Abstract
Rationale and Objectives
Relapse to alcohol use during abstinence or maladaptive eating habits during dieting is often provoked by stress. The anxiogenic drug yohimbine, which causes stress-like responses in humans and nonhumans, reliably reinstates alcohol and food seeking in a rat relapse model. Yohimibine is a prototypical alpha-2 adrenoceptor antagonist but results from studies on noradrenaline's role in yohimbine-induced reinstatement of drug and food seeking are inconclusive. Here we further addressed this issue by studying the effect of the alpha-1 adrenoceptor antagonist prazosin and the alpha-2 adrenoceptor agonist guanfacine on yohimbine-induced reinstatement.
Methods
In Exp. 1, we trained rats to self-administer alcohol (12% w/v, 1-h/day) and after extinction of alcohol-reinforced lever-pressing, we tested prazosin's (0.5, 1.0, and 2.0 mg/kg, i.p.) or guanfacine's (0.125, 0.25, and 0.5 mg/kg, i.p.) effect on yohimbine (1.25 mg/kg, i.p.)-induced reinstatement; we also examined prazosin's effect on intermittent-footshock-stress-induced reinstatement. In Exp. 2, we trained food-restricted rats to self-administer 45 mg food pellets and first examined prazosin's or guanfacine's effects on food-reinforced responding, and then, after extinction of lever presses, on yohimibine-induced reinstatement.
Results
Prazosin (0.5-2.0 mg/kg) blocked yohimbine-induced reinstatement of food and alcohol seeking, as well as footshock-induced reinstatement of alcohol seeking. Guanfacine attenuated yohimbine-induced reinstatement of alcohol seeking at the highest dose (0.5 mg/kg) but its effect on yohimbine-induced reinstatement of food seeking was not significant. Neither prazosin nor guanfacine affected high rate food-reinforced responding.
Conclusions
Results demonstrate an important role of postsynaptic alpha-1 adrenoceptors in stress-induced reinstatement of alcohol and food seeking.
Keywords: Alpha-1 adrenoceptors, Alpha-2 adrenoceptors, Diet, Alcohol self-administration, Noradrenaline, Reinstatement, Relapse, Stress
Introduction
A major problem in alcohol addiction treatment is high rates of relapse during abstinence (Brown et al. 1995). Similarly, a major problem in dietary treatments is high rates of relapse to the maladaptive eating habits (Peterson and Mitchell 1999). In both cases, relapse is often provoked by stress, anxiety, or negative mood states (Breese et al. 2010; Byrne et al. 2003; Grilo et al. 1989; Sinha 2007). The neuronal mechanisms underlying the effect of stress on relapse to alcohol use or maladaptive eating habits during dieting are unknown.
Over the last decade, investigators have used a rat reinstatement model (Shaham et al. 2003) to study neuronal mechanisms of stress-induced relapse to alcohol seeking (Le and Shaham 2002; Weiss et al. 2001). Since 2006, the reinstatement model has also been used to study mechanisms of stress-induced relapse to palatable food seeking (Ghitza et al. 2006; Nair et al. 2009a). In earlier studies with alcohol-experienced rats, the main stressor used to reinstate alcohol seeking was intermittent footshock (Le et al. 1999; Le et al. 1998; Liu and Weiss 2002). More recently, we and others have been using the pharmacological stressor yohimbine, which potently reinstates both alcohol and palatable food seeking (Ghitza et al. 2006; Le et al. 2005; Nair et al. 2009b; Richards et al. 2008). Yohimbine is a prototypical alpha-2 adrenoceptor antagonist that increases noradrenaline cell firing and noradrenaline release in terminal areas (Abercrombie et al. 1988; Aghajanian and VanderMaelen 1982), and induces anxiety-like responses in both humans and nonhumans (Bremner et al. 1996a; b; Holmberg and Gershon 1961; Lang and Gershon 1963). Yohimbine's effect on reinstatement of both alcohol and food seeking are likely due to the induction of a stress-like state, because this effect is blocked by systemic injections of antalarmin, a corticotropin-releasing factor (CRF) 1 receptor antagonist (Ghitza et al. 2006; Marinelli et al. 2007). The stress neurohormone CRF, via its action on extrahypothalamic brain sites, mediates footshock-stress-induced reinstatement of drug and alcohol seeking (Shaham et al. 2000a; Shalev et al. 2010).
As mentioned above, yohimbine is a prototypical alpha-2 adrenoceptor antagonist; thus it would be expected that activation of central noradrenergic systems mediate its potent effects on reinstatement of drug and food seeking. However, results from studies on the role of central noradrenergic systems in yohimbine-induced reinstatement are mixed. While the alpha-2 adrenoceptor agonist clonidine attenuates yohimbine-induced reinstatement of alcohol seeking in rats and cocaine seeking in monkeys (Le et al. 2009; Lee et al. 2004), clonidine has no effect on yohimbine-induced reinstatement of cocaine or food seeking in rats or yohimbine-induced reinstatement of cocaine conditioned place preference (CPP) in mice (Brown et al. 2009; Mantsch et al. 2010; Nair et al. 2009a). Additionally, 6-hydroxydopamine lesions of the ventral or dorsal noradrenergic bundles have no effect on yohimbine-induced reinstatement of alcohol seeking (Le et al. 2009). Additionally, yohimbine's effect on reinstatement of food or alcohol seeking is not mimicked by RS79948, a selective alpha-2 adrenoceptor antagonist (Le et al. 2009; Nair et al. 2009b). In contrast, in monkeys, yohimbine's effect on reinstatement is mimicked by RS79948 (Lee et al. 2004), and in mice this effect of yohimbine is mimicked by another selective alpha-2 adrenoceptor antagonist, BRL44408 (Mantsch et al. 2010). These authors also reported that yohimbine-induced reinstatement of cocaine CPP in mice is attenuated by the beta adrenoceptor antagonist, propranolol (Mantsch et al. 2010).
Here, we further characterized the role of noradrenaline in yohimbine-induced reinstatement of alcohol and food seeking by using the alpha-1 adrenoceptor antagonist prazosin (Cohen 1970) and the alpha-2 adrenoceptor agonist guanfacine (Scholtysik et al. 1975). We studied prazosin, because this drug decreases stress responses in laboratory animals (Handley and Mithani 1984; Manion et al. 2007). Additionally, Rasmussen et al. (2009) showed that prazosin decreases alcohol intake (two-bottle choice) in alcohol-preferring P rats, and Walker et al. (2008) showed that the drug decreases dependence-induced increases in operant alcohol self-administration in Wistar rats. Furthermore, to our knowledge, the effect of alpha-1 adrenoceptor antagonists on footshock-stress or yohimbine-induced reinstatement of alcohol seeking has not been determined. We studied guanfacine, because the drug decreases excitatory postsynaptic neurotransmission in medial prefrontal cortex (mPFC) (Ji et al. 2008) and bed nucleus of the stria terminalis (BNST) (Shields et al. 2009); these brain areas were recently implicated in yohimbine-induced reinstatement of drug and food seeking (Buffalari and See 2010; Nair et al. 2011). Additionally, guanfacine was recently approved for the treatment of attention deficit hyperactive disorder (ADHD) (Sallee and Eaton 2010). Thus, positive findings from a rat relapse model could prompt translational studies on the drug's efficacy for relapse prevention in humans.
Materials and Methods
The experimental procedures followed the “Principles of laboratory animal care” (NIH publication no. 85-23, 1996) and were approved by the local animal care and use committee.
Subjects and apparatus
Exp. 1: Alcohol
Forty-one male Wistar rats (Charles River, St-Constant, QC, Canada) weighing 150-200 g (age 7-8 weeks) at the start of the experiment were used. The rats were individually housed under a 12:12 h light-dark cycle (light on at 7:00 a.m. to 7:00 p.m.). Food and water were freely available in the home cage during all phases of the experiments and the temperature was maintained at 21±1°C. The self-administration chambers were constructed locally and were equipped with two levers, symmetrically centered on a side panel. During the self-administration sessions, responding on one lever (an active lever) activated an infusion pump (Razel Sci., Stamford, CT), while responding on the other lever (an inactive lever) was recorded, but did not activate the pump. Activation of the infusion pump resulted in the delivery of 0.19 ml of alcohol into a drinking receptacle located between the two levers and initiated a 5-sec timeout period. The grids of the self-administration chambers were connected to scrambled shockers (Med Associates, Georgia, VT).
Exp. 2: Food
Twelve male Long-Evans rats (Charles River, Raleigh, NC) weighing 350-450 g (age 11-14 weeks) at the start of the experiment were used. The rats were housed individually in the animal facility under a reverse 12-h:12-h light-dark cycle (lights off at 8 a.m.). All rats were kept on a restricted diet of 16 g/day of Purina rat chow (about 60-65% of their daily food intake) during the training phase and 20-22 g/day during the extinction and reinstatement test phases to maintain stable body weight. The experiments were conducted in standard self-administration chambers (Med Associates, Georgia, VT). Each chamber had two levers 9 cm above the floor, but only one lever (“active,” retractable lever) activated the pellet dispenser, which delivered 45-mg food pellets containing 12.7% fat, 66.7% carbohydrate and 20.6% protein (Catalogue # 1811155, TestDiet, Richmond, IN). This particular pellet was chosen based on pellet preference tests in food-restricted rats, using 6 pellet types (obtained from TestDiet and Bioserv) with different compositions of fat (0 to 35%) and carbohydrate (45% to 91% [sugar pellets]) at different flavors (no flavor, banana, chocolate, grape). The chambers were also equipped with photobeams to record head entries into the food receptacles.
Drugs
Alcohol solution was prepared by diluting 95% ethanol (Commercial Alcohols Inc., Tiverton, ON, Canada) in tap water. Guanfacine HCl (N-Amidino-2-(2,6-dichlorophenyl) acetamide hydrochloride) was dissolved in saline and prazosin HCl (1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)piperazine hydrochloride) and yohimbine HCl (17-Hydroxyyohimban-16-carboxylic acid methyl ester hydrochloride) were dissolved in distilled water; all drugs were obtained from Sigma (St. Louis, MO). In Exp. 1, guanfacine (0, 0.125, 0.25, and 0.5 mg/kg, i.p.), prazosin (0, 0.5, 1.0, and 2.0 mg/kg, i.p.), and yohimbine (0, 1.25 mg/kg, i.p.) were injected at a volume of 1 ml/kg, except the high prazosin dose that was injected at 2 ml/kg. In Exp. 2, the injection volume for yohimbine was 0.5 ml/kg and 2 ml/kg for prazosin and guanfacine. The yohimbine dose was based on our previous studies (Ghitza et al. 2006; Le et al. 2009; Le et al. 2005; Shepard et al. 2004). The doses of prazosin and guanfacine were based on previous reports (Dekeyne and Millan 2006; Forget et al. 2010; Handley and Mithani 1984; Manion et al. 2007; Rasmussen et al. 2009; Sagvolden 2006; Walker et al. 2008).
Procedures
Exp. 1: alcohol
Rats were trained to self-administer alcohol as described previously (Le et al. 2005; Le et al. 1998). Briefly, naïve rats were initially provided with access to alcohol solutions and tap water for 30 min/day in novel drinking cages (30x18x18 cm) containing Richter tubes. Alcohol solutions were provided in increasing concentrations: 3% (w/v) for the first 5 d, 6% (w/v) for the next 5 d and 12% (w/v) for the next 10 d. Subsequently, operant self-administration of alcohol (12%) was initiated in 1-h daily sessions on a fixed ratio-1 (FR-1) 5-s timeout reinforcement schedule for at least 5 d (1 h/d). During the timeout, the house light was turned off, a key light over the active lever was turned on, and white noise was emitted by a speaker. The beginning of the sessions was signalled by the illumination of a houselight located at the top of the self-administration chamber; at the end of the session, the houselight was turned off. The requirement for alcohol delivery was then increased to an FR-2 reinforcement schedule for 5 d. Subsequently, the schedule requirement was increased to an FR-3 schedule for at least 6 d, until the rats demonstrated 3 d of stable alcohol-taking behaviour (variability of less than 20% of the mean). When alcohol was left in the drinking receptacle after a self-administration session, it was measured and the volume was taken into account for intake calculation.
Exp. 1a: Effect of prazosin or guanfacine on yohimbine-induced reinstatement of alcohol seeking
Forty-one rats were trained to self-administer alcohol as described above. The rats then received 10 daily extinction sessions. These sessions were identical to the self-administration sessions, except that the infusion pumps used to deliver alcohol were inactivated. The extinction sessions continued until the rats reached an extinction criterion of fewer than 12 active lever presses/1-h during two consecutive days; during the last 4 extinction sessions, the rats received vehicle injections to habituate them to the injection procedure. Two groups of rats (n=9-10 per group), which were matched for alcohol intake and extinction responding, were assessed for the effect of prazosin on yohimbine-induced reinstatement, using a mixed design that included the between-subjects factor of Yohimbine Dose (0, 1.25 mg/kg) and the within–subjects factor of Prazosin Dose (0, 0.5, 1.0, and 2.0 mg/kg). Two other groups of rats (n=10-11 per group) were assessed for the effect of guanfacine on yohimbine-induced reinstatement, using a mixed design that included the between-subjects factor of Yohimbine Dose (0, 1.25 mg/kg) and the within–subjects factor of Guanfacine Dose (0, 0.125, 0.25, and 0.5 mg/kg). On the test days, each rat was injected with vehicle or one of the prazosin/guanfacine doses, and 15 min later with vehicle or yohimbine. Forty-five min after the second injection, the rats were placed in the self-administration chambers for the 1-h reinstatement test sessions. The order of the injections of the different prazosin/guanfacine doses was counterbalanced and tests were performed every other day, with regular extinction sessions in the intervening days.
Exp. 1b: Effect of prazosin on footshock-induced reinstatement of alcohol seeking
Twenty rats from Exp. 1a were used after the completion of yohimbine testing. They received 4 additional daily extinction sessions. The effect of prazosin on intermittent footshock-induced reinstatement was assessed using a mixed design that included the within-subjects factor of Footshock (no shock , shock; n=10 per condition) and the within–subjects factor of Prazosin Dose (0, and 0.5 mg/kg). Each rat was injected with vehicle or prazosin 15 min before two daily 1-h test sessions; the first day was the baseline (no shock) session and the second day was the footshock test session. The 5 min intermittent footshock stress was administered just prior to the start of the 1-h test session. The shock parameters of 0.8 mA, 0.5 sec ON, a mean OFF period of 40 sec, and a range of 10-70 sec between each shock delivery were based on our previous work (Le et al. 1998; Shaham and Stewart 1995).
Exp. 2: Food
Exp. 2a: Effect of prazosin or guanfacine on food-reinforced responding
The rats were first given 3-h daily sessions of “autoshaping” for 2 days during which pellets were administered non-contingently every 5 min into a receptacle located near the active lever. Pellet delivery was accompanied by a compound 5-sec tone (2900 Hz, 20 dB above background)-light (a 7.5-W white light located above the active lever) cue. Subsequently, the rats were trained to lever-press for the pellets on an FR1, 20-sec timeout reinforcement schedule for 3 h/d for 5 days. At the start of the session, the red houselight was turned on and the active lever was extended. Following each pellet delivery, the compound tone-light cue (termed “cue” herein) was turned on for 5 sec. During the training days, regular food (16 g Purina rat chow) was given after the daily session (approximately 4 h into the dark cycle).
After the initial 5 day training, we used a within-subjects design to assess the effect of prazosin (0, 1.0, and 2.0 mg/kg, n=6) or guanfacine (0, 0.25, and 0.5 mg/kg, n=6) on high-rate food-reinforced responding. The drugs or their vehicle were injected 60 min before the test sessions, which were performed every other day. The order of the different doses was counterbalanced and regular training days were performed in the intervening days.
Exp. 2b: Effect of prazosin or guanfacine on yohimbine-induced reinstatement of food seeking
After the completion of Exp. 2a, the rats’ active lever responding was extinguished over 12, daily 3-h sessions during which lever presses led to tone-light cue presentations but not food. The rats (n=12) underwent 6 test sessions, every other day, during which they were injected with yohimbine (1.25 mg/kg) or distilled water and then injected with the vehicles of prazosin or guanfacine (n=6 per vehicle condition), prazosin (2.0 mg/kg, n=12), or guanfacine (0.5 mg/kg, n=12). The order of test sessions was counterbalanced and the rats were given regular extinction sessions on the intervening days. Prazosin, guanfacine, or their vehicles were injected 60 min before the start of the test sessions, and yohimbine was injected 15 min after prazosin/guanfacine injections.
Statistical analysis
The statistical analyses were performed separately on previously active and inactive lever presses during the reinstatement tests. The food-reinforced responding data were analysed for total pellet intake, lever presses during the 20-sec timeouts after each pellet delivery, and number of magazine entries (photobeam counts). Data were analyzed with ANOVAs and significant interactions (p values < 0.05) were followed by a Newman-Keuls post-hoc test (Exp. 1) or a Fisher PLSD post-hoc test (Exp. 2).
Results
Exp. 1: Alcohol
The mean±SEM number of alcohol deliveries (1 h), alcohol intake (g/kg), and active and inactive lever presses (1 h) on the last training day under the FR-3 reinforcement schedule was 25.57±1.24, 1.49±0.07, 85.44±4.61, and 0.61±0.24, respectively (total n=41). The mean±SEM number of previously active and inactive lever presses on the first and last extinction session (prior to the start of the reinstatement tests) was 72.71±5.95, 0.66±0.24, 9.02±0.88, and 0.51±0.18, respectively (total n=41).
Exp. 1a: Effect of prazosin or guanfacine on yohimbine-induced reinstatement of alcohol seeking
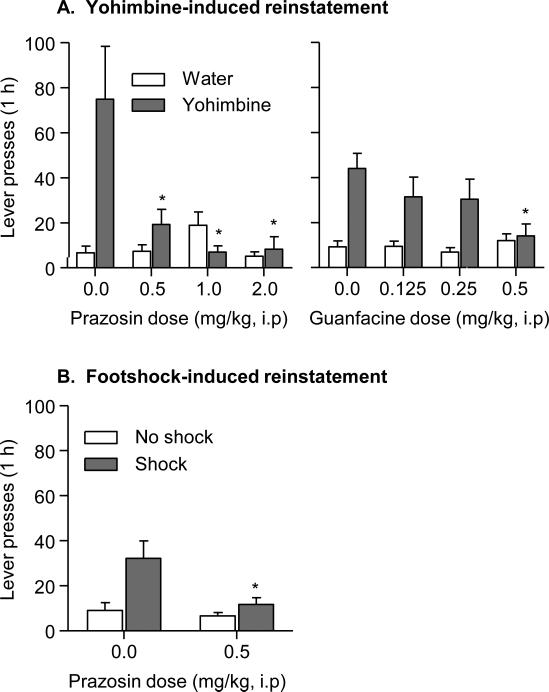
Both prazosin and guanfacine decreased yohimbine-induced reinstatement of alcohol seeking without affecting baseline extinction responding (Fig. 1a). The statistical analyses included the within-subjects factor of Prazosin Dose (0, 0.5, 1.0, 2.0 mg/kg) or Guanfacine Dose (0, 0.1, 0.25. 0.5 mg/kg), and the within-subjects factor of Yohimbine Dose (0, 1.25 mg/kg). The analyses of active lever presses showed significant Prazosin Dose × Yohimbine Dose [F(3,53)=6.3, p<0.05] and Guanfacine Dose × Yohimbine Dose [F(3,56)=3.2, p<0.05] interaction effects. The analyses of inactive lever data did not show significant effects and inactive lever presses were very low during testing (means of less than 5 presses per 1 h, data not shown). Post-hoc differences are indicated in Fig. 1a.
Figure 1. Exp. 1: alcohol: Effect of prazosin or guanfacine on yohimbine-induced reinstatement and effect of prazosin on intermittent footshock-induced reinstatement.
Data are mean±SEM presses on the previously active lever. (A) Effect of prazosin or guanfacine on yohimbine-induced reinstatement. (B) Effect of prazosin on intermittent footshock-induced reinstatement. In A, rats were injected with prazosin, guanfacine or their vehicle 60 min before the test sessions, and then injected with yohimbine or its vehicle 45 min before these sessions. In B, rats were injected with prazosin 40 min before the test sessions and intermittent footshock (5 min) was given (or not given) just prior to the start of the test session. * Different from the appropriate prazosin or the guanfacine vehicle condition, p < 0.05 (n=9-11 per drug condition).
Exp. 1b: Effect of prazosin on footshock-induced reinstatement of alcohol seeking
Prazosin decreased intermittent footshock-induced reinstatement of alcohol seeking without affecting baseline extinction responding (Fig. 1b). The statistical analyses included the between-subjects factor of Prazosin Dose (0, 0.5 mg/kg) and the within-subjects factor of Footshock (no shock, shock). The analysis of active lever presses showed a significant Prazosin Dose × Yohimbine Dose interaction effect [F(1,18)=5.3, p<0.05]. The analyses of inactive lever data did not show any significant effects and inactive lever presses were very low during testing (means of less than 4 presses per 1 h, data not shown). Post-hoc differences are indicated in Fig. 1b.
Exp. 2: Food
The mean±SEM number of pellet deliveries (3 h), timeout lever presses, inactive lever presses, and magazine entries on the last training day under an FR-1, 20-sec timeout reinforcement schedule was 239±26, 553±116, 1.0±0.6, and 670±114, respectively (total n=12). The mean±SEM number of previously active lever presses (3 h), inactive lever presses, and magazine entries on the 1st extinction session (prior to the start of the reinstatement tests) was 395±59, 6.0±2.7, and 213±42, respectively (total n=12). The mean±SEM number of previously active lever presses (3 h), inactive lever presses, and magazine entries on the 12th and last extinction session (prior to the start of the reinstatement tests) was 12±2, 0.8±0.4, and 28±6, respectively (total n=12).
Exp. 2a: Effect of prazosin and guanfacine on food-reinforced responding
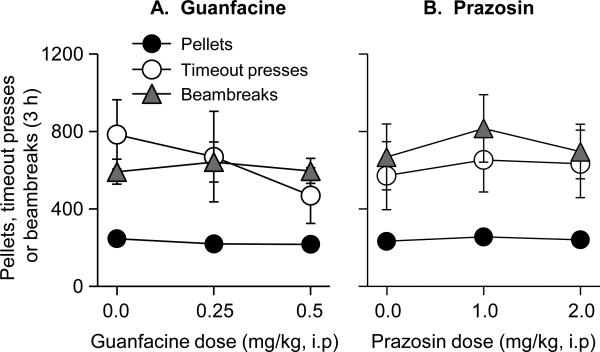
Neither prazosin nor guanfacine had an effect on pellet deliveries (3 h), timeout lever presses, and magazine entries (Fig. 2). These drugs also had no effect on inactive lever presses, which was very low during the dose-response determination (less than 3 presses per 3 h, data not shown).
Figure 2. Exp. 2: food: Effect of prazosin or guanfacine on food-reinforced responding.
Data are mean±SEM. Rats were injected with prazosin, guanfacine or their vehicle 60 min before the test sessions. The drugs had no significant effect on number of pellet earned, number of timeout lever presses, and magazine entries. Rats lever pressed for the food pellet under an FR-1 20 sec timeout reinforcement schedule (n=6 per dose, within-subjects design).
Exp. 2b: Effect of prazosin or guanfacine on yohimbine-induced reinstatement of food seeking
Prazosin decreased yohimbine-induced reinstatement of both active lever presses and magazine entries while guanfacine had a variable effect on this reinstatement (see below); neither prazosin nor guanfacine affected baseline extinction responding (Fig. 3a,b). The statistical analyses separately compared the vehicle condition (combining the 6 rats given the prazosin vehicle and the 6 rats that received the guanfacine vehicle together with yohimbine or its vehicle, total n=12) with the same 12 rats that received prazosin or guanfacine together with yohimbine or its vehicle (a total of 6 tests). The two analyses included the within-subjects factors of Prazosin Dose (0, 2.0 mg/kg) or Guanfacine Dose (0, 0.5 mg/kg) and Yohimbine Dose (0, 1.25 mg/kg).
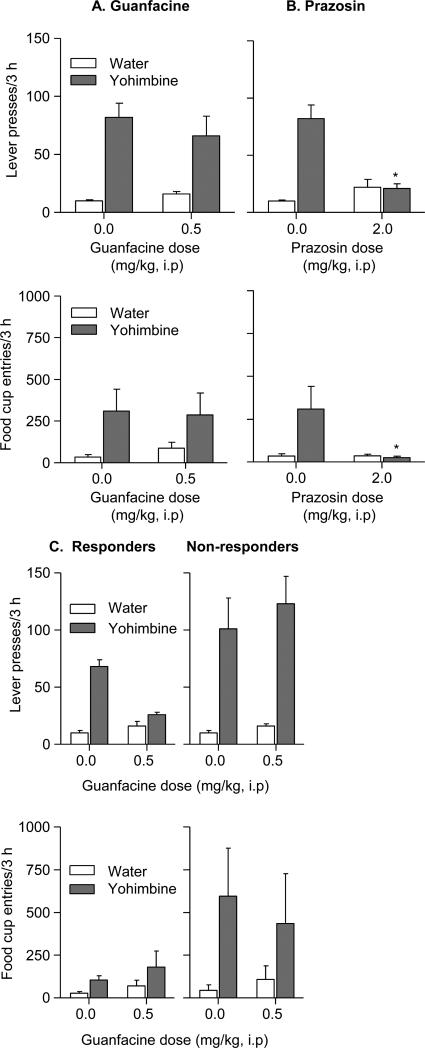
Figure 3. Exp. 2: Food: Effect of prazosin or guanfacine on yohimbine-induced reinstatement.
Data are mean±SEM presses on the previously active lever and mean±SEM magazine entries. (A, B) Effect of prazosin or guanfacine on yohimbine-induced reinstatement (n=12 per drug condition, within-subjects design). (C) Effect of guanfacine on yohimbine-induced reinstatement in groups of rats that either decreased (n=7) or not decreased (n=5) yohimbine-induced reinstatement after guanfacine injections. The rats were injected with prazosin, guanfacine or their vehicles 60 min before the test sessions, and then injected with yohimbine or its vehicle 45 min before these sessions. * Different from the appropriate prazosin or the guanfacine vehicle condition, p < 0.05.
Prazosin
The analysis of active lever presses and magazine entries showed significant Prazosin Dose × Yohimbine Dose interaction effects [F(1,11)=18.1, p<0.01, and F(1,11)=5.4, p<0.05, respectively]. Prazosin also reduced the increase in inactive lever presses after yohimbine injections (mean±SEM presses per 3 h of 14.5±5.5 and 0.8±0.2 in the vehicle+yohimbine and prazosin+yohimbine conditions, respectively, p<0.05). However, the result of a follow-up change-score analysis (active lever presses minus inactive lever presses) was similar to that of the active lever analysis (a significant Prazosin Dose × Yohimbine Dose interaction effect, [F(1,11)=21.3, p<0.01]). Post-hoc differences are indicated in Fig. 3a.
Guanfacine
Initial analysis showed a main effect of Yohimbine Dose [F(1,11)=19.4, p<0.01], but not of Guanfacine Dose or Guanfacine Dose × Yohimbine Dose. However, we observed large individual differences in the response to guanfacine with 5 rats showing no change or increased yohimbine-induced reinstatement, while in 7 other rats, guanfacine decreased yohimbine-induced reinstatement of lever presses [F(1,6)=28.2, p<0.01] but not magazine entries (p>0.1) (Fig. 3c). There was no significant effect of guanfacine on inactive lever presses (data not shown).
Discussion
The main finding of our study is that the alpha-1 adrenoceptor antagonist prazosin blocked yohimbine-induced reinstatement of alcohol and food seeking, as well as intermittent footshock stress-induced reinstatement of alcohol seeking. We also found that the alpha-2 agonist guanfacine attenuated yohimbine-induced reinstatement of alcohol seeking. In contrast, the effect of the highest dose of guanfacine on yohimbine-induced reinstatement of food seeking was inconsistent. Below we first discuss methodological issues related to data interpretation and then discuss these data in reference to the role of central noradrenergic systems in stress-induced reinstatement.
Methodological considerations
One issue to consider is that the effects of prazosin or guanfacine on yohimbine- and footshock-induced reinstatement are due to non-specific disruption of motor activity. At issue here is that at high doses both alpha-1 adrenoceptor antagonists and alpha-2 adrenoceptor agonists cause sedation, which would interfere with lever presses (Drew et al. 1979; Munzar and Goldberg 1999). This possibility is unlikely because at the dose range used here neither prazosin nor guanfacine decreased baseline extinction responding (Fig. 1 & 3), and more importantly, had no effect on high-rate food-reinforced responding (Fig. 2).
Another issue to consider is that in Exp. 2 yohimbine also increased inactive lever presses, suggesting a potential non-selective effect of yohimbine on motor performance. However, we suspect that this effect of yohimbine likely reflects response generalization rather than a non-selective effect on lever responding, because yohimbine had no effect on inactive lever responding in Exp. 1. Furthermore, in our previous studies on yohimbine-induced reinstatement of food seeking the drug's effect on inactive lever responding was inconsistent (Ghitza et al. 2006; Ghitza et al. 2007; Nair et al. 2009a; Nair et al. 2008; Nair et al. 2011). Additionally and perhaps more importantly, yohimbine's increases in active lever responding in Exp. 2 was associated with increases in magazine entries (Fig. 3), which indicates that yohimbine indeed caused “food seeking” rather than non-specific increase in activity.
An unexpected finding in our study was the different effect of guanfacine on yohimbine-induced reinstatement in alcohol-trained rats versus food-trained rats. Several procedural differences in the way the experiments were performed may account for these different results. These include the light-dark cycle assessment time (light cycle in alcohol-trained rats [Exp. 1] versus dark cycle in food-trained rats [Exp. 2]), the different rat strain (Wistar in Exp. 1 versus Long-Evans in Exp. 2), the food restriction conditions (food sated in Exp. 1 versus food restricted in Exp. 2), and the use of different rewards (alcohol versus food). It is currently unknown which of these factors might be involved in guanfacine's selective effect on yohimbine-induced reinstatement of alcohol but not food seeking. We suspect that the critical factor might be the reward type, because the present results extend those from our previous studies demonstrating that the alpha-2 agonist clonidine decreased yohimbine-induced reinstatement of alcohol seeking but not food seeking (Le et al. 2009; Nair et al. 2009a).
These present and previous findings raise the possibility that the neuronal mechanisms of yohimbine-induced reinstatement of alcohol seeking and yohimbine-induced reinstatement of palatable food seeking during dieting are not identical. Support for this notion is the finding that the hypocretin 1 receptor antagonist SB 334,847 decreased yohimbine-induced reinstatement of alcohol seeking but not food seeking (Nair et al. 2008; Richards et al. 2008). However, this interpretation of the present data should be made with caution, because we only tested one guanfacine dose against yohimbine-induced reinstatement in food-trained rats. Thus, it is possible that a higher guanfacine dose would result in a higher proportion of rats that are sensitive to guanfacine's effect on yohimbine-induced reinstatement.
A pharmacological issue to consider is the receptor type(s) through which prazosin or guanfacine act to decrease yohimbine-induced and footshock-induced reinstatement. While we interpret these data to suggest a role of alpha-1 and alpha-2 adrenoceptors in this reinstatement, other receptor types might be involved. Specifically, while prazosin is often used as an alpha-1 adrenoceptor antagonist, it also binds with modest affinity to alpha-2 adrenoceptors (Ki=109-219) (Boyajian and Leslie 1987). However, prazosin's affinity to the alpha-1 adrenoceptor is significantly higher (Ki=0.12-0.31) (Doxey et al. 1984; Millan et al. 2000) and its effect on yohimbine-induced reinstatement was not reproduced in food-trained rats by guanfacine. Thus, the most parsimonious pharmacological interpretation of our data is that prazosin's effect on stress-induced reinstatement is due to blockade of alpha-1 adrenoceptors. As for guanfacine, the drug also binds at a moderate degree to the 5-HT1a, but its affinity to the alpha-2 receptor is significantly higher (Newman-Tancredi et al. 1998). Thus, it is likely that at the dose range used here, the effect of guanfacine on yohimbine-induced reinstatement of alcohol seeking is due to its actions on alpha-2 adrenoceptors.
Finally, our data suggest that targeting alpha-1 adrenoceptors with prazosin is a more effective way to reduce yohimbine-induced reinstatement than targeting the alpha-2 adrenoceptors (the presumed receptor target of yohimbine) with guanfacine. However, this conclusion should be made with caution, because it is possible that guanfacine doses that are higher than the ones we tested in our study would decrease yohimbine-induced reinstatement more effectively.
Role of noradrenaline in stress-induced reinstatement of alcohol and food seeking
The robust effect of prazosin on footshock-induced reinstatement of alcohol seeking in the present study extend previous work on the role of central noradrenaline systems in footshock-stress-induced reinstatement of drug seeking (Bossert et al. 2005; Brown et al. 2009) and further identify a potential postsynaptic adrenoceptor (alpha-1) that mediates this effect. In the present study we only injected prazosin systemically and thus its effect on footshock-induced (or yohimbine-induced) reinstatement might be due to its peripheral effects. However, this possibility is relatively unlikely because results from previous studies using intracranial injection and lesion methods and peripheral injections of non-lipophilic drugs demonstrate that central but not peripheral noradrenaline systems mediate the effect of intermittent-footshock-stress on reinstatement of drug seeking (Erb et al. 2000; Leri et al. 2002; Shaham et al. 2000b; Wang et al. 2001; Yamada and Bruijnzeel 2010).
The inhibitory effect of prazosin or guanfacine on yohimbine-induced reinstatement of alcohol seeking in the present results also supports a role for central noradrenergic systems in yohimbine-induced reinstatement of alcohol seeking. However, while these data are in agreement with our previous finding, that yohimbine-induced reinstatement is inhibited by clonidine, they are not in agreement with our findings that lesions of the dorsal or ventral noradrenergic bundle have no effect on yohimbine-induced reinstatement (Le et al. 2009). The dorsal noradrenergic bundle comprises locus coeruleus neurons that project to many forebrain areas and provide the sole noradrenergic input to cortical areas such as the hippocampus and the frontal cortex (Foote et al. 1983; Moore and Bloom 1979). The ventral noradrenergic bundle is comprised of neurons from the lateral tegmental nuclei that innervate a smaller number of forebrain areas (most of which are also innervated by locus coeruleus neurons) and its major projection areas include the hypothalamus, central amygdala, septum, nucleus accumbens, and BNST (Aston-Jones et al. 1999; Fritschy and Grzanna 1991; Pacak et al. 1998).
The reasons for the discrepant results between the effect of systemic injections of adrenoceptor drugs and central noradrenaline lesions are unknown. One possibility is that this discrepancy reflects peripheral effects of the pharmacological agents. However, since results from recent studies using reversible inactivation methods and intracranial drug injections indicate central actions for yohimbine's effect on reinstatement of drug and food seeking (Buffalari and See 2010; Nair et al. 2011), this possibility is unlikely. We speculate that the discrepancy between systemic drug injections and central noradrenergic lesions might reflect the limitation of the 6-OHDA lesion methodology used in our previous study to lesion the dorsal and ventral noradrenergic bundle (Le et al. 2009). In particular, even highly effective 6-OHDA monoamine lesions (90-95%) do not decrease extracellular concentration of the monoamines, often do not decrease behavioral responses to systemic drug injections, and may lead to post-synaptic receptor supersensitivity (Kostrzewa et al. 2003; Pappas and Ings 1987; Robinson and Whishaw 1988; Tassin et al. 1986).
A question that we did not address our study is the role of post-synaptic beta-adrenoceptors in yohimbine-induced reinstatement of alcohol and food seeking. Based on some evidence, we suspect that activation of beta adrenoceptors also contributes to this reinstatement. Thus, systemic injections of the beta adrenoceptor antagonist propranolol decreased yohimbine-induced reinstatement of cocaine CPP (Mantsch et al. 2010). Additionally, propranolol injections attenuated the effect of yohimbine on the induction of Fos (the protein product of the immediate early gene c-fos; (Curran and Morgan 1995) in the mPFC (Bing et al. 1992), a brain area recently implicated in yohimbine-induced reinstatement of food seeking (Nair et al. 2011).
Finally, we previously found that systemic injections of the CRF1 receptor antagonists (CP-154,526 and antalarmin) respectively decreased intermittent footshock-induced and yohimbine-induced reinstatement of alcohol seeking (Le et al. 2000; Marinelli et al. 2007). We also found that antalarmin decreased yohimbine-induced reinstatement of food seeking (Ghitza et al. 2006). Additionally, we found that both yohimbine injections and intermittent footshock exposure increased CRF mRNA expression in the BNST (Funk et al. 2006), a brain area in which blockade of CRF receptors decreased footshock-induced reinstatement of cocaine seeking (Erb and Stewart 1999). Recently, it was reported that reversible inactivation of the BNST with a mixture of the GABAa and GABAb agonists muscimol and baclofen decreased yohimbine-induced reinstatement of cocaine seeking (Buffalari and See 2010). Together, these observations, our present and previous data, as well as results from other studies (Brown et al. 2009; Leri et al. 2002; Shalev et al. 2010), suggest that an interaction between CRF and noradrenaline in the BNST plays an important role in both yohimbine- and intermittent footshock-induced reinstatement of alcohol and food seeking.
Concluding remarks and implications for treatment
Prazosin and guanfacine have been used in the past for the treatment PTSD and ADHD, respectively (Byers et al. 2010; Sallee and Eaton 2010). Guanfacine was also recently approved by the FDA for the treatment of ADHD. Here, we showed that both guanfacine and prazosin blocked stress-induced reinstatement of alcohol seeking. Thus, to the extent that the reinstatement procedure provides a valid animal model of craving and relapse (Epstein et al. 2006), we propose that both prazosin and guanfacine, which are both safe and well-tolerated in humans, should be tested in clinical studies in alcoholics for their effects on stress-induced craving in the laboratory and stress-induced relapse in the alcoholic home environment. We also hope that our data will lead human studies on prazosin's effect on stress-induced food craving and relapse to maladaptive eating habits during dieting.
Acknowledgments
This work was supported by a grant from the NIAAA (AA13108) to A.D. Lê and by the Intramural Research Program of the National Institute on Drug Abuse. We thank Dr. Donna Calu and Ms. Thi Kuch for their help in conducting Exp. 2.
References
- Abercrombie ED, Keller RW, Jr., Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, VanderMaelen CP. alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis: A target site for noradrenergic actions in opiate withdrawal. Ann. N. Y. Acad. Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Bing G, Stone EA, Zhang Y, Filer D. Immunohistochemical studies of noradrenergic-induced expression of c-fos in the rat CNS. Brain Res. 1992;592:57–62. doi: 10.1016/0006-8993(92)91658-2. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: An update and clinical implications. Eur. J. Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Boyajian CL, Leslie FM. Pharmacological evidence for alpha-2 adrenoceptor heterogeneity: differential binding properties of [3H]rauwolscine and [3H]idazoxan in rat brain. J Pharmacol Exp Ther. 1987;241:1092–8. [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2010 doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. preclinical studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II.clinical studies. Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J. Stud. Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D'Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2009;203:121–30. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berl) 2010;213:19–27. doi: 10.1007/s00213-010-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers MG, Allison KM, Wendel CS, Lee JK. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J Clin Psychopharmacol. 2010;30:225–9. doi: 10.1097/JCP.0b013e3181dac52f. [DOI] [PubMed] [Google Scholar]
- Byrne S, Cooper Z, Fairburn C. Weight maintenance and relapse in obesity: a qualitative study. Int J Obes Relat Metab Disord. 2003;27:955–62. doi: 10.1038/sj.ijo.0802305. [DOI] [PubMed] [Google Scholar]
- Cohen BM. Prazosin hydrochloride (CP-12,299-1), an oral anti-hypertensive agent: preliminary clinical observations in ambulatory patients. J Clin Pharmacol J New Drugs. 1970;10:408–17. [PubMed] [Google Scholar]
- Curran T, Morgan JI. Fos: an immediate-early transcription factor in neurons. J. Neurobiol. 1995;26:403–12. doi: 10.1002/neu.480260312. [DOI] [PubMed] [Google Scholar]
- Dekeyne A, Millan MJ. Discriminative stimulus properties of the selective and highly potent alpha2-adrenoceptor agonist, S18616, in rats: mediation by the alpha2A subtype, and blockade by the atypical antidepressants, mirtazapine and mianserin. Neuropharmacology. 2006;51:718–26. doi: 10.1016/j.neuropharm.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Doxey JC, Lane AC, Roach AG, Virdee NK. Comparison of the alpha-adrenoceptor antagonist profiles of idazoxan (RX 781094), yohimbine, rauwolscine and corynanthine. Naunyn Schmiedebergs Arch. Pharmacol. 1984;325:136–144. doi: 10.1007/BF00506193. [DOI] [PubMed] [Google Scholar]
- Drew GM, Gower AJ, Marriott AS. Alpha 2-adrenoceptors mediate clonidine-induced sedation in the rat. Br J Pharmacol. 1979;67:133–41. [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J. Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol. Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, Le Foll B. Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology. 2010;35:1751–60. doi: 10.1038/npp.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Grzanna R. Selective effects of DSP-4 on locus coeruleus axons: are there pharmacologically different types of noradrenergic axons in the central nervous system? Prog. Brain Res. 1991;88:257–268. doi: 10.1016/s0079-6123(08)63815-7. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF(1) receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Nair SG, Golden SA, Gray SM, Uejima JL, Bossert JM, Shaham Y. Peptide YY3-36 decreases reinstatement of high-fat food seeking during dieting in a rat relapse model. J. Neurosci. 2007;27:11522–11532. doi: 10.1523/JNEUROSCI.5405-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Shiffman S, Wing RR. Relapse crises and coping among dieters. J. Consult. Clin. Psychol. 1989;57:488–495. doi: 10.1037//0022-006x.57.4.488. [DOI] [PubMed] [Google Scholar]
- Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- Holmberg G, Gershon S. Autonomic and psychic effects of yohimbine hydrochloride. Psychopharmacologia. 1961;2:93–106. doi: 10.1007/BF00592678. [DOI] [PubMed] [Google Scholar]
- Ji XH, Ji JZ, Zhang H, Li BM. Stimulation of alpha2-adrenoceptors suppresses excitatory synaptic transmission in the medial prefrontal cortex of rat. Neuropsychopharmacology. 2008;33:2263–71. doi: 10.1038/sj.npp.1301603. [DOI] [PubMed] [Google Scholar]
- Kostrzewa RM, Kostrzewa JP, Brus R. Dopamine receptor supersensitivity: an outcome and index of neurotoxicity. Neurotox Res. 2003;5:111–8. doi: 10.1007/BF03033376. [DOI] [PubMed] [Google Scholar]
- Lang WJ, Gershon S. Effects of psychoactive drugs on yohimbine induced responses in conscious dogs. A proposed screening procedure for anti-anxiety agents. Arch. Int. Pharmacodyn. Ther. 1963;142:457–472. [PubMed] [Google Scholar]
- Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol. Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology. 2009;204:477–88. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Watchus W, Juzytsch W, Shalev U, Shaham Y. The role of corticotropin- releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus W, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress in rats. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzystch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology. 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha(2)-arenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced, but not cocaine-induced reinstatement, by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J. Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J. Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manion ST, Gamble EH, Li H. Prazosin administered prior to inescapable stressor blocks subsequent exaggeration of acoustic startle response in rats. Pharmacol Biochem Behav. 2007;86:559–65. doi: 10.1016/j.pbb.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–78. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF(1) receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology. 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Audinot V, Cussac D, Lejeune F, Nicolas JP, Coge F, Galizzi JP, Boutin JA, Rivet JM, Dekeyne A, Gobert A. Agonist and antagonist actions of yohimbine as compared to fluparoxan at alpha(2)-adrenergic receptors (AR)s, serotonin (5-HT)(1A), 5-HT(1B), 5-HT(1D) and dopamine D(2) and D(3) receptors. Significance for the modulation of frontocortical monoaminergic transmission and depressive states. Synapse. 2000;35:79–95. doi: 10.1002/(SICI)1098-2396(200002)35:2<79::AID-SYN1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholaimine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu. Rev. Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg SR. Noradrenergic modulation of the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology (Berl) 1999;143:293–301. doi: 10.1007/s002130050950. [DOI] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Epstein DH, Shaham Y. The neuropharmacology of relapse to food seeking: methodology, main findings, and comparison with relapse to drug seeking. Prog Neurobiol. 2009a;89:18–45. doi: 10.1016/j.pneurobio.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Pickens CL, Smith DG, Shaham Y. Effects of the MCH1 receptor antagonist SNAP 94847 on high-fat food-reinforced operant responding and reinstatement of food seeking in rats. Psychopharmacology. 2009b;205:129–140. doi: 10.1007/s00213-009-1523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br. J. Pharmacol. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Navarre BM, Cifani C, Pickens CL, Bossert JM, Shaham Y. Role of dorsal medial prefrontal cortex dopamine D1-family receptors in relapse to high-fat food seeking induced by the anxiogenic drug yohimbine. Neuropsychopharmacology. 2011;36:497–510. doi: 10.1038/npp.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A, Nicolas JP, Audinot V, Gavaudan S, Verriele L, Touzard M, Chaput C, Richard N, Millan MJ. Actions of alpha2 adrenoceptor ligands at alpha2A and 5-HT1A receptors: the antagonist, atipamezole, and the agonist, dexmedetomidine, are highly selective for alpha2A adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:197–206. doi: 10.1007/pl00005243. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Kopin IJ, Goldstein DS. Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Front. Neuroendocrinol. 1998;16:89–150. doi: 10.1006/frne.1995.1004. [DOI] [PubMed] [Google Scholar]
- Pappas BA, Ings R. Neonatal 6-hydroxydopamine lesion of spinal noradrenergic terminals: nociception, clonidine analgesia and spinal alpha two adrenoceptors. Brain Res Bull. 1987;18:221–5. doi: 10.1016/0361-9230(87)90193-6. [DOI] [PubMed] [Google Scholar]
- Peterson CB, Mitchell JE. Psychosocial and pharmacological treatment of eating disorders: a review of research findings. J. Clin. Psychol. 1999;55:685–697. doi: 10.1002/(sici)1097-4679(199906)55:6<685::aid-jclp3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2009;33:264–72. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology. 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Whishaw IQ. Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res. 1988;450:209–224. doi: 10.1016/0006-8993(88)91560-0. [DOI] [PubMed] [Google Scholar]
- Sagvolden T. The alpha-2A adrenoceptor agonist guanfacine improves sustained attention and reduces overactivity and impulsiveness in an animal model of Attention-Deficit/Hyperactivity Disorder (ADHD). Behav Brain Funct. 2006;2:41. doi: 10.1186/1744-9081-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee FR, Eaton K. Guanfacine extended-release for attention-deficit/hyperactivity disorder (ADHD). Expert Opin Pharmacother. 2010;11:2549–56. doi: 10.1517/14656566.2010.517523. [DOI] [PubMed] [Google Scholar]
- Scholtysik G, Lauener H, Eichenberger E, Burki H, Salzmann R, Muller-Schweinitzer E, Waite R. Pharmacological actions of the antihypertensive agent N-amidino-2-(2,6-dichlorophenyl)acetamide hydrochloride (BS 100-141). Arzneimittelforschung. 1975;25:1483–91. [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res. Brain Res. Rev. 2000a;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs JM, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of the locus coeruleus noradrenergic neurons. Eur. J. Neurosci. 2000b;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin self-administration behavior in drug-free animals: An effect mimicking heroin, not withdrawal. Psychopharmacology. 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol. Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Shields AD, Wang Q, Winder DG. alpha2A-adrenergic receptors heterosynaptically regulate glutamatergic transmission in the bed nucleus of the stria terminalis. Neuroscience. 2009;163:339–51. doi: 10.1016/j.neuroscience.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9:388–95. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Tassin JP, Studler JM, Herve D, Blanc G, Glowinski J. Contribution of noradrenergic neurons to the regulation of dopaminergic (D1) receptor denervation supersensitivity in rat prefrontal cortex. J Neurochem. 1986;46:243–8. doi: 10.1111/j.1471-4159.1986.tb12953.x. [DOI] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–7. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Cen X, Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur. J. Pharmacol. 2001;432:153–161. doi: 10.1016/s0014-2999(01)01487-x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann. N. Y. Acad. Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Yamada H, Bruijnzeel AW. Stimulation of alpha2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropharmacology. 2010;60:303–11. doi: 10.1016/j.neuropharm.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]