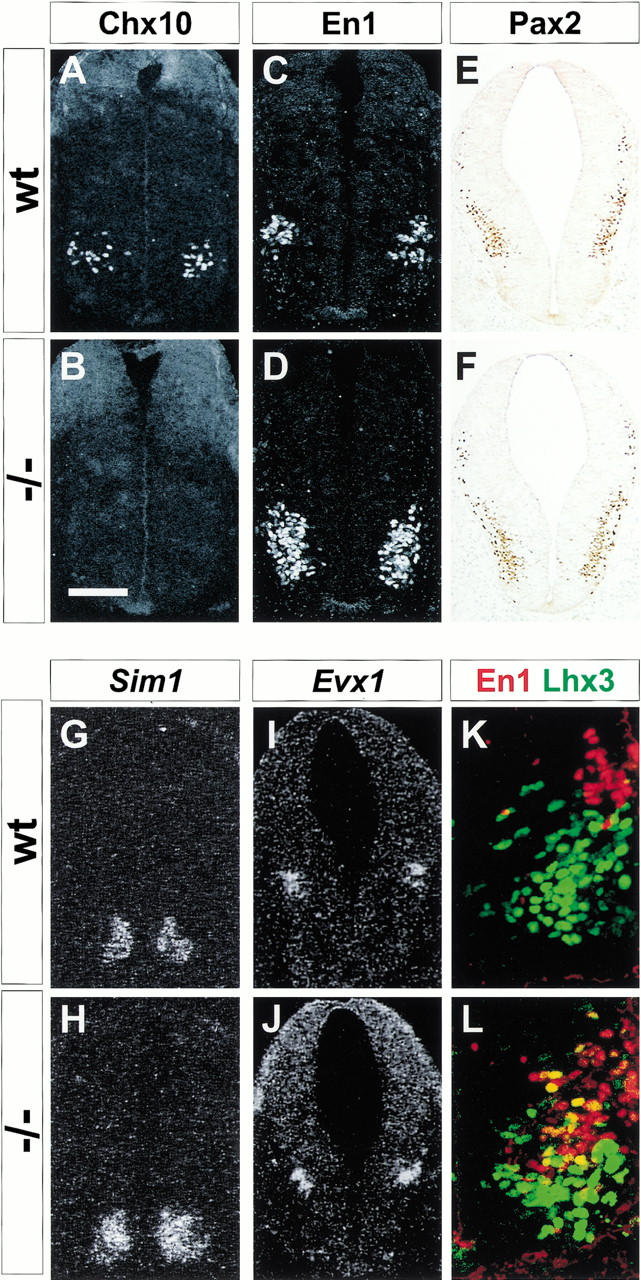
A switch in ventral interneuron fates in Nkx6.1 mutant mice. Chx10 expression in V2 neurons at rostral cervical levels of E10.5 wild-type (A) and Nkx6.1 mutant (B) embryos. En1 expression by V1 neurons at rostral cervical levels of wild-type (C) and Nkx6.1 mutant (D) embryos. Pax2 expression in a set of interneurons that includes V1 neurons (Burrill et al. 1997) at caudal hindbrain levels of wild-type (E) and Nkx6.1 mutant (F) embryos. (G,H) Sim1 expression by V3 neurons in the cervical spinal cord of wild-type (G) and Nkx6.1 mutant (H) embryos. Evx1 expression by V0 neurons at caudal hindbrain levels of wild-type (I) and Nkx6.1 mutant (J) embryos. En1 (red) and Lhx3 (green) expression by separate cell populations in the ventral spinal cord of E11 wild-type (K) and Nkx6.1 mutant (L) embryos. Scale bar shown in B = 60 μm (A–D), 75 μm (E,F), 70 μm (G,J,H,J), or 35μ m (K,L).
Evx1+, Pax2+ V1 interneurons derive from progenitor cells located dorsal to the Nkx6.1 progenitor domain (Fig. 4B) within a domain that expresses Dbx2 but not Dbx1 (Burrill et al. 1997; Matise and Joyner 1997; Pierani et al. 1999). Because Dbx2 expression undergoes a marked ventral expansion in Nkx6.1 mutants, we examined whether there might be a corresponding expansion in the domain of generation of V1 neurons. In Nkx6.1 mutants, the region that normally gives rise to V2 neurons and motor neurons now also generated V1 neurons, as assessed by the ventral shift in expression of the En1 and Pax2 homeodomain proteins (Fig. 4B,C,E,F). Consistent with this, there was a two- to threefold increase in the total number of V1 neurons generated in Nkx6.1 mutants (Fig. 4C,D). In contrast, the domain of generation of Evx1/2 V0 neurons, which derive from the Dbx1 progenitor domain (Pierani et al. 1999), was unchanged in Nkx6.1 mutants (Fig. 4I,J). Thus, the ventral expansion in Dbx2 expression is accompanied by a selective switch in interneuronal fates from V2 neurons to V1 neurons. In addition, we observed that some neurons within the ventral spinal cord of Nkx6.1 mutants coexpressed the V1 marker En1 and the V2 marker Lhx3 (Fig. 4K,L). The coexpression of these markers is rarely if ever observed in single neurons in wild-type embryos (Ericson et al. 1996). Thus, within individual neurons in Nkx6.1 mutants, the ectopic program of V1 neurogenesis appears to be initiated in parallel with a residual, albeit transient, program of V2 neuron generation. This result complements observations in Hb9 mutant mice, in which the programs of V2 neuron and motor neuron generation coincide transiently within individual neurons (Arber et al. 1999; Thaler et al. 1999).
Taken together, our findings reveal an essential role for the Nkx6.1 homeobox gene in the specification of regional pattern and neuronal fate in the ventral half of the mammalian CNS. Within the broad ventral domain within which Nkx6.1 is expressed (Fig. 5A), its activity is required to promote MN and V2 interneuron generation and to restrict the generation of V1 interneurons (Fig. 5B). We favor the idea that the loss of MN and V2 neurons is a direct consequence of the loss of Nkx6.1 activity, as the depletion of these two neuronal subtypes is evident at stages when only low levels of Dbx2 are expressed ectopically in most regions of the ventral neural tube. Nonetheless, we can not exclude that low levels of ectopic ventral Dbx2 expression could contribute to the block in motor neuron generation. Consistent with this view, the ectopic expression of Nkx6.1 is able to induce both motor neurons and V2 neurons in chick neural tube (Briscoe et al. 2000). V3 interneurons and cranial visceral motor neurons derive from a set of Nkx6.1 progenitors that also express Nkx2.2 and Nkx2.9 (Briscoe et al. 1999; Fig. 5A). The generation of these two neuronal subtypes is unaffected by the loss of Nkx6.1 activity, suggesting that the actions of Nkx2.2 and Nkx2.9 dominate over that of Nkx6.1 within these progenitors. The persistence of some spinal motor neurons and V2 neurons in Nkx6.1 mutants could reflect the existence of a functional homologue within the caudal neural tube.
Figure 5.
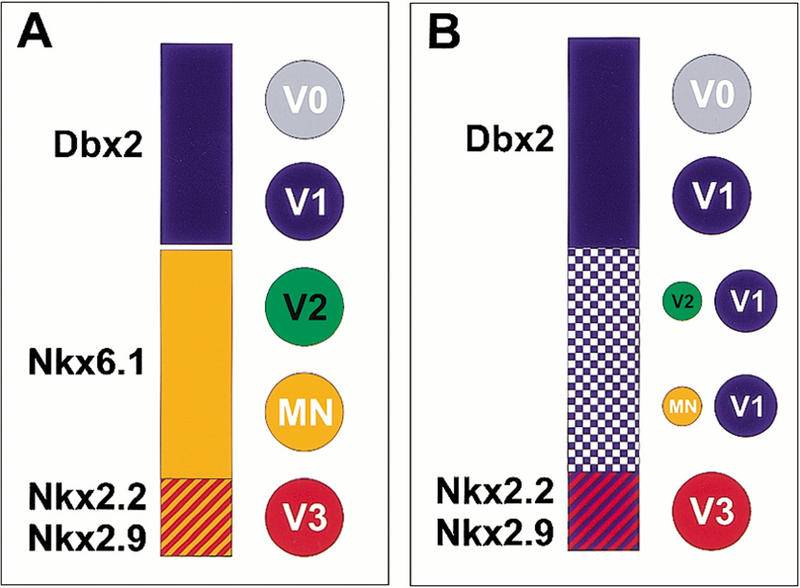
Changes in progenitor domain identity and neuronal fate in the spinal cord of Nkx6.1 mutant embryos. (A) In wild-type mouse embryos, cells in the Nkx6.1 progenitor domain give rise to three classes of ventral neurons: V2, motor (MN), and V3 neurons. V3 neurons derive from cells in the ventral most region of Nkx6.1 expression that also express Nkx2.2 and Nkx2.9. V1 neurons derive from progenitor cells that express Dbx2 but not Nkx6.1. (B). In Nkx6.1 mutant embryos the domain of Dbx2 expression by progenitor cells expands ventrally and by embryonic day 12 (E12) occupies the entire dorsoventral extent of the ventral neural tube, excluding the floor plate. Checked blue indicates the gradual onset of ventral Dbx2 expression. This ventral shift in Dbx2 expression is associated with a marked decrease in the generation of V2 and MN neurons and a ventral expansion in the domain of generation of V1 neurons. A virtually complete loss of MN and V2 neurons is observed at cervical levels of the spinal cord. The generation of V3 neurons (and cranial visceral motor neurons at hindbrain levels) is unaffected by the loss of Nkx6.1 or by the ectopic expression of Dbx2.
The role of Nkx6.1 revealed in these studies, taken together with previous findings, suggests a model in which the spatially restricted expression of Nkx genes within the ventral neural tube (Fig. 5) has a pivotal role in defining the identity of ventral cell types induced in response to graded Shh signaling. Strikingly, in Drosophila, the Nkx gene NK2 has been shown to have an equivalent role in specifying neuronal fates fate in the ventral nerve cord (Chu et al. 1998; McDonald et al. 1998). Moreover, the ability of Nkx6.1 to function as a repressor of the dorsally expressed Gsh1/2 homeobox genes parallels the ability of Drosophila NK2 to repress Ind, a Gsh1/2-like homeobox gene (Weiss et al. 1998). Thus, the evolutionary origin of regional pattern along the dorsoventral axis of the central nervous system may predate the divergence of invertebrate and vertebrate organisms.
Materials and methods
Generation of Nkx6.1 null mutation
A null mutation in Nkx6.1 was generated by gene targeting in 129-strain ES cells by excising an 800-bp NotI fragment containing part of exon1 and replacing it by a PGK-neo cassette (M. Sander and M. German, unpubl.). Mutants were born at Mendelian frequency and died soon after birth; they exhibited movements only upon tactile stimulation.
Immunocytochemistry and in situ hybridization
Localization of mRNA was performed by in situ hybridization following the method of Schaeren-Wiemers and Gerfin-Moser (1993). The Dbx2 riboprobe comprised the 5′ EcoR1 fragment of the mouse cDNA (Pierani et al. 1999). Probes for other cDNAs were cited in the text and used as described therein. Protein expression was localized by indirect fluorescence immunocytochemistry or peroxidase immunohistochemistry (Briscoe et al. 1999; Ericson et al. 1997b). Nkx6.1 was detected with a rabbit antiserum (Briscoe et al. 1999). Antisera against Shh, Pax7, Isl1/2, HB9, Lhx3, Chx10, Phox2a/b, En1, and Pax2 have been described (Briscoe et al. 1999; Ericson et al. 1997b). Fluorescence detection was carried out using an MRC 1024 Confocal Microscope (BioRad).
Acknowledgments
We thank K. MacArthur and S. Yu for help in preparing the manuscript; the following people for cDNAs: P. Gruss, S. Potter, F.Ruddle, R. McInnes, A. Joyner, G. Martin, M.Tessier-Lavigne, and C. Gall; J.F. Brunet for Phox2; and H. Westphal for Lhx3 antibodies. JLRR is supported by Nina Ireland, NARSAD, NIDA (R01DA12462), and NIMH K02 MH01046–01, J.B. is a research associate of the Howard Hughes Medical Institute, J.E. is supported by the Swedish Foundation for Strategic research, the Swedish National Science Research Council, the Karolinska Institute, and the Harald and Greta Jeanssons Foundation. M.G. is supported by NIH (grants DK41822 and DK21344). M.S. was supported by the Deutsche Forschungsgemeinschaft and the Juvenile Diabetes Foundation International. T.J. is supported by NIH and is an Investigator of the Howard Hughes Research Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL jlrr@cgl.ucsf.edu; FAX (415) 502-7618.
Article and publication are at www.genesdev.org/cgi/doi/10.1101/gad.820400.
References
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: Dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O'Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson E. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Burrill JD, Moran L, Goulding MD, Saueressig H. PAX2 is expressed in multiple spinal cord interneurons, including a population of EN1+ interneurons that require PAX6 for their development. Development. 1997;124:4493–4503. doi: 10.1242/dev.124.22.4493. [DOI] [PubMed] [Google Scholar]
- Chu H, Parras C, White K, Jimenez F. Formation and specification of ventral neuroblasts is controlled by vnd in Drosophila neurogenesis. Genes & Dev. 1998;12:3613–3624. doi: 10.1101/gad.12.22.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Briscoe J, Rashbass P, van Heyningen V, Jessell TM. Graded sonic hedgehog signaling and the specification of cell fate in the ventral neural tube. Cold Spring Harbor Symp Quant Biol. 1997a;62:451–466. [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997b;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Goulding MD, Chalepakis G, Deutsch U, Erselius JR, Gruss P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 1991;10:1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt M, Brook A, McMahon AP. The world according to hedgehog. Trends Genet. 1997;13:14–21. doi: 10.1016/s0168-9525(96)10051-2. [DOI] [PubMed] [Google Scholar]
- Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes & Dev. 1998;12:1705–1713. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden A, Krumlauf R. Patterning the vertebrate neuraxis. Science. 1996;274:1109–1115. doi: 10.1126/science.274.5290.1109. [DOI] [PubMed] [Google Scholar]
- Matise MP, Joyner AL. Expression patterns of developmental control genes in normal and Engrailed-1 mutant mouse spinal cord reveal early diversity in developing interneurons. J Neurosci. 1997;17:7805–7816. doi: 10.1523/JNEUROSCI.17-20-07805.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JA, Holbrook S, Isshiki T, Weiss J, Doe CQ, Mellerick DM. Dorsoventral patterning in the Drosophila central nervous system: The vnd homeobox gene specifies ventral column identity. Genes & Dev. 1998;12:3603–3612. doi: 10.1101/gad.12.22.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst O, Herbrand H, Arnold HH. Nkx2–9 is a novel homeobox transcription factor which demarcates ventral domains in the developing mouse CNS. Mech Dev. 1998;73:85–93. doi: 10.1016/s0925-4773(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- Pierani A, Brenner-Morton S, Chiang C, Jessell TM. A sonic hedgehog–independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell. 1999;97:903–915. doi: 10.1016/s0092-8674(00)80802-8. [DOI] [PubMed] [Google Scholar]
- Qiu M, Shimamura K, Sussel L, Chen S, Rubenstein JL. Control of anteroposterior and dorsoventral domains of Nkx-6.1 gene expression relative to other Nkx genes during vertebrate CNS development. Mech Dev. 1998;72:77–88. doi: 10.1016/s0925-4773(98)00018-5. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Beachy PA. Patterning of the embryonic forebrain. Curr Opin Neurobiol. 1998;8:18–26. doi: 10.1016/s0959-4388(98)80004-4. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Shimamura K, Martinez S, Puelles L. Regionalization of the prosencephalic neural plate. Annu Rev Neurosci. 1998;21:445–477. doi: 10.1146/annurev.neuro.21.1.445. [DOI] [PubMed] [Google Scholar]
- Sander M, Kalamaras J, German MS. Keystone symposium on vertebrate development. 1998. Steamboat Springs, Colorado, April 8, 1998. [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: In situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Jessell TM. Motor neuron–derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell. 1998;94:503–514. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: Evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Jessell TM. Diversity and pattern in the developing spinal cord. Science. 1996;274:1115–1123. doi: 10.1126/science.274.5290.1115. [DOI] [PubMed] [Google Scholar]
- Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, Pfaff SL. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23:675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Valerius MT, Li H, Stock JL, Weinstein M, Kaur S, Singh G, Potter SS. Gsh-1: A novel murine homeobox gene expressed in the central nervous system. Dev Dyn. 1995;203:337–351. doi: 10.1002/aja.1002030306. [DOI] [PubMed] [Google Scholar]
- Weiss JB, Von Ohlen T, Mellerick DM, Dressler G, Doe CQ, Scott MP. Dorsoventral patterning in the Drosophila central nervous system: The intermediate neuroblasts defective homeobox gene specifies intermediate column identity. Genes & Dev. 1998;12:3591–3602. doi: 10.1101/gad.12.22.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]