Abstract
Progressive supranuclear palsy (PSP) is associated with pathological changes along the dentatorubrothalamic tract and in premotor cortex. We aimed to assess whether functional neural connectivity is disrupted along this pathway in PSP, and to determine how functional changes relate to changes in structure and diffusion. Eighteen probable PSP subjects and 18 controls had resting-state (task-free) fMRI, diffusion tensor imaging and structural MRI. Functional connectivity was assessed between thalamus and the rest of the brain, and within the basal ganglia, salience and default mode networks (DMN). Patterns of atrophy were assessed using voxel-based morphometry, and patterns of white matter tract degeneration were assessed using tract-based spatial statistics. Reduced in-phase functional connectivity was observed between the thalamus and premotor cortex including supplemental motor area (SMA), striatum, thalamus and cerebellum in PSP. Reduced connectivity in premotor cortex, striatum and thalamus were observed in the basal ganglia network and DMN, with subcortical salience network reductions. Tract degeneration was observed between cerebellum and thalamus and in superior longitudinal fasciculus, with grey matter loss in frontal lobe, premotor cortex, SMA and caudate. SMA functional connectivity correlated with SMA volume and measures of cognitive and motor dysfunction, while thalamic connectivity correlated with degeneration of superior cerebellar peduncles. PSP is therefore associated with disrupted thalamocortical connectivity that is associated with degeneration of the dentatorubrothalamic tract and the presence of cortical atrophy.
Keywords: Resting state fMRI, functional connectivity, white matter tracts, atrophy, dentatorubrothalamic tract
INTRODUCTION
Progressive supranuclear palsy (PSP) is a neurodegenerative disorder characterized by a symmetrical akinetic-rigid syndrome with vertical supranuclear gaze palsy and early falls[1]. Previous imaging studies have demonstrated subcortical and cortical atrophy[2, 3] and degeneration of white matter tracts in PSP[4, 5], particularly the dentatorubrothalamic tract running from the dentate nucleus of the cerebellum, through superior cerebellar peduncles to ventrolateral thalamus. The ventrolateral thalamus then in turn projects to the premotor cortex. Pathological studies have also found degeneration and activated microglia along this tract[6, 7]. Functional connections between regions on this tract, and subsequent connections to the cortex, may hence be abnormal in PSP.
Resting-state fMRI, also known as task-free fMRI, has emerged as an important technique to assess functional connectivity in the brain. Spontaneous neural activity occurs in the brain during rest and is organized into specific functional networks in which the low frequency changes in fMRI signal intensity are highly correlated or in-phase[8]. These networks relate to structurally connected neuroanatomical systems[9, 10], and it has been shown that these networks are altered in subjects with neurodegenerative disease[11, 12]. For example, Alzheimer’s disease affects the default mode network (DMN)[11] which is thought to be important for episodic memory processing, and frontotemporal dementia affects the salience network[12] which is involved in processing homeostatically relevant stimuli. The DMN and salience network are anticorrelated to each other in healthy brain suggesting an inverse relationship between these neural systems. However, it is unknown whether functional connectivity is disrupted in PSP, or whether functional abnormalities are anatomically related to regions of structural damage.
We aimed to assess functional connectivity in PSP using resting-state fMRI, and to relate these changes to patterns of atrophy and white matter tract degeneration. Since we were particularly interested in assessing the dentatorubrothalamic tract we assessed functional connectivity from the thalamus using a seed-based approach. We also performed secondary analyses assessing connectivity within the basal ganglia network, and also in the widely studied DMN and salience networks to assess cortical changes.
MATERIALS AND METHODS
Subjects
Between August 1st 2009 and April 30th 2010, we prospectively recruited all consecutive PSP subjects that were referred to the Department of Neurology at the Mayo Clinic, Rochester, Minnesota. All subjects were evaluated by a neurodegenerative specialist and PSP expert (KAJ), received a clinical diagnosis of PSP, and underwent a standardized imaging protocol including resting-state fMRI, DTI and volumetric MRI.
Inclusion criteria
Subjects must have met the NINDS-SPSP criteria for probable PSP[13]. Specifically, subjects must have been age 40 or older with symptoms being gradually progressive, and presence of vertical (upward or downward gaze) supranuclear palsy and prominent postural instability with falls in the first year. In addition, there could be no evidence of another disease to explain the symptoms and patients would have to meet mandatory exclusion criteria[13]. All subjects must have completed a usable resting-state fMRI, DTI and volumetric MRI.
Exclusion criteria
Subjects were excluded from the study if they met NINDS-SPSP criteria for possible PSP[13] or did not have all three usable imaging sequences.
Over this time period, 28 subjects were evaluated that met NINDS-SPSP criteria for possible or probable PSP[13]. Seven were excluded because they only met criteria for possible PSP and three were excluded because the resting-state fMRI sequence was unusable. Therefore, 18 subjects were included in this study. Informed consent was obtained from all subjects for participation in the studies, which were approved by the Mayo IRB. All subjects underwent detailed clinical and neurological examination with standardized and valid tests (Table 1). We recruited 18 healthy control subjects that were matched by age and gender to the 18 PSP subjects and who had the identical imaging acquisition. Subject demographics are shown in Table 1.
TABLE 1.
Subject demographics
| Controls (n=18) | PSP (n=18) | |
|---|---|---|
| Gender (% female) | 10 (56%) | 10 (56%) |
| Education (yrs) | 14.5 ± 2.1 | 14.5 ± 2.5 |
| Age at exam (yrs) | 68.3 ± 6.7 | 67.8 ± 7.3 |
| Age at onset (yrs) | NA | 64.4 ± 7.2 |
| Time onset to exam (yrs) | NA | 3.3 ± 1.8 |
| MMSE (/30) | 29.1 ± 0.9 | 27.9 ± 2.6 |
| FAB (/18) | NA | 13.5 ± 2.3 |
| PSPRS (/100) | NA | 33.2 ± 13.2 |
| UPDRS Part III | NA | 38.5 ± 15.8 |
Data shown as mean ± standard deviation. FAB = Frontal Assessment Battery (Dubois et al. Neurology 2000;55:1261–6); PSPRS = PSP Rating Scale (Golbe et al. Brain 2007;130:1552–65); MMSE = Mini-Mental State Examination (Folstein et al. Archives of General Psychiatry 1983;40:812); UPDRS = Unified Parksinon’s Disease Rating Scale (Goetz et al. Mov. Disord. 2007;22:41–7). No significant differences were observed across controls and PSP subjects in any variable.
Image Acquisition Protocols
A standardized protocol was performed on a 3T GE scanner. The resting-state fMRI signal time series was acquired using a gradient echo-planar (EPI) sequence (TR/TE = 3000/30 ms, 90° flip angle, slice thickness 3.3mm, in-plane resolution 3.3mm, and 103 volumes). Subjects were instructed to keep their eyes open during scanning. The DTI acquisition consisted of a single-shot EPI pulse sequence (TR= 10,200ms; in-plane matrix 128/128; in-plane resolution 2.7mm, FOV 35cm; phase field of view (FOV) 0.66; 42 diffusion encoding steps and four non-diffusion weighted T2 images; 2.7mm isotropic resolution). Parallel imaging with a sensitivity encoding (SENSE) factor of two was used. A 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) was also acquired (TR/TE/T1, 2300/3/900 ms; flip angle 8°, 26-cm FOV; 256 × 256 in-plane matrix with a phase FOV of 0.94, slice thickness of 1.2 mm, in-plane resolution 1.0mm). All MPRAGE images underwent pre-processing correction for gradient non-linearity and intensity non-uniformity.
Processing of Resting-state fMRI
Preprocessing and data analysis was performed utilizing SPM5 and the resting-state fMRI data analysis toolkit (REST) (http://www.restfmri.net). Preprocessing steps included discarding the first 3 volumes to obtain steady state magnetization, realignment, slice time correction, normalization to ICBM EPI template, smoothing with 4 mm full- width half maximum Gaussian kernel, linearly detrending to correct for signal drift, and 0.01–0.08 HZ bandpass filtering to reduce non-neuronal contributions to BOLD fluctuations. In addition, regression correction for spurious variables included rigid-body transformation motion effects, global mean signal, white matter and cerebral spinal fluid[14, 15]. These preprocessed images were analyzed using seed-based connectivity analysis and also Independent Component Analysis (ICA).
Seed-based analysis
A thalamic seed was defined using the automated anatomical labeling (AAL) atlas[16]. The average BOLD signal time course within the thalamic seed was correlated to every voxel in the brain for each subject using Pearson’s correlation coefficient. Before group comparison, the correlation coefficients were converted to z-scores using the Fischer r-to-z transformation. Regions that have positive z-scores between two fluctuating time-courses indicate “in-phase” connections and the regions with negative z-scores indicate “out-of-phase” connections. These z-score images were entered into the statistical analysis.
ICA Analysis
The independent components were first identified using model-free ICA (MELODIC)[17] in the control subjects with estimation of 20 independent components. Three independent component networks (ICNs) of interest were identified by visual inspection of the group independent components: the basal ganglia network[18], salience network and the DMN. Individual ICNs were then derived for all subjects utilizing the spatial and temporal dual regression method[19]. The individual components were entered into statistical analysis.
Identical statistical analyses were performed for the seed-based and ICA approach: 1) One sample t-tests were used to display the voxel-wise connectivity maps in controls and PSP subjects. 2) Two-sample two-sided t-tests were performed to compare voxel-wise connectivity between PSP subjects and controls. In order to assess only in-phase connections the group comparisons were masked by the out-of-phase connectivity map identified in the one sample t-tests of controls. 3) The two-sample t-tests were repeated controlling for atrophy on a voxel-wise basis by entering each subjects gray matter probability maps as a covariate using the Biological Parametric Mapping toolbox[20]. All analyses were assessed corrected for multiple comparisons using family wise error (FWE) at the cluster level (p<0.05) (http://www.sph.umich.edu/nichols/JG2/CorrClusTh.m).
Processing of Structural MRI
Voxel-based morphometry (VBM)[21], using SPM5, was employed to assess gray and white matter volume loss in PSP subjects compared to controls. All MPRAGE scans were normalized to a customized template consisting of all subjects in the study and segmented using unified segmentation. Grey and white matter images were modulated and smoothed at 8 mm FWHM. Two-sided t-tests were used to assess patterns of loss in the PSP subjects compared to controls. Since results did not survive FWE correction, all results were assessed uncorrected at p<0.001.
Processing of DTI
Each of the 42 diffusion-weighted images was registered to the non-diffusion weighted b0 volumes using affine transformations. Images were brain-extracted[22] and fractional anisotropy (FA) maps were generated[23]. Voxel-wise statistical analysis of the FA data was performed using tract-based Spatial statistics (TBSS)[24] (http://www.fmrib.ox.ac.uk/fsl). The FA images for each subject were aligned into common space using a non-linear registration, and then affinely transformed into MNI space. A mean FA image was created from all subjects, and was thinned to create a mean FA skeleton which represents the centers of all tracts common to the group. The FA skeleton was thresholded at >0.25 to exclude peripheral tracts with inter-subject variability and partial volume effects. Each subject’s aligned FA data was then projected onto this skeleton and the resulting data were fed into voxel-wise cross-group statistics. Results were assessed after correction for multiple comparisons using FWE at p<0.05.
Functional and structural metrics for correlation analyses
In order to correlate functional and structural changes, summary metrics were generated for each imaging modality. For the primary thalamic seed-based functional connectivity analysis, atlas-based parcellation was employed using SPM5 and the AAL atlas[16] to calculate mean signal intensity for each subject, for the supplemental motor area (SMA), thalamus, basal ganglia and cerebellum; regions where PSP subjects showed reduced connectivity compared to controls. Atlas-based parcellation was also used to generate grey matter volumes for each of these regions, as previously described[25]. Total intracranial volume (TIV) was calculated and used to correct regional grey matter volumes for differences in head size. For DTI, since we hypothesized that abnormalities in the dentatorubrothalamic tract could be related to functional changes in PSP, we measured mean FA in a superior cerebellar peduncle region-of-interest for each subject[5].
Statistical analysis
Statistical analyses were performed utilizing the JMP computer software (JMP Software, version 8.0; SAS Institute Inc, Cary, NC) with α set at 0.05. Binary data were compared with Chi-square test while t-tests were used for continuous data. Pair-wise correlation analyses were performed within PSP subjects to assess correlations between the regional functional connectivity measures (mean signal intensity in SMA, thalamus, basal ganglia and cerebellum) and 1) clinical measures, 2) grey matter volumes of SMA, thalamus, basal ganglia and cerebellum, and 3) mean FA in the superior cerebellar peduncle.
RESULTS
Resting-state fMRI
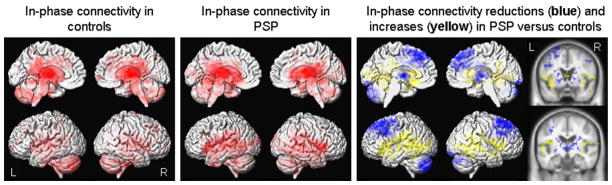
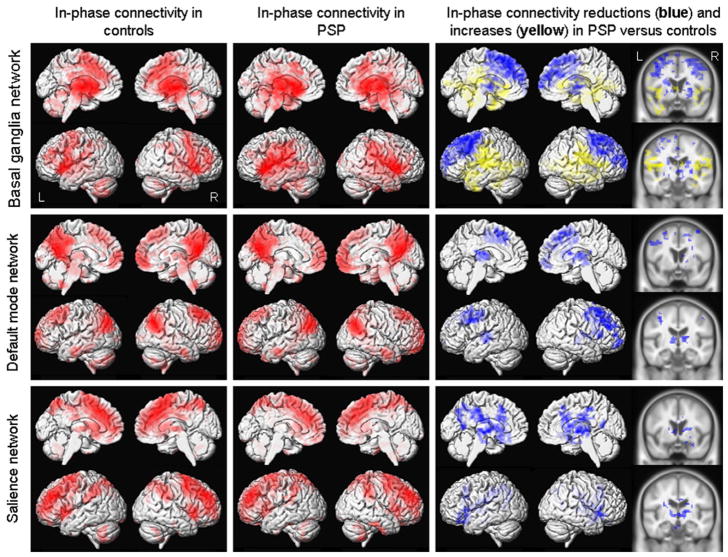
The result of the seed-based analysis of the thalamus is shown in Figure 1. Significantly reduced in-phase connectivity was observed in the premotor cortex, including SMA, extending into the posterior prefrontal cortex, thalamus, basal ganglia and cerebellum in PSP compared to controls. Increased in-phase connectivity was also observed in PSP predominantly in regions surrounding the perisylvian fissure. Similar patterns of altered in-phase connectivity were observed in the basal ganglia network using the ICA analysis (Figure 2), with reduced in-phase connectivity observed in PSP in the premotor cortex, including SMA, extending into the prefrontal cortex, striatum and thalamus, with increases observed surrounding the perisylvian fissure, compared to controls.
Figure 1.
Resting-state fMRI results from seed-based analysis of the thalamus. Left: Patterns of in-phase voxel-wise connectivity observed in control subjects; Middle: Patterns of in-phase voxel-wise connectivity observed in PSP subjects; Right: Patterns of reduced (shown in blue) and increased (shown in yellow) in-phase connectivity in the PSP subjects compared to controls, shown on both 3D renders and slices through the basal ganglia and thalamus. Results are shown after cluster-level correction for multiple comparisons at p<0.05.
Figure 2.
Resting-state fMRI results from ICA-based analyses of the basal ganglia network, DMN and salience network. Left: Patterns of in-phase voxel-wise connectivity observed in control subjects; Middle: Patterns of in-phase voxel-wise connectivity observed in PSP subjects; Right: Patterns of reduced (shown in blue) and increased (shown in yellow) in-phase connectivity in the PSP subjects compared to controls, shown on both 3D renders and slices through the basal ganglia and thalamus. Results are shown after cluster-level correction for multiple comparisons at p<0.05.
Reduced in-phase functional connectivity was also observed in PSP in both cortical networks using the ICA analysis (Figure 2). In the DMN, reduced in-phase connectivity was observed in premotor cortex, including SMA, extending into the prefrontal cortex, temporal lobe, thalamus and striatum in PSP compared to controls. In the salience network, reduced in-phase connectivity was observed predominantly in subcortical regions in PSP, including striatum and thalamus. No regions showed increased in-phase connectivity in PSP compared to controls in either cortical network.
The reductions of functional connectivity observed across the thalamic seed-based analysis and the basal ganglia, DMN and salience network ICA analyses remained the same after the correction for atrophy. However, the regions of increased connectivity observed with the thalamus seed and basal ganglia network were no longer present.
Structural MRI and DTI
Grey matter loss was observed in posterior frontal lobes, SMA, caudate and thalamus in PSP compared to controls, with white matter loss observed in cerebellum, brainstem and regions of the posterior frontal lobes (Figure 3A). The DTI analysis showed significantly reduced FA in superior cerebellar peduncles, body of the corpus callosum, regions of the superior longitudinal fasciculus located predominantly in the posterior frontal lobes, inferior longitudinal fasciculus, thalamus and fornix in PSP compared to controls (Figure 3B). No regions showed increased FA in PSP compared to controls.
Figure 3.
Structural changes in subjects with PSP compared to controls. Panel A shows patterns of grey (shown in red) and white matter (shown in green) volume loss in PSP compared to controls assessed using voxel-based morphometry. Results are shown uncorrected for multiple comparisons at p<0.001 on 3D renders and slices through the basal ganglia and thalamus. Panel B shows regions of reduced fractional anisotropy (shown in red) in PSP subjects compared to controls displayed on the TBSS white matter tract skeleton (shown in green). Results are shown after correction for multiple comparisons at p<0.05. ILF = inferior longitudinal fasciculus; SLF = superior longitudinal fasciculus
Correlation analyses
Functional connectivity changes in the SMA were correlated with measures of cognitive and motor performance, as well as grey matter volume of the SMA (Supplemental Table 1). Mean FA in the superior cerebellar peduncles was correlated with functional connectivity changes in the thalamus. No significant correlations were observed between functional connectivity and disease severity or executive function measures, or volume of the thalamus, striatum or cerebellum.
DISCUSSION
Resting state fMRI provides a unique method for assessing functional connectivity in the brain. This technique was utilized in this study and demonstrated disruption of in-phase functional connectivity in subjects with PSP.
Reduced functional connectivity was observed between the thalamus and a network of gray matter regions including premotor cortex, with SMA, prefrontal cortex, striatum and cerebellum in the seed-based analysis. Structural changes were also observed in these regions, with white matter loss observed in cerebellum and brainstem and both gray and white matter loss in premotor cortex extending into prefrontal cortex and striatum; consistent with patterns previously reported in pathologically confirmed PSP[3]. The reductions in functional connectivity remained even after correction for grey matter volume suggesting that functional changes are not confounded by volume loss. As we originally hypothesized, these findings suggest both structural damage and altered functional connectivity along the dentatorubrothalamic tract which runs from the cerebellum, through the superior cerebellar peduncles synapsing in the ventrolateral thalamus. Projections from the ventrolateral thalamus then terminate in premotor areas of the frontal lobe. The DTI analysis confirms involvement of this tract by demonstrating degeneration of the superior cerebellar peduncles in these subjects. White matter tract degeneration was also observed in the anterior portions of the superior longitudinal fasciculus which projects into the posterior frontal lobe. These patterns of reduced functional connectivity were also observed using the independent ICA-based analysis of the basal ganglia network, which included thalamus and basal ganglia. Once again, reduced in-phase connectivity was observed in premotor and prefrontal cortex and thalamus. The reason for the lack of changes in cerebellum is unclear but could be because the basal ganglia network includes both the thalamus and basal ganglia; the later of which does not directly receive fibers from, or send projections to, the cerebellum.
As well as observing a close spatial relationship between structural and functional changes in PSP, a significant correlation was observed between volume and functional connectivity within the SMA. One could hypothesize that disruption in functional connectivity between thalamus and SMA results in the break-down of tissue integrity in the SMA, although we can not rule out the possibility that atrophy precedes functional disruption. A significant correlation was also observed between FA in the superior cerebellar peduncles and functional connectivity within the thalamus. The superior cerebellar peduncles project from the dentate nucleus of the cerebellum to the thalamus and are typically affected in subjects with PSP[5, 7]. Our results suggest that breakdown in this white matter tract could be causing a disruption in functional connectivity within the thalamus. Associations between functional and structural connectivity measured using DTI have previously been observed[9, 10, 26], with findings suggesting that reductions in functional connectivity occur after white matter tract damage[26]. The lack of any direct association between superior cerebellar peduncle degeneration and functional connectivity in the striatum or SMA could be because the superior cerebellar peduncle does not provide fibers directly to these structures.
Correlations were also observed between SMA functional connectivity and scores on cognitive and motor tests, suggesting that disruption of thalamocortical connections may be contributing to these clinical deficits in PSP. The premotor cortex is intricately connected with prefrontal cortex which is involved in reasoning and executive function; hence important for cognitive performance. Extrapyramidal features are associated with basal ganglia dysfunction[27], and likely result from disruption of basal ganglia influence on motor control via projections to thalamus, which in turn project to frontal motor cortices. Indeed, pathological changes, including atrophy, neuronal loss, gliosis and the presence of tau-positive inclusions, have been shown to affect the thalamocortical motor pathways in PSP, including the ventrolateral motor thalamus and SMA[28]. Saccadic abnormalities are also a characteristic feature of PSP and have been related to brainstem neural circuits[29], although there is evidence for some cortical control from prefrontal cortex[30].
Interestingly, we also observed regions of increased in-phase connectivity in PSP in the analyses assessing thalamic connectivity and the basal ganglia network. These increases were observed in regions that did not show volume loss and hence may suggest a reorganization of functional connectivity in PSP with new connectivity formed in local regions of relatively unaffected portions of the brain. The significance of these changes is however unclear, especially since these findings were not present in the analyses that corrected for atrophy. The changes after atrophy correction reflected increased statistical power which served to increase the spatial extent of the out-of-phase connectivity mask, and therefore reduce the amount of voxels that were considered in-phase and hence analyzed in the two sample t-tests.
The cortical networks also provided insight into the disease. Reduced in-phase connectivity was once again observed in thalamus and striatum in both the DMN and salience network, and in premotor cortex, including SMA, in the DMN. The fact that the DMN, which heavily involves medial and lateral parietal lobes, showed greater reductions in posterior frontal connectivity than the salience network suggests that the disease is preferentially affecting long range fibers connecting the parietal and frontal lobes. Indeed, we observed degeneration of the superior longitudinal fasciculus which projects between the frontal and parietal lobes.
The strength of this study was that we assessed resting-state fMRI, DTI and VBM in combination which allowed us to look at all aspects of functional and structural change in PSP. These analyses showed that PSP is associated with a defined and focal pattern of abnormalities centered on the dentatorubrothalamic tract and subsequent connections to the premotor cortex. These results were confirmed using two different resting-state fMRI analysis methods. It should be acknowledged however that this study focused solely on assessing in-phase connectivity, and hence any changes in out-of-phase connectivity would not have been identified. This approach was appropriate since the biological interpretation of out-of-phase connectivity remains unclear and data reduction was important to improve clarity. This study increases our knowledge of changes that occur in the brain of PSP subjects and highlights the close relationship between functional and structural changes.
Supplementary Material
Acknowledgments
This work was supported by the Dana Foundation; National Institutes of Health (grant numbers R01-DC010367, R01-AG037491, R21-AG38736, and R01-AG11378) and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation. We would like to acknowledge Drs. Scott Eggers, J. Eric Ahlskog and Joseph Matsumoto for referring patients for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steele JC, Richardson JC, Olszewski J. Progressive Supranuclear Palsy. a Heterogeneous Degeneration Involving the Brain Stem, Basal Ganglia and Cerebellum with Vertical Gaze and Pseudobulbar Palsy, Nuchal Dystonia and Dementia. Archives of neurology. 1964 Apr;10:333–59. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- 2.Brenneis C, Seppi K, Schocke M, Benke T, Wenning GK, Poewe W. Voxel based morphometry reveals a distinct pattern of frontal atrophy in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2004 Feb;75(2):246–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008 Feb;29(2):280–9. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padovani A, Borroni B, Brambati SM, Agosti C, Broli M, Alonso R, et al. Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2006 Apr;77(4):457–63. doi: 10.1136/jnnp.2005.075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitwell JL, Master AV, Avula R, Kantarci K, Eggers SD, Edmonson HA, et al. Clinical correlates of white matter tract degeneration in PSP. Archives of neurology. doi: 10.1001/archneurol.2011.107. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishizawa K, Dickson DW. Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration. Journal of neuropathology and experimental neurology. 2001 Jun;60(6):647–57. doi: 10.1093/jnen/60.6.647. [DOI] [PubMed] [Google Scholar]
- 7.Tsuboi Y, Slowinski J, Josephs KA, Honer WG, Wszolek ZK, Dickson DW. Atrophy of superior cerebellar peduncle in progressive supranuclear palsy. Neurology. 2003 Jun 10;60(11):1766–9. doi: 10.1212/01.wnl.0000068011.21396.f4. [DOI] [PubMed] [Google Scholar]
- 8.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews. 2007 Sep;8(9):700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 9.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 200 Jan;19(1):72–8. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Human brain mapping. 2009 Oct;30(10):3127–41. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America. 2004 Mar 30;101(13):4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010 May;133(Pt 5):1352–67. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996 Jul;47(1):1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005 Jul 5;102(27):9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009 Oct 1;47(4):1408–16. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002 Jan;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 17.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE transactions on medical imaging. 2004 Feb;23(2):137–52. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 18.Robinson S, Basso G, Soldati N, Sailer U, Jovicich J, Bruzzone L, et al. A resting state network in the motor control circuit of the basal ganglia. BMC neuroscience. 2009;10:137. doi: 10.1186/1471-2202-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proceedings of the National Academy of Sciences of the United States of America. 2009 Apr 28;106(17):7209–14. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, et al. Biological parametric mapping: A statistical toolbox for multimodality brain image analysis. Neuroimage. 2007 Jan 1;34(1):137–43. doi: 10.1016/j.neuroimage.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000 Jun;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 22.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 23.Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003 Nov;50(5):1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006 Jul 15;31(4):1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain. 2009 Nov;132(Pt 11):2932–46. doi: 10.1093/brain/awp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston JM, Vaishnavi SN, Smyth MD, Zhang D, He BJ, Zempel JM, et al. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci. 2008 Jun 18;28(25):6453–8. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cordato NJ, Duggins AJ, Halliday GM, Morris JG, Pantelis C. Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain. 2005 Jun;128(Pt 6):1259–66. doi: 10.1093/brain/awh508. [DOI] [PubMed] [Google Scholar]
- 28.Halliday GM, Macdonald V, Henderson JM. A comparison of degeneration in motor thalamus and cortex between progressive supranuclear palsy and Parkinson’s disease. Brain. 2005 Oct;128(Pt 10):2272–80. doi: 10.1093/brain/awh596. [DOI] [PubMed] [Google Scholar]
- 29.Revesz T, Sangha H, Daniel SE. The nucleus raphe interpositus in the Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Brain. 1996 Aug;119( Pt 4):1137–43. doi: 10.1093/brain/119.4.1137. [DOI] [PubMed] [Google Scholar]
- 30.Bodis-Wollner I, Bucher SF, Seelos KC. Cortical activation patterns during voluntary blinks and voluntary saccades. Neurology. 1999 Nov 10;53(8):1800–5. doi: 10.1212/wnl.53.8.1800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.