Abstract
Objective
This study examined a possible association of dietary exposure to polybrominated biphenyls (PBBs), a brominated flame retardant, and self-reported abnormal Pap test results and cervical dysplasia as a precursor to cervical cancer.
Methods
Women in Michigan who ingested contaminated poultry, beef, and dairy products in the early 1970s were enrolled in a population-based cohort study in Michigan. Serum PBB and serum polychlorinated biphenyl (PCB) concentrations were measured. Reproductive history and health information, including Pap test results, were self-reported by participants.
Results
Of the women, 23% (223 of 956) reported an abnormal Pap test. In unadjusted analyses, self-reporting an abnormal Pap test was associated with younger age, current smoking (hazard ratio [HR] 1.61, 95% confidence interval [CI] 1.19-2.17), and longer duration of lifetime use of oral contraceptives (≥10 years; HR 1.92, 95% CI 1.21-3.06). When adjusting for PCB exposure, age at the interview, and smoking history, there was a slightly elevated risk of self-reporting an abnormal Pap test among the highly exposed women compared to women with nondetectable PBB concentrations (PBB≥13 μg/L, HR 1.23, 95% CI 0.74-2.06); however, the CI was imprecise. When breastfeeding duration after the initial PBB measurement was taken into account, there was a reduced risk of self-reporting an abnormal Pap test among the highly exposed women who breastfed for ≥12 months (HR 0.41, 95% CI 0.06-3.03; referent group: women with nondetectable PBB concentrations who did not breastfeed).
Conclusions
It remains important to evaluate the potential reproductive health consequences of this class of chemicals as well as other potential predictors of abnormal Pap tests.
Introduction
In 1973, a brominated flame retardant containing polybrominated biphenyls (PBBs) was accidentally mixed with livestock feed on Michigan farms. In the months that followed (1973–1974), many Michigan residents unknowingly ate poultry, beef, and dairy products contaminated with high concentrations of PBBs. The Michigan Department of Community Health established a registry of exposed individuals in 1976 to study possible health effects from this agricultural accident.1,2 This cohort provides a unique opportunity to examine important health outcomes related to PBB exposure. A variety of reproductive health outcomes have been examined among women who were born before the PBB incident occurred (before July 1973) and who were likely to have dietary exposure to PBBs.3–7
PBBs belong to a family of structurally similar chemicals known as polyhalogenated aromatic hydrocarbons, which also includes polychlorinated biphenyls (PCB), dioxins, and furans. PBBs, manufactured chemicals added to electrical devices and plastics, were discontinued in the United States in the late 1970s, although structurally similar brominated flame retardants continue to be produced.8,9 PBBs are stable persistent pollutants, with half-lives of 11–29 years depending on the initial level of exposure.10,11 Like PBBs, PCBs are lipophilic and can remain in the body for many years.12 Although the United States stopped the manufacture of PCBs in the 1970s based on evidence of their persistence and toxicity, the general population is still exposed through diet, mainly fish. Examining the long-term health effects of PBBs, PCBs, and other similar chemicals remains an important and relevant clinical and public health issue.
The Papanicolaou (Pap) test aims to identify cervical dysplasia, a precursor to cervical cancer, by sampling cells from the transformation zone of the cervix.13 Because environmental pollutants have been suspected as possible cofactors for the development of cervical cancer, we hypothesize that women with PBB or PCB exposure may be at an increased risk of having Pap test abnormalities, which may be caused by a direct carcinogenic effect. Although human papillomavirus (HPV) is central to the development of cervical dyplasia and cervical cancer, infection with HPV alone is not sufficient to cause cancer, as most HPV infections are transient.14 It is possible that exposure to PBB or PCB might modify the local response to HPV infection. Furthermore, PBB exposure has been shown to cause immunosuppression in laboratory animals15; immunosuppression resulting from such conditions as systemic lupus erythematosus16 and AIDS17 is associated with an increased risk of cervical dysplasia and cancer. Because of their role as potential endocrine disruptors, PBBs or PCBs might induce dysplastic changes in squamous or glandular cells of the ectocervix or endocervix and might affect the transformation zone where cells are sampled for Pap tests and where cervical cancers arise.
In this study, we explore whether established risk factors of cervical cancer18,19 (e.g., smoking, long-term use of oral contraceptives, high parity, and young age at first full-term pregnancy) are consistent or associated with abnormal Pap tests. In addition, we examine a possible association of PBB exposure and self-reported Pap test abnormalities. As PCB concentrations were measured in this cohort, we also examine a possible association of PCB exposure and Pap test abnormalities.
Materials and Methods
Study population
The Michigan Department of Community Health began enrolling individuals into the Michigan Long-Term PBB Study during 1976–1978. Participants either resided on farms that had been quarantined or had consumed food products produced from quarantined farms. An enrollment questionnaire (1976–1978) solicited a detailed medical history, reproductive history, and information on lifestyle and exposures. Most participants also provided a serum sample.
The Female Health Study, a collaboration between Emory University and Michigan Department of Community Health, was designed to investigate reproductive and endocrine outcomes among female participants of the Michigan Long-Term PBB Study. Women were invited to participate in a telephone interview in 1997–1998. Of the 1530 women eligible for participation, 88 (6%) could not be located, 9 (0.6%) were deceased, and 8 (0.5%) were too ill to participate in the study. Of the remaining 1425 women, 1185 (83%) agreed to participate in the study, and of those, 1046 provided at least one serum sample since 1976, and 1005 were exposed to PBB through diet (n=41 women exposed to PBB in utero were excluded).The Institutional Review Boards at Emory University and the Michigan Department of Community Health approved the protocols, and participants gave written informed consent.
Exposure assessment
Participants were exposed to a mixture of PBBs that contained mostly PBB-153 or 2,2′4,4′5,5′-hexabromobiphenyl (60% of the mixture).8 Serum samples were collected at enrollment into the cohort (1976–1978) and analyzed by the Michigan Department of Community Health Bureau of Laboratories. PBBs were detected and quantitated using gas chromatography, with electron capture detection.20 Serum was analyzed for PCB exposure as Aroclor 1254, using a similar analytic method as that for PBB. The limit of detection (LOD) was 1.0 μg/L for the PBB serum samples and 5.0 μg/L for the PCB serum samples. Serum PBB and PCB measurements were collected from nonfasting women and were not adjusted for serum lipid levels.
Outcome assessment
Self-reported information about a woman's Pap test history was collected during the telephone interviews described. Women were asked a series of related questions: (1) Have you ever had a Pap smear? (2) When was your most recent Pap smear screening test (within the last year, past 2 years, past 3 years, or more than 3 years ago)? (3) Have you ever had an abnormal Pap smear? (4) How many times have you had an abnormal Pap smear result? (5) How old were you when you had each abnormal Pap smear result? (6) Was any procedure or treatment performed because of this abnormal Pap smear? Based on the responses, we restricted our sample of 1005 by excluding those who either refused (n=2) or answered no (n=12) to question (1); answered don't know (n=5) to question (3); or had an abnormal Pap test of unknown dates (n=9) or before the PBB exposure period (n=21). After exclusions, the final sample size was 956.
Verification of abnormal Pap tests
To confirm Pap test abnormalities, we sent medical record consent forms to women who reported having an abnormal Pap test during the interview (n=223). We requested Pap test reports, colposcopy reports, and biopsy and histology records from their physicians. Thirty-one women (14%) could not be contacted to obtain consent for medical record release, 36 women (16%) did not provide consent, 39 (17%) had medical records that were unavailable or incomplete, and 10 (4%) had no record of an abnormal Pap test, according to their physician. Medical records for the 105 remaining women were received and reviewed by an obstetrician/gynecologist (D.J.J.).
Statistical analyses
PBB and PCB exposure was based on the initial serum sample collected at enrollment (mostly during 1976–1979). Because of their skewed distributions, we categorized exposures into three groups using the distribution of serum levels among the women who self-reported an abnormal Pap test: < LOD (<1 μg/L for PBB and <5 μg/L for PCB), ≥LOD up to the median value (3 μg/L for PBB and 7 μg/L for PCB), and ≥ the median. To capture the highly exposed women, we categorized exposures as <LOD, up to the 90th percentile (13 μg/L for PBB and 11 μg/L for PCB), and ≥ the 90th percentile of exposure.
We performed survival analysis techniques to account for censoring and time-varying risk factors collected during the interviews. We examined the risk of self-reporting an abnormal Pap test and determined if risk varied by exposure or risk factors. We analyzed Kaplan-Meier curves and unadjusted hazard ratios (HRs) with the outcome and assessed the proportional hazards assumption by examination of log-log survival curves. In multivariable analyses, we performed extended Cox models to allow for time-dependent covariates. We used age as the time scale21,22 so that participants entered the risk set at their age during the PBB exposure period (defined as age on July 1, 1973). Women contributed person-time until the age when they self-reported an abnormal Pap test or the age when they participated in the telephone interview for women who were censored and did not report having an abnormal Pap test. To control for cohort effects or secular trends in screening, all models were stratified by birth cohort (born before 1940, 1940–1949, 1950–1959, 1960–1973).
We assume that the risk factors for cervical cancer may be relevant and consistent for abnormal Pap tests. These risk factors were considered as potential confounders and identified from the American Cancer Society (ACS)18 and the American College of Obstetricians and Gynecologists (ACOG)19 guidelines.
The potential risk factors for abnormal Pap tests that were treated independent of time were categorized as follows: education level (≤high school or some college or higher), household income (<$35,000 or ≥$35,000), and health insurance status (no or yes), which were used as proxies for socioeconomic status (SES); diethylstilbestrol (DES) exposure in utero (no, yes, or don't know), which was asked of women born during 1940–1971; and lifetime duration of oral contraceptive use (never, 1–5, 6–9, or ≥10 years). Because duration of oral contraceptive use was asked as a lifetime measure, we could not examine it as a time-dependent covariate.
We were able to treat several potential risk factors as time-dependent covariates. For parity (categorized as no live births, 1–2 live births, or ≥3 live births), women who never had a live birth were assigned a value of 0. Otherwise, parity was assigned a value of 1 if a woman had 1–2 live births or a value of 2 if she had ≥3 live births before the survival time of interest (abnormal Pap test date or interview date). Age at first live birth was categorized as no live births, <20 years, 20–24 years, or ≥25 years. A woman who experienced an abnormal Pap test or was interviewed (if censored) before she had her first live birth was classified as having no live births. For smoking history (categorized as never, former, or current), we used the ages when the women reported they smoked regularly (at least one cigarette a day). A never smoker was assigned a value of 0. A former or current smoker was assigned a value of 0 for any time prior to when she smoked regularly, which could then change to reflect whether she was a current smoker for the times during follow-up that she smoked regularly (assigned a value of 2) or a former smoker for the times after she had stopped smoking regularly (assigned a value of 1).
We did not have information on HPV infections, dietary factors, sexual history factors, or sexually transmitted diseases (STDs). In addition to the common ACS/ACOG risk factors, we considered several other descriptive covariates. Age at the interview and body mass index (BMI) at enrollment were time-independent covariates. Age at the interview (categorized as 24–34, 35–39, 40–49, ≥50 years) was considered because older age has been associated with a decreased risk for abnormal Pap tests.23,24 In addition, because the interview was 24 years after the PBB exposure incident, the younger ages (24–34 and 35–39) would reflect women exposed to PBB during vulnerable periods from childhood to midadolescence (<16 years). BMI, calculated from height and weight collected at enrollment, was used as a measure of adiposity for women who were at least 16 years of age at enrollment. BMI was based on standard classifications25: underweight and normal (<25 kg/m2; a small number of women were classified as underweight, n=29, so they were combined with the normal weight women), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2).
We also ascertained if a woman had a history of pelvic inflammatory disease (PID) or a history of cervical cancer (no or yes). PID was included as a time-dependent covariate (categorized as no or yes) in the models, such that a woman was initially assigned a value of 0 (for no), which would change to 1 (for yes) at the age when she developed the condition.
Because lactation may reduce a woman's body burden of exposure to environmental contaminants, we examined the combined effect of PBB exposure by breastfeeding duration. Breastfeeding duration, calculated by summing all breastfeeding periods from the initial PBB measurement up to the abnormal Pap test date or interview date, was included as a time-dependent covariate and categorized as no breastfeeding, <12 months, and ≥12 months.
Self-reported abnormal Pap tests were used in the main analyses. However, we performed a subanalysis limiting the women who reported abnormal Pap tests to those verified by medical records. We used serum PBB and PCB concentrations collected during the enrollment period (1976–1979) to represent peak exposure levels. In a sensitivity analysis, we excluded 19 women who had their initial serum samples taken at later times (1980s–1990s).
We examined the crude associations between potential risk factors and self-reporting an abnormal Pap test status with unadjusted HRs. We then examined the crude associations between potential risk factors and PBB exposure status using chi-square tests (exposure split as <LOD, up to median, ≥ median). We found that PCB exposure, age at the interview, and BMI at enrollment were significantly associated with PBB exposure (chi-square p≤0.05). Women with higher PBB concentrations had higher PCB concentrations. Older women (≥40 years) were more likely to have lower PBB concentrations compared to the younger women. Women classified as overweight or obese were more likely to have lower PBB concentrations. Effect modification was assessed for interactions that were biologically plausible, which included PBB with PCB exposure, PBB with BMI at enrollment, and PBB with breastfeeding duration.
Using a backward elimination modeling strategy, covariates that were either statistically significantly associated with self-reporting an abnormal Pap test (p<0.05) or exhibited substantial confounding (>10% change in the odds ratio [OR]) or effect modification (p<0.05) with PBB exposure were retained in the multivariable models. All statistical analyses were performed with SAS statistical software version 9.1 (SAS Institute Inc., Cary, NC).
Results
The study included 956 women who had a mean age of 22 years during the PBB exposure period (based on age in July 1973) and had their serum PBB measurements collected at a mean age of 26 years. Overall, 23% (223 of 956) of women reported an abnormal Pap test. Women with a self-reported history of abnormal Pap tests were younger during the PBB exposure period (minimum–maximum: infancy–60 years; mean age 16 years; median age 14 years) than women who did not report having an abnormal Pap test (minimum–maximum: infancy–62 years; mean age 23 years; median age 21 years). There was no difference in the median PBB concentration for women with a self-reported history of abnormal Pap tests (median 2 μg/L; minimum–maximum: not detectable–707 μg/L) and women without a self-reported history of abnormal Pap tests (median 2 μg/L; minimum–maximum: not detectable–1745 μg/L). Similarly, there was no difference in the median PCB concentration for women with a self-reported history of abnormal Pap tests (median 5 μg/L; minimum–maximum: not detectable–31 μg/L) and women without a self-reported history of abnormal Pap tests (median 5 μg/L; minimum–maximum: not detectable–78 μg/L).
Of the 223 women who self-reported an abnormal Pap test after the PBB exposure period, 88 (39%) reported more than one abnormal screening. The average age at the first reported abnormal Pap screening was 33 years (median 30 years; minimum–maximum: 17–68 years). The average time from exposure (1973) to the abnormal Pap test differed slightly by levels of PBB exposure. Women with PBB concentrations ≥3 μg/L (the median) had a longer time since exposure to the abnormal Pap test date (minimum–maximum: 3–24 years; mean 17.5 years; median 18 years) than women with PBB exposure below the limit of detection (minimum–maximum: 2–24 years: mean 14.2 years; median 14 years). Women with PBB concentrations between 1 and <3 μg/L were similar to those with PBB concentrations ≥3 μg/L (minimum–maximum: 2–24 years: mean 16.5 years; median 18 years).
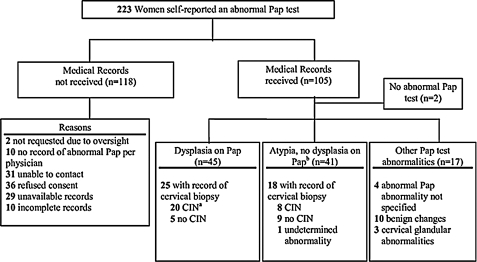
Pap test cytology results were reviewed for 105 of the 223 women (Fig. 1). Two women (2%) had no Pap test abnormality documented in their medical records. Of the remaining 103 women, 93 (90%) had evidence of an abnormality in their medical records. This included 45 (43%) who were noted to have dysplasia and 41 (39%) who had atypia, and the remaining 7 women had other Pap test abnormalities (4 with abnormality not specified and 3 with cervical glandular abnormalities). Among the 43 women with cervical biopsies, 28 (65%) had evidence of cervical intraepithelial neoplasia (CIN) on histologic study. Among the other Pap test abnormalities, 10 women had benign changes.
FIG. 1.
Flow chart of medical verification of self-reported abnormal Pap tests among women in the Michigan Female Health Study. Records were requested from 223 women, and 105 charts were obtained and reviewed. aCIN, cervical intraepithelial neoplasia. bIncludes terms, atypia and atypical squamous cells of undetermined significance (ASCUS).
Table 1 presents crude HRs describing characteristics of women by self-reported abnormal Pap test status. For the time-independent covariates, we found a decreased risk of self-reporting an abnormal Pap test among women who were ≥40 years at the interview (40–49 years: HR 0.29, 95% CI 0.14-0.60; ≥50 years: HR 0.11, 95% CI 0.04-0.33). This would equate to a reduced risk for women who were exposed to PBB when they were ≥16 years of age (the interview was 24 years after exposure). BMI at enrollment, education level, and household income at the interview were not associated with self-reporting an abnormal Pap test. Although it did not reach statistical significance, women who self-reported an abnormal Pap test were more likely to have health insurance at the interview. Eleven women born during 1940–1971 reported having been exposed to DES in utero. Among these women, there was a slightly increased risk of self-reporting an abnormal Pap test (HR 1.81, 95% CI 0.79-4.12). A longer duration of lifetime use of oral contraceptives was observed among women who self-reported an abnormal Pap test, which increased for more years used (≥10 years: HR 1.92, 95% CI 1.21-3.06). Seventeen women, who had an abnormal Pap test reported having a history of cervical cancer diagnosed within 1 year of when the abnormal Pap test occurred.
Table 1.
Crude Hazard Ratios of Characteristics of Women by Self-Reported Abnormal Pap Test Status (n=956)
| |
Self-reported an abnormal Pap test |
|
|
|
|---|---|---|---|---|
| Characteristic | Yes n (%) | No n (%) | HRa | 95% CI |
| Age at interview, years | ||||
| 24–34 | 80 (35.9) | 177 (24.1) | 1.00 | – |
| 35–39 | 48 (21.5) | 99 (13.5) | 0.76 | 0.48-1.22 |
| 40–49 | 48 (21.5) | 148 (20.2) | 0.29 | 0.14-0.60 |
| ≥50 | 47 (21.1) | 309 (42.2) | 0.11 | 0.04-0.33 |
| Body mass index at enrollment, kg/m2 | ||||
| <25 | 98 (44.1) | 304 (41.8) | 1.00 | – |
| 25–29.9 | 25 (11.3) | 132 (18.2) | 0.73 | 0.47-1.15 |
| ≥30 | 6 (2.7) | 83 (11.4) | 0.31 | 0.14-0.72 |
| Missingb | 93 (41.9) | 208 (28.6) | 1.17 | 0.61-2.25 |
| Education level at interview | ||||
| ≤High school | 96 (43.1) | 352 (48.0) | 1.00 | – |
| Some college or higher | 127 (56.9) | 381 (52.0) | 0.96 | 0.73-1.26 |
| Household income/year at interview | ||||
| <$35, 000 | 82 (39.8) | 309 (46.0) | 1.00 | – |
| ≥$35, 000 | 124 (60.2) | 363 (54.0) | 0.95 | 0.71-1.26 |
| Health insurance status at interview | ||||
| No | 13 (5.8) | 56 (7.6) | 1.00 | – |
| Yes | 210 (94.2) | 676 (92.4) | 1.37 | 0.78-2.40 |
| History of DES exposure in uteroc | ||||
| No | 131 (68.2) | 355 (71.1) | 1.00 | – |
| Yes | 6 (3.1) | 5 (1.0) | 1.81 | 0.79-4.12 |
| Don't know | 55 (28.7) | 139 (27.9) | 1.05 | 0.76-1.44 |
| Lifetime duration of oral contraceptive use, years | ||||
| Never | 35 (15.7) | 222 (30.3) | 1.00 | – |
| 1–5 | 95 (42.6) | 317 (43.3) | 1.20 | 0.77-1.85 |
| 6–9 | 36 (16.1) | 94 (12.8) | 1.43 | 0.86-2.37 |
| ≥10 | 57 (25.6) | 100 (13.6) | 1.92 | 1.21-3.06 |
| History of cervical cancer | ||||
| No | 206 (92.4) | 733 (100.0) | 1.00 | – |
| Yes | 17 (7.6) | 0 (0.0) | 7.15 | 4.29-11.92 |
| Parityd | ||||
| No live births | 71 (31.8) | 101 (13.8) | 1.00 | – |
| 1–2 live births | 80 (35.9) | 257 (35.0) | 1.00 | 0.70-1.44 |
| ≥3 live births | 72 (32.3) | 375 (51.2) | 0.89 | 0.58-1.36 |
| Age at first live birth, yearsd | ||||
| No live births | 71 (31.8) | 101 (13.8) | 0.87 | 0.55-1.38 |
| <20 | 41 (18.4) | 152 (20.7) | 0.80 | 0.51-1.26 |
| 20–24 | 73 (32.7) | 316 (43.1) | 0.82 | 0.55-1.21 |
| ≥25 | 38 (17.1) | 164 (22.4) | 1.00 | – |
| History of smoking regularlyd | ||||
| Never | 138 (61.9) | 529 (72.2) | 1.00 | – |
| Former | 39 (17.5) | 108 (14.7) | 1.37 | 0.86-2.19 |
| Current | 46 (20.6) | 96 (13.1) | 1.61 | 1.19-2.17 |
| History of pelvic inflammatory diseased | ||||
| No | 214 (97.3) | 712 (97.3) | 1.00 | – |
| Yes | 6 (2.7) | 20 (2.7) | 1.12 | 0.50-2.54 |
| Breastfeeding duration, monthsd,e | ||||
| No breastfeeding | 159 (71.3) | 510 (69.6) | 1.00 | – |
| <12 | 49 (22.0) | 137 (18.7) | 1.17 | 0.82-1.65 |
| ≥12 | 15 (6.7) | 86 (11.7) | 0.85 | 0.49-1.49 |
Models stratified by birth cohort (<1940, 1940–1949, 1950–1959, 1960–1973).
Body mass index at enrollment missing if <16 years at enrollment.
Diethylstilbestrol (DES) exposure in utero not asked if participant was born before 1940 or after 1971.
Treated as time-dependent covariates in the extended Cox models; frequencies and proportions are based on status at the event (abnormal Pap test date) or at right censoring (interview date) (for parity, age at first live birth, history of breastfeeding, smoking, or pelvic inflammatory disease).
Breastfeeding duration between the initial PBB measurement up to the event (abnormal Pap test date) or right censoring (interview date).
CI, confidence interval; HR, hazard ratio.
For the time-dependent covariates, the frequencies and proportions in Table 1 correspond to a woman's status at the event (abnormal Pap test date) or right censoring (interview date). Higher parity or earlier age at first live birth was not associated with self-reporting an abnormal Pap test. A greater proportion of women with self-reported abnormal Pap tests (38%) had ever smoked cigarettes compared to 28% of women who did not report an abnormal Pap test. In addition, women who were current smokers (at the event) had a significantly higher risk of reporting an abnormal Pap screening (HR 1.61, 95% CI 1.19-2.17). History of PID was not associated with self-reporting an abnormal Pap test. Breastfeeding duration was not associated with self-reporting an abnormal Pap test, although the HR for breastfeeding ≥12 months was slightly protective (HR 0.85, 95% CI 0.49-1.49).
Table 2 gives the crude and adjusted HRs of initial PBB exposure and risk of self-reporting an abnormal Pap test. The HRs for self-reporting an abnormal Pap test did not vary greatly by PBB concentrations when split at the median levels (crude or adjusted for PCB exposure split at the median levels, age at the interview, and smoking history). In the highly exposed women, there was a slight increase in risk (PBB≥13 μg/L: HR 1.23, 95% CI 0.74-2.06) for self-reporting an abnormal Pap test compared to women with nondetectable PBB concentrations when adjusted for PCB exposure split at the 90th percentile, age at the interview, and smoking history, but the CI was imprecise. The HRs for PCB exposure (PCB≥11 μg/L: HR 1.48, 95% CI 0.94-2.33) and smoking history (current smokers: HR 1.63, 95% CI 1.20-2.20) were also elevated in the adjusted PBB model for the highly exposed women.
Table 2.
Hazard Ratios for Risk of Self-Reported Abnormal Pap Test, by Initial Polybrominated Biphenyl Exposure (n=956)
| Serum concentrations | n (%) | Crude HR | 95% CI | Adjusted HRa | 95% CI |
|---|---|---|---|---|---|
| Model 1: Median PBB=3 μg/L | |||||
| <1 (not detectable) | 189 (19.8) | 1.00 | – | 1.00 | – |
| 1–<3 | 355 (37.1) | 1.01 | 0.69-1.48 | 1.14 | 0.77-1.68 |
| ≥3 | 412 (43.1) | 1.02 | 0.71-1.48 | 1.14 | 0.78-1.67 |
| Model 2: 90th percentile PBB=13 μg/L | |||||
| <1 (not detectable) | 189 (19.8) | 1.00 | – | 1.00 | – |
| 1–<13 | 669 (70.0) | 1.00 | 0.70-1.41 | 1.13 | 0.79-1.62 |
| ≥13 | 98 (10.2) | 1.18 | 0.72-1.95 | 1.23 | 0.74-2.06 |
Models adjusted for polychlorinated biphenyl (PCB) concentrations (median PCB in model 1 and 90th percentile PCB in model 2), age at the interview (24–34, 35–39, 40–49, ≥50 years), and smoking history (never, former, and current), where smoking history's treated as a time-dependent covariate, and models stratified by birth cohort (<1940, 1940–1949, 1950–1959, 1960–1973).
Overall, interaction terms for PBB with BMI at enrollment and PBB with PCB exposure were not significant. For PBB and PCB exposure combined, we found an elevated risk (HR 1.46, 95% CI 0.78-2.78) for women with PBB and PCB concentrations ≥ median compared to women with nondetectable PBB and PCB exposure, although this was based on small numbers (PBB and PCB concentrations ≥ median, n=148 women, 35 who self-reported an abnormal Pap test; PBB and PCB concentrations <LOD, n=74 women, 14 who self-reported an abnormal Pap test).
The overall interaction term for PBB (when split at the median and 90th percentile) by breastfeeding duration in the adjusted models was not significant. However, when PBB exposure was split at the 90th percentile, there was a decreased risk of self-reporting an abnormal Pap test for women with high PBB exposure (PBB≥13 μg/L) who breastfed for ≥12 months (HR 0.41, 95% CI 0.06-3.03) compared to women with nondetectable PBB levels who did not breastfeed, but the CI was imprecise (Table 3). Conversely, there was an elevated risk of self-reporting an abnormal Pap test for women with high PBB exposure (PBB≥13 μg/L) who did not breastfeed (HR 1.43, 95% CI 0.80-2.54) compared to women with nondetectable PBB levels who did not breastfeed. This was also true for several other categories of PBB exposure and breastfeeding duration (Table 3).
Table 3.
Adjusted Hazard Ratios for Risk of Self-Reported Abnormal Pap Test, by Initial Polybrominated Biphenyl Exposure and Breastfeeding Duration (n=956)
| |
Breastfeeding durationa |
|||||
|---|---|---|---|---|---|---|
| |
No breastfeeding |
1–<12 months |
≥12 months |
|||
| Serum PBB concentrations | Adjusted HRb | 95% CI | Adjusted HRb | 95% CI | Adjusted HRb | 95% CI |
| Model 1: Median PBB=3 μg/L | ||||||
| <1 (not detectable) | 1.00 | Referent | 1.03 | 0.42-2.52 | 1.31 | 0.39-4.44 |
| 1–<3 | 1.22 | 0.78-1.91 | 1.16 | 0.64-2.12 | 0.82 | 0.34-2.02 |
| ≥3 | 1.11 | 0.72-1.72 | 1.52 | 0.87-2.67 | 0.88 | 0.36-2.16 |
| Model 2: 90th percentile PBB=13 μg/L | ||||||
| <1 (not detectable) | 1.00 | Referent | 1.01 | 0.41-2.46 | 1.34 | 0.40-4.53 |
| 1–<13 | 1.12 | 0.74-1.69 | 1.37 | 0.83-2.27 | 0.96 | 0.47-1.97 |
| ≥13 | 1.43 | 0.80-2.54 | 1.18 | 0.41-3.41 | 0.41 | 0.06-3.03 |
Breastfeeding duration is time-dependent and includes breastfeeding episodes between the initial PBB measurement and the event (abnormal Pap test date) or right censoring (interview date).
Models adjusted for PCB concentrations (median PCB in model 1 and 90th percentile PCB in model 2), age at the interview (24–34, 35–39, 40–49, ≥50 years), and smoking history (never, former, and current), where smoking history's treated as a time-dependent covariate, and models stratified by birth cohort (<1940, 1940–1949, 1950–1959, 1960–1973).
We compared the characteristics of the participants who self-reported an abnormal Pap test by medical record status (received or not received) (Table 4). There were no differences in their PBB or PCB exposure concentrations or other descriptive characteristics, except for history of cervical cancer. Of the women who had a history of cervical cancer, a greater proportion had medical records received.
Table 4.
Characteristics of Participants, by Whether Medical Records Were Received to Verify Self-Reported Abnormal Pap Test (n=221)
| |
Medical records received |
||
|---|---|---|---|
| Characteristic | Yes n (%) | No n (%) | chi-square p value |
| Serum PBB concentrations | 0.88 | ||
| <1 (not detectable) | 20 (19.4) | 20 (17.0) | |
| 1–<3 | 39 (37.9) | 45 (38.1) | |
| ≥3 | 44 (42.7) | 53 (44.9) | |
| Serum PCB concentrations | 0.48 | ||
| <5 (not detectable) | 37 (35.9) | 43 (36.4) | |
| 5–<7 | 21 (20.4) | 29 (24.6) | |
| ≥7 | 35 (34.0) | 30 (25.4) | |
| Missing | 10 (9.7) | 16 (13.6) | |
| Age at interview, years | 0.26 | ||
| 24–34 | 35 (34.0) | 44 (37.4) | |
| 35–39 | 17 (16.5) | 30 (25.4) | |
| 40–49 | 26 (25.2) | 22 (18.6) | |
| ≥50 | 25 (24.3) | 22 (18.6) | |
| BMI at interview, kg/m2 | 0.11 | ||
| <25 | 42 (40.8) | 56 (47.9) | |
| 25–29.9 | 30 (29.1) | 40 (34.2) | |
| ≥30 | 31 (30.1) | 21 (18.0) | |
| Education level at interview | 0.38 | ||
| ≤High school | 47 (45.6) | 47 (39.8) | |
| Some college or higher | 56 (54.4) | 71 (60.2) | |
| Household income/year at interview | 0.78 | ||
| <$35, 000 | 39 (40.2) | 41 (38.3) | |
| ≥$35, 000 | 58 (60.0) | 66 (61.7) | |
| Health insurance status at interview | 0.97 | ||
| No | 6 (5.8) | 7 (5.9) | |
| Yes | 97 (94.2) | 111 (94.1) | |
| History of oral contraceptive use, years | 0.06 | ||
| Never | 21 (20.4) | 14 (11.9) | |
| 1–5 | 36 (34.9) | 59 (50.0) | |
| 6–9 | 21 (20.4) | 15 (12.7) | |
| ≥10 | 25 (24.3) | 30 (25.4) | |
| History of DES exposure in uteroa | 0.52b | ||
| No | 59 (68.6) | 70 (67.3) | |
| Yes | 4 (4.7) | 2 (1.9) | |
| Don't know | 23 (26.7) | 32 (30.8) | |
| History of cervical cancer | 0.01 | ||
| No | 90 (87.4) | 114 (96.6) | |
| Yes | 13 (12.6) | 4 (3.4) | |
| Parityc | 0.17 | ||
| No live births | 29 (28.2) | 42 (35.6) | |
| 1–2 live births | 34 (33.0) | 44 (37.3) | |
| ≥3 live births | 40 (38.8) | 32 (27.1) | |
| Age at first live birth, yearsc | 0.06 | ||
| No live births | 29 (28.2) | 42 (35.6) | |
| <20 | 19 (18.5) | 21 (17.8) | |
| 20–24 | 42 (40.8) | 30 (25.4) | |
| ≥25 | 13 (12.6) | 25 (21.2) | |
| History of smoking regularlyc | 0.46 | ||
| Never | 63 (61.2) | 75 (63.6) | |
| Former | 21 (20.4) | 17 (14.4) | |
| Current | 19 (18.4) | 26 (22.0) | |
| History of pelvic inflammatory diseasec | 1.00b | ||
| No | 99 (98.0) | 114 (97.4) | |
| Yes | 2 (2.0) | 3 (2.6) | |
| Breastfeeding duration (months)c,d | 0.23 | ||
| No breastfeeding | 69 (67.0) | 88 (74.6) | |
| <12 | 24 (23.3) | 25 (21.2) | |
| ≥12 | 10 (9.7) | 5 (4.2) | |
DES exposure in utero not asked if participant was born before 1940 or after 1971.
Fisher's exact test.
Frequencies and proportions are based on status at the event (abnormal Pap test date) for parity, age at first live birth, smoking history, pelvic inflammatory disease, and history of breastfeeding duration.
Breastfeeding duration since the initial PBB measurement up to the event (abnormal Pap test date) or right censoring (interview date).
BMI, body mass index.
The sensitivity analysis limiting the cases to the 93 medically verified abnormal Pap tests (excludes the 2 women whose medical records were not verified and the 10 women with benign changes) resulted in similar HRs that were in some cases of higher magnitude than those presented in Table 2. In the model (adjusted for PCB exposure, age at the interview, and smoking history), there was an increase in the risk for self-reporting an abnormal Pap test among the highly exposed women (n=85 women: HR 1.41, 95% CI 0.67-2.95) compared to women with nondetectable PBB concentrations. In addition, the analysis excluding the 19 women who had their initial serum PBB sample taken during the 1980s–1990s found minimal changes to the HRs.
Discussion
Our study, which to our knowledge is the first to explore the relationship between PBB or PCB exposure and abnormal Pap tests, has several key strengths, including a population-based cohort design along with a well-defined window of PBB exposure. This cohort also provided a convenient opportunity to study PCB exposure among women whose PCB concentrations reflect those found in the general population.26
Our study has several limitations. First, for our outcome variable, we relied on self-reported Pap test results, and there is some suggestion that women may overreport their Pap screening history.27 For women who self-reported an abnormal Pap test in our study and had medical records available, the majority (>90%) had evidence of an abnormality on medical record review. However, a large proportion (53%) did not have medical records available.
We considered risk factors for cervical cancer18,19 to determine if they were consistent with the reporting of an abnormal Pap test. We were limited in that several of the risk factors were captured either as of the interview date and not before their Pap test history (e.g., education, income, or health insurance) or as a lifetime measure (for duration of oral contraceptives). We found consistent associations for history of smoking and longer years of oral contraceptive use. For duration of oral contraceptive use, however, we did not ask women when they used oral contraceptives in relation to when they reported their Pap tests. Women who reported a history of DES exposure in utero appeared to have increased risk, but this was limited because of small numbers (n=11 reported yes). Further, a number of women (n=194, 28%) did not know if they had a history of DES exposure in utero, which is a possible source of misclassification. We did not find consistent associations for parity (≥3 live births) or age at first live birth (younger age) when they occurred before the abnormal Pap test date or interview date. Further, we did not have information on HPV infections, dietary factors, sexual history factors, or STDs.
When we explored a possible effect modification of PBB exposure by breastfeeding duration, the results suggested a reduced risk of self-reporting an abnormal Pap test among the highly exposed women who breastfed for ≥12 months. This seems plausible, as lactation may reduce a woman's body burden of exposure. The risk of self-reporting an abnormal Pap test was elevated in the highly exposed women who did not breastfeed.
Although we reviewed medical records to confirm self-reported abnormalities, we did not review the medical records of women who did not report an abnormal Pap test. In a small study conducted in Australia, among the 82 women who self-reported a normal Pap test, 9 (11%) had abnormal results documented in their medical records.28 Second, as some of the Pap test reports were prior to the adoption of the initial Bethesda system,29 current standard terminology was not consistently used in those medical records. However, the obstetrician/gynecologist (D.J.J.) reviewing the medical records was able to use the descriptions found in the medical records to classify these Pap tests into the categories used in this study. Lastly, it is possible that our null findings are due to our small sample size rather than a true lack of effect. There were 98 highly exposed women (top 10%) for PBB (n=26 who self-reported an abnormal Pap test) and 104 highly exposed women for PCB (n=27 who self-reported an abnormal Pap test). When considering the highly exposed women, the HRs for PBB or PCB exposure were increased (1.23 and 1.48, respectively), but the CIs included the null value.
For the current study, we examined risk of cervical dysplasia, a precursor to cervical cancer. However, it is important to note that because most HPV infections are transient,14 cytologic manifestions of active HPV infection (i.e., abnormal Pap tests) often regress. This means that most women infected with HPV do not develop cervical cancer; the two factors most closely linked to development of cervical cancer are persistence of HPV infection and infection with high-risk types.30,31 Therefore, the risk factors for women who develop abnormal Pap tests may be different from those for the subset of women who develop cervical cancer.
Because long-term exposure to PBBs and PCBs in animals has been associated with neoplasias,32 we wanted to evaluate a potential carcinogenic effect. For example, as PBB can cause immunosuppression15 and immunosuppression can increase the risk of cervical cancer,16,17 it is possible that PBB exposure might include the development of cervical cancer via immunotoxicity. In addition, because polyhalogenated aromatic hydrocarbons have been shown to disrupt endocrine function,33 the question has been raised of whether they can affect the reproductive system. In this Michigan cohort, prior analyses of women exposed to PBB through diet have not found associations of PBB or PCB exposure with risk of spontaneous abortion,4 time of menopause,5 or benign breast disease.6 In the Davis et al. study,7 no overall association was found between PBB concentrations and menstrual function; however, the study suggested that PBB exposure may impact menstrual function among a subset of women who had recently lost weight. There was an increased incidence of endometriosis among women in this cohort with moderate and high PCB levels compared to women with low PCB levels; however, PBB exposure was not associated with endometriosis.3
In this study, we found that younger women were more likely to report a history of an abnormal Pap test. This may reflect the high prevalence of HPV and cervical dysplasia among younger women.34 Most HPV infections are transient, however, particularly in younger women.14 Once infected, older women are more likely to have persistent HPV infections and, therefore, are at increased risk of cervical dysplasia and cancer. Therefore, it will be critical to continue to follow this cohort of exposed women, as the highest incidence of cervical cancer is among women in their late 40s.35 In addition, since the advent of the HPV vaccine, future studies of cervical dysplasia will need to incorporate vaccination information into their design.31 Finally, although the current study includes women who had dietary exposure to PBBs, future research may consider the Pap test history of daughters who were exposed to PBBs in utero and through breastfeeding.
Conclusions
Consistent with other studies,23,24,36,37 we found associations between risk of reporting an abnormal Pap test and younger age, history of smoking, and a long history of oral contraceptive use. Our results do not indicate significant associations between risk of reporting an abnormal Pap test and PBB or PCB exposure. However, the nonsignificant but elevated HRs with higher exposure levels do not rule out the possibility that there may be an association that we were unable to detect, given the small number of women with high-level exposures. The type of high-level PBB exposure that occurred in Michigan is extremely unusual, but exposures to lower levels of similar chemicals are not uncommon. Therefore, it is critical that reproductive health consequences from this class of chemicals be evaluated further.
Acknowledgments
Funding for this research was provided by the National Institute of Environmental Health Sciences (R01–ES08341 and R01–ES012014) and by Centers for Disease Control and Prevention cooperative agreement U37/CCU500392. We thank the staffs of the Michigan Department of Community Health for access to archived data on cohort participants and the Michigan Public Health Institute for their assistance with data collection, and we thank the participants of the Michigan Long-Term PBB study. We also thank Lynn Zanardi for her early work on these analyses.
Disclosure Statement
No competing financial interests exist.
References
- 1.Fries GF. The PBB episode in Michigan: An overall appraisal. Crit Rev Toxicol. 1985;16:105–156. doi: 10.3109/10408448509056268. [DOI] [PubMed] [Google Scholar]
- 2.Landrigan PJ. Wilcox KR., Jr Silva J., Jr Humphrey HE. Kauffman C. Heath CW., Jr Cohort study of Michigan residents exposed to polybrominated biphenyls: Epidemiologic and immunologic findings. Ann NY Acad Sci. 1979;320:284–294. doi: 10.1111/j.1749-6632.1979.tb56611.x. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman CS. Small CM. Blanck HM. Tolbert P. Rubin C. Marcus M. Endometriosis among women exposed to polybrominated biphenyls. Ann Epidemiol. 2007;17:503–510. doi: 10.1016/j.annepidem.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Small CM. Cheslack-Postava K. Terrell M, et al. Risk of spontaneous abortion among women exposed to polybrominated biphenyls. Environ Res. 2007;105:247–255. doi: 10.1016/j.envres.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanck HM. Marcus M. Tolbert PE, et al. Time to menopause in relation to PBBs, PCBs, and smoking. Maturitas. 2004;49:97–106. doi: 10.1016/j.maturitas.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser R. Marcus M. Blanck HM, et al. Polybrominated biphenyl exposure and benign breast disease in a cohort of U.S. women. Ann Epidemiol. 2003;13:16–23. doi: 10.1016/s1047-2797(02)00256-9. [DOI] [PubMed] [Google Scholar]
- 7.Davis SI. Blanck HM. Hertzberg VS, et al. Menstrual function among women exposed to polybrominated biphenyls: A follow-up prevalence study. Environ Health a Global Access Sci Source. 2005;4:15. doi: 10.1186/1476-069X-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agency for Toxic Substances and Disease Registry. Toxicological profile for polybrominated biphenyls and polybrominated diphenyl ethers. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2004. [Google Scholar]
- 9.Juhasz AL. Smith E. Weber J. Brominated flame retardants—Safety at what cost? Lancet. 2007;370:1813. doi: 10.1016/S0140-6736(07)61757-7. [DOI] [PubMed] [Google Scholar]
- 10.Blanck HM. Marcus M. Hertzberg V, et al. Determinants of polybrominated biphenyl serum decay among women in the Michigan PBB cohort. Environ Health Perspect. 2000;108:147–152. doi: 10.1289/ehp.00108147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen DH. Flanders WD. Friede A. Humphrey HE. Sinks TH. Half-life of polybrominated biphenyl in human sera. Environ Health Perspect. 1995;103:272–274. doi: 10.1289/ehp.95103272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews HB. Dedrick RL. Pharmacokinetics of PCBs. Annu Rev Pharmacol Toxicol. 1984;24:85–103. doi: 10.1146/annurev.pa.24.040184.000505. [DOI] [PubMed] [Google Scholar]
- 13.Michalas SP. The Pap test: George N. Papanicolaou (1883–1962). A screening test for the prevention of cancer of uterine cervix. Eur J Obstet Gynecol Reprod Biol. 2000;90:135–138. doi: 10.1016/s0301-2115(00)00260-8. [DOI] [PubMed] [Google Scholar]
- 14.Ho GY. Bierman R. Beardsley L. Chang CJ. Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 15.Damstra T. Jurgelski W., Jr Posner HS, et al. Toxicity of polybrominated biphenyls (PBBs) in domestic and laboratory animals. Environ Health Perspect. 1982;44:175–188. doi: 10.1289/ehp.8244175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klumb EM. Araújo ML. Jesus GR, et al. Is higher prevalence of cervical intraepithelial neoplasia in women with lupus due to immunosuppression? J Clin Rheumatol. 2010;16:153–157. doi: 10.1097/RHU.0b013e3181df5261. [DOI] [PubMed] [Google Scholar]
- 17.Chaturvedi AK. Madeleine MM. Biggar RJ. Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101:1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Cancer Society. What are the risk factors for cervical cancer? 2010. www.cancer.org. [Aug;2010 ]. www.cancer.org
- 19.American College of Obstetricians and Gynecologists. Practice bulletin No. 35: Diagnosis and treatment of cervical carcinomas. Obstet Gynecol. 2002;99:855–867. [PubMed] [Google Scholar]
- 20.Needham LL. Burse VW. Price HA. Temperature-programmed gas chromatographic determination of polychlorinated and polybrominated biphenyls in serum. J Assoc Off Anal Chem. 1981;64:1131–1137. [PubMed] [Google Scholar]
- 21.Korn EL. Graubard BI. Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: Choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 22.Thiebaut AC. Benichou J. Choice of time-scale in Cox's model analysis of epidemiologic cohort data: A simulation study. Stat Med. 2004;23:3803–3820. doi: 10.1002/sim.2098. [DOI] [PubMed] [Google Scholar]
- 23.Sawaya GF. Kerlikowske K. Lee NC. Gildengorin G. Washington AE. Frequency of cervical smear abnormalities within 3 years of normal cytology. Obstet Gynecol. 2000;96:219–223. doi: 10.1016/s0029-7844(00)00882-6. [DOI] [PubMed] [Google Scholar]
- 24.Benard VB. Eheman CR. Lawson HW, et al. Cervical screening in the National Breast and Cervical Cancer Early Detection Program, 1995–2001. Obstet Gynecol. 2004;103:564–571. doi: 10.1097/01.AOG.0000115510.81613.f0. [DOI] [PubMed] [Google Scholar]
- 25.National Institutes of Health. Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. Obes Res. 1998;6(Suppl 2):51–209S. [PubMed] [Google Scholar]
- 26.Kreiss K. Studies on populations exposed to polychlorinated biphenyls. Environ Health Perspect. 1985;60:193–199. doi: 10.1289/ehp.8560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howard M. Agarwal G. Lytwyn A. Accuracy of self-reports of Pap and mammography screening compared to medical record: A meta-analysis. Cancer Causes Control. 2009;20:1–13. doi: 10.1007/s10552-008-9228-4. [DOI] [PubMed] [Google Scholar]
- 28.Newell S. Girgis A. Sanson-Fisher R. Ireland M. Accuracy of patients' recall of Pap and cholesterol screening. Am J Public Health. 2000;90:1431–1435. doi: 10.2105/ajph.90.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Cancer Institute Workshop. The 1988 Bethesda system for reporting cervica/vaginal cytological diagnosis. JAMA. 1989;262:931–934. [PubMed] [Google Scholar]
- 30.Collins Y. Einstein MH. Gostout BS, et al. Cervical cancer prevention in the era of prophylactic vaccines: A preview for gynecologic oncologists. Gynecol Oncol. 2006;102:552–562. doi: 10.1016/j.ygyno.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 31.Frazer IH. Prevention of cervical cancer through papillomavirus vaccination. Natl Rev Immunol. 2004;4:46–54. doi: 10.1038/nri1260. [DOI] [PubMed] [Google Scholar]
- 32.Silberhorn EM. Glauert HP. Robertson LW. Carcinogenicity of polyhalogenated biphenyls: PCBs and PBBs. Crit Rev Toxicol. 1990;20:440–496. doi: 10.3109/10408449009029331. [DOI] [PubMed] [Google Scholar]
- 33.Crain DA. Janssen SJ. Edwards TM, et al. Female reproductive disorders: The roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90:911–940. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markowitz LE. Sternberg M. Dunne EF. McQuillan G. Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003–2004. J Infect Dis. 2009;200:1059–1067. doi: 10.1086/604729. [DOI] [PubMed] [Google Scholar]
- 35.Ries LAG. Eisner MP. Kosary CL, et al. SEER Cancer Statistics Review, 1973–1999. Bethesda, MD: National Cancer Institute; 2002. [Google Scholar]
- 36.Winkelstein W., Jr Smoking and cervical cancer—Current status: A review. Am J Epidemiol. 1990;131:945–957. doi: 10.1093/oxfordjournals.aje.a115614. [DOI] [PubMed] [Google Scholar]
- 37.Moreno V. Bosch FX. Munoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: The IARC multicentric case-control study. Lancet. 2002;359:1085–1092. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]