Abstract
Ca2+ signaling in striated muscle cells is critically dependent upon thin filament proteins tropomyosin (Tm) and troponin (Tn) to regulate mechanical output. Using in vitro measurements of contractility, we demonstrate that even in the absence of actin and Tm, human cardiac Tn (cTn) enhances heavy meromyosin MgATPase activity by up to 2.5-fold in solution. In addition, cTn without Tm significantly increases, or superactivates sliding speed of filamentous actin (F-actin) in skeletal motility assays by at least 12%, depending upon [cTn]. cTn alone enhances skeletal heavy meromyosin's MgATPase in a concentration-dependent manner and with sub-micromolar affinity. cTn-mediated increases in myosin ATPase may be the cause of superactivation of maximum Ca2+-activated regulated thin filament sliding speed in motility assays relative to unregulated skeletal F-actin. To specifically relate this classical superactivation to cardiac muscle, we demonstrate the same response using motility assays where only cardiac proteins were used, where regulated cardiac thin filament sliding speeds with cardiac myosin are >50% faster than unregulated cardiac F-actin. We additionally demonstrate that the COOH-terminal mobile domain of cTnI is not required for this interaction or functional enhancement of myosin activity. Our results provide strong evidence that the interaction between cTn and myosin is responsible for enhancement of cross-bridge kinetics when myosin binds in the vicinity of Tn on thin filaments. These data imply a novel and functionally significant molecular interaction that may provide new insights into Ca2+ activation in cardiac muscle cells.
Introduction
In striated muscle cells, thin filament proteins troponin (Tn) and tropomyosin (Tm) modulate muscle function by conferring Ca2+ dependence of contractility (Gordon et al., 2000). Tm binds along actin filaments, and each Tm is associated with a complex of three troponin subunits: troponin I (TnI), troponin C (TnC), and troponin T (TnT). In the absence of Ca2+, Tm-Tn blocks strong binding of myosin to actin. When TnC binds Ca2+, Tn-Tm moves away from myosin binding sites on actin, relieving inhibition and allowing strong actomyosin interactions—and thus muscle contraction—to proceed with accompanying hydrolysis of MgATP by myosin's motor domain, subfragment 1 (S1).
In vitro assays are commonly used to study specific molecular interactions that occur within living cells by reconstituting key elements of the intracellular environment, then observing molecular-level effects of protein–protein interactions. Solution ATPase assays have been widely used to study not only the molecular mechanism of MgATP hydrolysis by myosin (Trentham et al., 1976; Homsher and Kean, 1978; Taylor, 1979; Eisenberg and Greene, 1980), but also to dissect Ca2+-dependent molecular interactions within the thin filament that affect myosin's MgATP activity (mATPase) (Trybus and Taylor, 1980; Chalovich et al., 1981; Gordon et al., 2000). Ca2+ regulation of actomyosin interactions can also be reconstituted for study at the level of single, regulated thin filaments using in vitro motility (IVM) assays. IVM assays can be performed with either cardiac or skeletal muscle proteins such that thin filaments stop at low [Ca2+] and exhibit graded sliding speed (Vf) over the range of physiological [Ca2+], with saturation of Vf at high [Ca2+] (Homsher et al., 1996; Gordon et al., 1997).
Ca2+ regulatory proteins Tm and Tn enhance actomyosin interactions at saturating Ca2+ levels as indicated by elevation of both mATPase in solution and Vf in IVM assays (Lehrer and Morris, 1982; Fraser and Marston, 1995; Homsher et al., 1996; Gordon et al., 1997; Lehrer and Geeves, 1998; Homsher et al., 2000; Schoffstall et al., 2006a), and also by increased maximum force of cardiac preparations (Homsher et al., 2000; Fujita et al., 2002). Interestingly, addition of Tn (without Tm) to IVM assays significantly increases Vf, but addition of Tm (without Tn) does not (Gordon et al., 1998; Homsher et al., 2000). The mechanism of regulatory protein enhancement of actomyosin function is unknown but could be due to altered structural dynamics of filamentous actin (F-actin) caused by Ca2+-Tn-Tm (Borovikov et al., 2009). While this explanation has intuitive appeal, high-resolution structures of F-actin have not yet provided adequate clarification for this question. If actomyosin interactions are enhanced by Tn in the absence of Tm, this explanation, however, falls short. We propose an alternative and novel hypothesis that Tn modulates actomyosin activity through a direct interaction of Tn with myosin. We present evidence that Tn alone is responsible for enhancement of mATPase and superactivation of thin filament sliding speed in solution assays; human cardiac Tn (cTn) enhances mATPase in a concentration-dependent manner and with sub-micromolar affinity, even in the absence of actin, Tm, and Ca2+.
In addition, we explore the possibility that cTnI's extreme COOH-terminus (the mobile domain, “MD”) (Murakami et al., 2005; Blumenschein et al., 2006; Pirani et al., 2006) could be the region of cTn that directly influences myosin function. During the cardiac cycle, MD binds actin at low [Ca2+] and is thought to maintain Tm in the closed position, thus preventing strong actomyosin interactions. A number of DNA mutations that lead to familial hypertrophic cardiomyopathy (FHC) are clustered in the MD (Gomes and Potter, 2004), and some of these mutations further enhance maximum Vf for thin filaments (Köhler et al., 2003). A direct interaction of MD with myosin could be significant to clinical pathology of FHC. To explore the possibility that MD alters myosin function, we utilized a recombinant cTn mutant, K164Δ, that is truncated after cTnI residue K164 and is thus missing the 46 COOH-terminal amino acids that comprise MD in human cTn. Our mATPase and IVM data indicate that MD is not the region on cTn responsible for functional enhancement of myosin activity.
In light of recent structural observations that cross-bridges can be found in the vicinity of Tn in actively contracting insect flight muscle (Wu et al., 2009), our observations suggest a previously unrecognized, secondary regulatory role for Tn beyond Ca2+-dependent structural changes in the thin filament.
Materials and Methods
Proteins
Recombinant human cTn and cTm (αα-Tm) were bacterially expressed and purified as published (Schoffstall et al., 2006a). K164Δ, a truncation mutant of human cTnI missing the COOH- terminal MD, was generated by introducing stop codons in place of the codon for cTnI residue K164 in our cTn co-expression construct. Rabbit skeletal muscle myosin, heavy meromyosin (HMM), and actin were prepared as described (Kron et al., 1991; Gordon et al., 1997; Chase et al., 2000; Schoffstall et al., 2005). All animal procedures involving rabbits were approved by Florida State University's Institutional Animal Care and Use Committee. Cardiac myosin was prepared from porcine hearts as described (Schoffstall et al., 2006b) and cardiac actin was extracted from acetone powder prepared from porcine hearts as described for rabbit skeletal actin (Pardee and Spudich, 1982). Fresh porcine hearts were obtained from a local butcher.
IVM assays
IVM assays were performed to measure the Vf of fluorescently labeled F-actin or thin filaments propelled by rabbit skeletal HMM or porcine cardiac myosin. IVM methods were essentially as described (Chase et al., 2000; Mihajlović et al., 2004; Schoffstall et al., 2006a). Rabbit skeletal or porcine cardiac F-actin was fluorescently labeled with rhodamine phalloidin (RhPh). Immediately before motility assays with regulatory proteins, cTn, cTm, or equimolar concentrations of both cTn and cTm were applied to labeled F-actin in the flow cell as described for thin filaments (Köhler et al., 2003; Liang et al., 2003; Schoffstall et al., 2006a); cTn, cTm, or cTn and cTm were also added to motility buffers. Solution composition for assays with thin filaments was calculated as described (Köhler et al., 2003; Liang et al., 2003; Schoffstall et al., 2006a) and consisted of 2 mM MgATP, 1 mM Mg2+, 10 mM ethylene glycol tetraacetic acid (EGTA), sufficient Ca(CH3COO)2 to achieve the desired pCa (pCa 9–4), 50 mM K+, 15 mM Na+, 20 mM 3-(N-morpholino) propanesulfonic acid (MOPS), pH 7.00 at 30°C, 0.3% methyl cellulose, and ionic strength was adjusted to 0.085 mM with TrisOH/CH3COOH). IVM assays were conducted at 30°C. To minimize photo-oxidative damage, motility buffers also contained 3 mg mL−1 glucose, 100 μg mL−1 glucose oxidase, 18 μg mL−1 catalase and 40 mM dithiothreitol. IVM assays and analysis for skeletal muscle actin/HMM were as described (Schoffstall et al., 2006a). Cardiac muscle actin/myosin IVM was observed by fluorescence microscopy on an Olympus BX60 microscope (Olympus, Center Valley, PA), imaging 5–6 fields from varying areas on each flow cell. Field images were recorded as 30 s clips using a Q-Imaging Rolexa-XR Fast 1394 camera (Q-Imaging, Surrey, BC Canada) with StreamPix Software (Norpix, Montreal, Quebec, Canada). Movie clips were processed into stacks consisting of 900–1000 frames using Virtual Dub 1.6.17 (Avery Lee, 2007). Stacks were imported into ImageJ 1.39s (National Institutes of Health, Bethesda, MD) to threshold .avi movies into binary images. Binary movies were analyzed using custom motility analysis software from the University of Washington.
HMM MgATPase assays
HMM mATPase assays were performed as previously described (White, 1982; Schoffstall et al., 2006a) to measure the rate of MgATP hydrolysis by HMM in solution at room temperature (22°C–24°C). Control and experimental samples were negative control (no proteins), HMM alone, and HMM plus cTn in variable molar ratios. Reaction buffer was 1 mM dithiothreitol, 5 mM MgCl2, 1 mM EGTA, 25 mM Tris (pH 7.0), and 50 mM KCl, with 0.2 μM HMM and varying concentrations of cTn. The reaction was started with addition of 1 mM ATP, and stopped at intervals from 30 s to 300 min with 13.3% sodium dodecyl sulfate in 0.12 M ethylenediaminetetraacetic acid pH 7.0. Indicator solution (0.5% ammonium molybdate [w/v], 0.5 M H2SO4, and 5 mg mL−1FeSO4) was added, incubated for 15 min at 37°C, and absorbance measured at 550 nm on a Beckman Coulter DU640 spectrophotometer (Beckman Coulter, Fullerton, CA). All reactions were performed at room temperature (20°C–24°C) leading up to the 37°C incubation, after which all solutions returned to room temperature before the absorbance reading. Absorbance was then converted to inorganic phosphate (Pi) concentration. Rate of MgATP hydrolysis per myosin S1 was assumed to be equal to the rate of Pi production, which was calculated as the slope of the linear regression of Pi versus time and dividing by twice the concentration of HMM.
Data analysis
Regression analyses were performed with Excel (versions 2000 and 2007; Microsoft, Redmond, WA) and SigmaPlot 2002 and 2009 for Windows (versions 8.0 and 9.01; SPSS, Chicago, IL). Estimates of apparent affinity were obtained by nonlinear regression analysis. Statistical significance was determined using Student's t-test.
Results
Effect of cTn on filament sliding speed in motility assays in the presence and absence of cTm and Ca2+
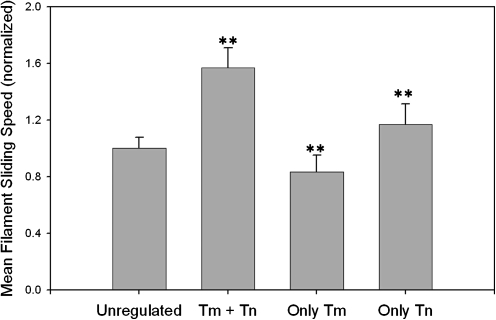
Previous IVM observations that cTn alone—without Tm—is primarily responsible for superactivation of maximum Vf of thin filaments were made using regulatory proteins purified from bovine heart (Homsher et al., 2000). To determine whether human cTn and cTm act similarly, we conducted IVM assays to compare Vf for F-actin with thin filaments at saturating Ca2+, and also F-actin in the presence of human cTm only or human cTn only (Fig. 1). In agreement with previous observations (Homsher et al., 1996, 2000; Gordon et al., 1997, 1998; Schoffstall et al., 2006a), we found that (i) maximum Ca2+-activated Vf in motility assays with thin filaments reconstituted with human cTn and cTm is substantially faster than Vf of unregulated F-actin; (ii) adding human cTm (no cTn) to F-actin does not increase Vf, and slightly inhibits the sliding speed; and conversely, and (iii) adding human cTn (no cTm) significantly increases Vf over that of unregulated F-actin (Fig. 1).
FIG. 1.
Cardiac Tn is primarily responsible for superactivation of maximum Ca2+-activated sliding speed of regulated thin filaments in motility assays. Rhodamine phalloidin-labeled filaments were F-actin (unregulated); thin filaments reconstituted with equimolar concentrations of human cTn + cTm at pCa 5 (Tm + Tn); addition of cTm, but not Tn (only Tm); or addition of cTn, but not Tm (only Tn). Speeds of at least 40 filaments per level were averaged, and then normalized to that of unregulated F-actin. Average unregulated filament sliding speed was 5.1 μm/s (±0.4). Statistical significance p < 0.01 is indicated by **. Error bars represent standard deviation. Note that addition of only cTn significantly superactivates sliding speed as observed with thin filaments reconstituted with both cTn + cTm at pCa 5, whereas addition of cTm alone significantly slows sliding speed. Tm, tropomyosin; Tn, troponin; cTn, cardiac Tn; F-actin, filamentous actin.
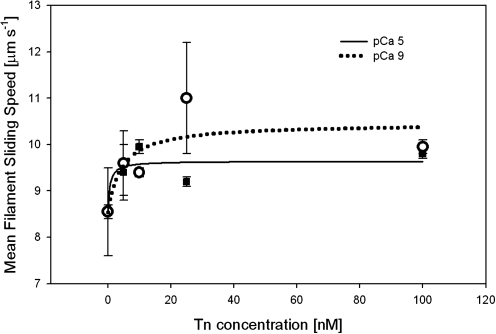
Because cTn binds Ca2+ during muscle contraction, it was important to determine whether [Ca2+] affects superactivation of Vf by cTn. Figure 2 illustrates that there was little or no effect of Ca2+ on cTn's superactivation of Vf over a range of cTn concentrations; in this experiment, we compared pCa 5 and pCa 9, that is, Ca2+ concentrations that would induce maximum Vf or stop filament sliding, respectively, for thin filaments reconstituted with Tn + Tm (Fig. 1). The [cTn] plotted (Fig. 2) are total concentrations. In IVM assays there is a vast excess of cTn over available binding sites on actin; free [cTn] is therefore approximated by [total cTn], and affinity should be similar to that obtained from regression analysis. Over the range of cTn concentrations tested, sliding speed generally increased with [cTn] with an apparent affinity of cTn for HMM estimated to be on the order of 1–10 nM (Fig. 2). Thus, we find that human cTn superactivates actomyosin interaction—in the absence of Tm—in a concentration-dependent, but not Ca2+-dependent, manner (Fig. 2).
FIG. 2.
[Ca2+] has little or no effect on cTn's superactivation of F-actin sliding speed. Varying concentrations of cTn (but no Tm) were added to rhodamine phalloidin-labeled F-actin in motility assays. Sliding speeds of filaments over skeletal HMM were measured at pCa5 (closed symbols and solid line) and pCa 9 (open symbols and dotted line). Error bars represent SEM. These data indicate that cTn affects actomyosin cycling with apparent affinity on the order of 1–10 nM. HMM, heavy meromyosin; SEM, standard error of the mean.
Effect of cTn on HMM MgATPase assays in the absence of actin-Tm
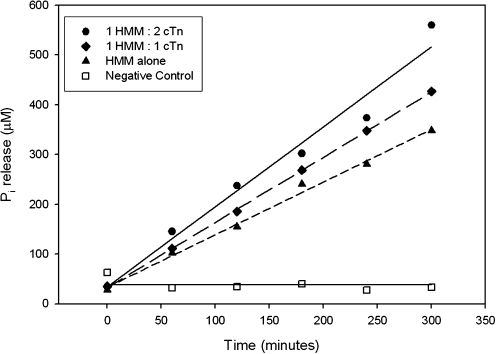
While it is possible that cTn alters the structure of actin, which in turn upregulates actomyosin interaction (Homsher et al., 2000), an alternative and simpler hypothesis is that cTn interacts directly with myosin to enhance mATPase. To test this latter idea, we designed an actin-free experiment to compare the rate of MgATP hydrolysis by skeletal HMM alone versus HMM and cTn in 1:1 or 1:2 molar ratios (HMM:cTn). In three replicate experiments we found that cTn consistently increased mATPase over that of HMM alone (one representative assay is presented in Fig. 3), and that the 1:2 ratio mATPaserate was faster than the 1:1 ratio mATPase rate. The mean rates of the three replicate experiments were HMM alone, 0.04 ± 0.002 Pi s−1 S1−1; 1 HMM:1cTn, 0.05 ± 0.005 Pi s−1 S1−1; and 1 HMM:2 cTn, 0.06 ± 0.006 Pi s−1 S1−1. Interestingly, these data suggest that cTn and HMM interact in solution—in the absence of actin and Tm—in a manner that enhances myosin's MgATP hydrolysis activity.
FIG. 3.
cTn enhances HMM MgATP hydrolysis rate in the absence of actin-Tm. Time course of Pi appearance was determined as described in Materials and Methods using skeletal HMM alone (solid triangles, short dashed line), HMM and total cTn in a 1:1 molar ratio (solid diamonds, long dashed line), and HMM and total cTn in a 1:2 molar ratio (solid circles, bold solid line at top); little or no ATP was hydrolyzed in the absence of HMM (negative control; open squares, thin horizontal line at bottom). Results are from one representative assay. These results suggest cTn interacts with HMM in solution to enhance MgATPase.
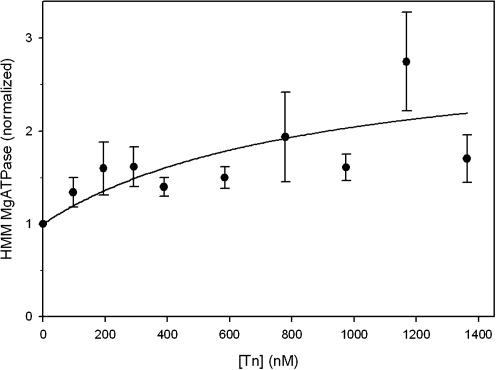
We then extended our observations over a wider range of HMM:cTn ratios (Fig. 4). To control for effects of any nonspecific protein binding, replicate MgATPases were also run adding bovine serum albumin instead of cTn (data not shown), with no effect on rates. In the MgATPase assay, total [cTn] is reported although a significant proportion of cTn may be bound to HMM; thus, estimates of apparent affinity constants obtained from regression analyses are upper limits, and actual binding affinities may be stronger. The data in Figure 4 are, therefore, consistent with saturable binding of cTn to HMM withan upper limit of the apparent affinity estimated to be on the order of 100 nM, and support our hypothesis that cTn can modulate actomyosin activity through a direct interaction with myosin. We have considered that our data (Figs. 2 and 4) might suggest more than one binding event between cTn and myosin; however, we have not yet expanded our experimental study to investigate this possibility. This finding could have significant implications toward a more complete understanding of Ca2+ activation of striated muscle, especially in cardiac muscle.
FIG. 4.
HMM MgATP hydrolysis activity is enhanced by cTn in a concentration-dependent manner. HMM MgATPase activity in the presence of varying concentrations of total cTn (no actin-Tm) was normalized to that obtained in the absence of cTn (0.011 + 0.002 Pi/s/S1; N = 15). [HMM] was 200 nM. Error bars represent SEM. The data are consistent with saturable binding of cTn to HMM such that cTn affects myosin cycling with a maximum apparent affinity on the order of 100 nM. S1, subfragment 1 of myosin.
Lack of influence of cTnI mobile domain on enhancement of HMM MgATPase in the absence of actin-Tm
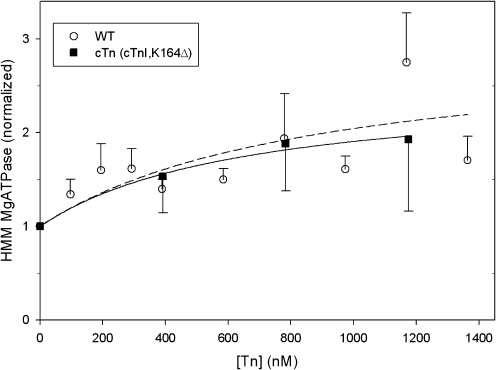
To determine whether the COOH-terminus MD of cTnI could be the portion of cTn that interacts with myosin to effect superactivation, we utilized a modified human cTn containing a truncation mutant of cTnI, K164Δ. Using our actin and Tm-free MgATPase protocols (above), we compared skeletal HMM mATPase in the presence of wild type (WT) cTn to that in the presence of cTn (cTnI K164Δ) (Fig. 5). We found no significant difference in the enhancement of mATPase by these two cTn constructs, suggesting that cTnI's MD is not involved in cTn's enhancement of mATPase.
FIG. 5.
The COOH-terminal mobile domain of cTnI is not required for cTn's enhancement of HMM MgATPase activity. HMM MgATPase activity in the absence of actin-Tm was measured using varying concentrations of cTn (TnI, K164Δ) (closed squares, solid line); data for WT cTn (open circles, dotted curve) were replotted from Figure 4 for comparison. As in Figure 4, HMM MgATPase activity in the presence of cTn constructs (no actin-Tm) was normalized to that obtained with HMM alone (0.009 ± 0.007 Pi/s/S1; N = 5). Error bars represent SEM. There was no significant difference between WT and cTn (TnI, K164Δ) in enhancement of MgATPase activity.
Lack of influence of cTnI mobile domain on superactivation of regulated cardiac thin filament maximum sliding speed
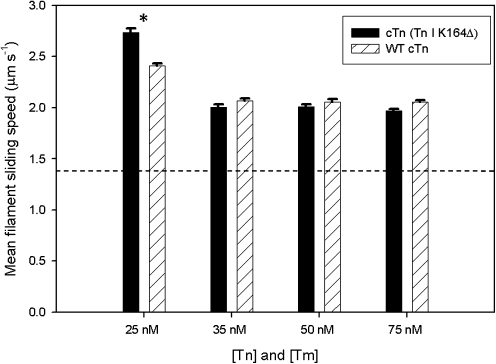
To further investigate the correlation between mATPase and motility assay results, we focused specifically on Ca2+ activation of cardiac muscle contraction by using an all-cardiac muscle protein model in the IVM assay (Materials and Methods); this assay utilized recombinant human cTn and cTm with porcine cardiac actin and myosin. Superactivation of the maximum sliding speed of regulated thin filaments was consistent with that previously published using cardiac and skeletal muscle proteins from various sources and in various combinations (Introduction). At pCa 5, we confirmed that cardiac thin filaments regulated with WT cTn have a significantly faster Vf than unregulated cardiac F- actin (Fig. 6); cardiac thin filaments slide ∼40%–50% faster than unregulated F-actin (Fig. 6), comparable to the effect of cTn on skeletal HMM (Fig. 1). Vf of cardiac thin filaments reconstituted with cTn (cTnI K164Δ) was similarly increased over that of cardiac F-actin (Fig. 6). At most concentrations of regulatory proteins tested, cTn (TnI K164Δ) superactivated Vf of thin filaments to the same extent as WT cTn. The only [Tn-Tm] that exhibited any significant difference was at 25 nM, where maximum Vf of cardiac thin filaments reconstituted with cTn (TnI K164Δ) was faster than those reconstituted with WT cTn. These data confirm that the COOH-terminus of cTnI is not the site of interaction with myosin that leads to cTn's superactivation of myosin activity, and further supports the possibility that cTn enhancement of mATPase is at least partly responsible for superactivation of maximum Vf by cTn or Ca2+-cTn-cTm in motility assays.
FIG. 6.
The COOH-terminal mobile domain of cTnI is not required for cTn's superactivation of maximum Ca2+-activated cardiac thin filament sliding speed. Thin filaments were reconstituted with varying equimolar concentrations of cTm and either WT cTn (striped bar) or cTn (TnI K164Δ) (solid bar). Error bars represent SEM. *represents a statistically significant difference (P < 0.05) between mutant and wild type. Comparison of mean filament sliding speeds shows that both cTn (TnI K164Δ) and WT cTn, at all concentrations tested, superactivate actomyosin cycling significantly and similarly over that with unregulated (dashed line) F-actin.
Superactivation of myosin activity by regulated thin filaments is similar, whether using skeletal HMM (Figs. 1 and 2), or cardiac myosin (Fig. 6) in the assay. We have previously established (Schoffstall et al., 2006a) that the Ca2+ sensitivity of regulated cardiac thin filament sliding does not depend on myosin isoform. Although absolute sliding speeds are quite different between the isoforms, we have previously validated the use of either myosin isoform in in vitro assays to study the effects of cardiac thin filament proteins on sliding speed.
Discussion and Conclusions
While many of the molecular interactions responsible for regulation of the contractile cycle in cardiac muscle cells have been described (Gordon et al., 2000), there is a growing body of evidence that additional proteins and previously unrecognized molecular interactions are also important (Clark et al., 2002; Pyle and Solaro, 2004). We have determined that, even in the absence of Tm, actin, and Ca2+, human cTn is able to directly interact with myosin in such a way that it enhances mATPase in solution assays (Figs. 3 and 4). We estimate that cTn enhances mATPase by binding to myosin with a maximum apparent affinity on the order of 100 nM (Fig. 4). Furthermore, we have established that thin filaments exhibit significantly faster sliding speed in the IVM assay than unregulated F-actin using both skeletal and cardiac muscle protein models (Figs. 1 and 6). We have shown that this superactivation is not associated with the presence of Tm on thin filaments, but rather is primarily due to Tn (Figs. 1 and 2). The observed superactivation of thin filament sliding speed in motility assays (Figs. 1 and 6) (Fraser and Marston, 1995; Homsher et al., 1996, 2000; Gordon et al., 1997; Schoffstall et al., 2006a)—and possibly enhanced force generation in the cell's intact sarcomere (Fujita et al., 2002)—may thus be a result of enhanced mATPase due to a previously unrecognized interaction between Tn and myosin. The data we present is derived from in vitro solution assays; the stoichiometry of muscle protein binding events in these assays is different from that in an intact sarcomere, where Tn binds along the thin filament every seven actin monomers. Ongoing work will extend our in vitro solution assay findings to investigations of these effects in permeabilized muscle fibers and/or myocyte cultures, where sarcomere structure is intact.
Our observation that cTn modulates myosin activity via a direct molecular interaction is unique and novel, and is perhaps surprising for these well-studied proteins. We note, however, that protein–protein interactions just within the thin filament are not yet fully resolved. Early reports indicated that in the absence of Tm, high concentrations of Tn actually inhibit actin-activated myosin ATPase activity (Potter and Gergely, 1974). Our MgATPase experiments, however, were performed in the absence of both actin and Tm, explicitly to demonstrate that an interaction between Tn and myosin—the only proteins in the assay—is responsible for enhancement of myosin ATPase activity, which is dependent upon [cTn] (Fig. 4). Recognizing the importance of cTnI's COOH-terminal MD in muscle activation (Murakami et al., 2005; Blumenschein et al., 2006; Pirani et al., 2006), we asked whether this domain is the site of interaction with myosin that is responsible for enhancement of myosin activity. cTn containing cTnI MD truncation mutant cTn (TnI K164Δ) enhanced HMM MgATPase activity to the same extent as WT (Fig. 5) and superactivated maximum Ca2+-activated Vf of reconstituted thin filaments in IVM assays using all cardiac muscle proteins (Fig. 6). We have thus determined that the COOH-terminal MD of cTnI is not the myosin interaction site. Furthermore, it seems unlikely that the cardiac-specific NH2-terminus of cTnI is the responsible structure in cTn, considering that reconstitution of thin filaments with either cardiac or skeletal muscle Tn-Tm leads to superactivation of maximal Ca2+-activated Vf. These results are consistent with investigations of sTnI and cTnI deletion mutants on Ca2+- and actin-activated S1 or myofibrillar MgATPase in the presence of Tm (Rarick et al., 1997; Van Eyk et al., 1997). Ongoing work will focus on determining what other portion(s) of TnI, TnC, or TnT could be responsible for functional enhancement of myosin activity (Potter et al., 1995; Bing et al., 1997; Szczesna et al., 2000).
Although much is currently understood about the contractile cycle within the intact muscle cell sarcomere, there is not yet a complete explanation of how conformational changes in Tn brought about by Ca2+ binding to cTnC are coupled with actomyosin binding and cross-bridge cycling. Time-resolved, X-ray diffraction studies indicate that structural changes in thick filaments occur before force development, and include initial movement of S1 away from thick filaments toward myosin binding sites on thin filaments (Huxley and Faruqi, 1983; Bagni et al., 1994). During steady, Ca2+-activated contraction, structural studies on insect flight muscle suggest that some cross-bridges form in the vicinity of Tn (Wu et al., 2009). A Tn-S1 interaction in working muscle could provide an explanation for these structural observations. The broader implication is that a direct molecular interaction between Tn and myosin could be a key component of the thin filament's multiple roles in Ca2+ activation of muscle contraction, especially in cardiac muscle cells.
Proteins such as titin and myosin-binding protein C are believed to interact with both thick and thin filaments, and modulate striated muscle function in health and disease (Clark et al., 2002; Pyle and Solaro, 2004). Thick filament protein myosin-binding protein C affects not only structure and function of the thick filament, but the thin filament as well (Korte et al., 2003; Stelzer et al., 2006, 2007). This implies that thick and thin filaments must have direct links other than just actomyosin cross-bridges. A consequence of Tn-myosin interactions as suggested here would be that changes in the thin filament would result in changes in the thick filament (and vice versa). Such interactions could be at least partly responsible for cross-bridge dependent cooperative activation of thin filaments (Gordon et al., 2000; Liang et al., 2003). Many FHC mutations have been identified in Tn as well as the thick filament proteins. The Tn-myosin interaction may be relevant to some of the hyper-Ca2+-sensitivity and changes in cross-bridge kinetics seen in cTn and/or myosin mutations implicated as causative agents of FHC (Köhler et al., 2003; Gomes and Potter, 2004). It is possible that the interaction site (for either cTn or myosin) is essential for normal cardiac muscle regulation, so mutations at or near these sites could ultimately be relevant to development of cardiac hypertrophy.
Acknowledgments
We thank Dr. Michael Regnier, University of Washington, for permission to use the HAMM Lab's custom-designed IVM automated analysis program, and Drs. Albert M. Gordon, University of Washington, and James D. Potter, University of Miami, for critical comments on the article. This work was supported by NIH grants HL63972 and GM079592 (P.B.C.) and MBRS-RISE 2R25 GM059244-09 (Barry University, supporting V.I. and D.M.). N.M.B. was supported by American Heart Association fellowship 0315097B. A.K.T. was supported by American Heart Association fellowship 0615164B. N.L.M. was supported by an FSU MARTECH fellowship. V.A.L. was supported by an FSU Mentored Research and Creative Endeavors Award. L.H. and S.H. were supported by the FSU Young Scholars Program.
Disclosure Statement
No competing financial interests exist.
References
- Bagni M.A. Cecchi G., et al. Development of stiffness precedes cross-bridge attachment during the early tension rise in single frog muscle fibres. J Physiol. 1994;481(Pt 2):273–278. doi: 10.1113/jphysiol.1994.sp020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing W. Fraser I.D., et al. Troponin I and troponin T interact with troponin C to produce different Ca2+-dependent effects on actin-tropomyosin filament motility. Biochem J. 1997;327(Pt 2):335–340. doi: 10.1042/bj3270335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenschein T.M. Stone D.B., et al. Dynamics of the C-terminal region of TnI in the troponin complex in solution. Biophys J. 2006;90:2436–2444. doi: 10.1529/biophysj.105.076216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikov Y.S. Karpicheva O.E., et al. Modulation of the effects of tropomyosin on actin and myosin conformational changes by troponin and Ca2+ Biochim Biophys Acta. 2009;1794:985–994. doi: 10.1016/j.bbapap.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Chalovich J.M. Chock P.B., et al. Mechanism of action of troponin. tropomyosin. Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin. J Biol Chem. 1981;256:575–578. [PMC free article] [PubMed] [Google Scholar]
- Chase P.B. Chen Y., et al. Viscosity and solute dependence of F-actin translocation by rabbit skeletal heavy meromyosin. Am J Physiol Cell Physiol. 2000;278:C1088–C1098. doi: 10.1152/ajpcell.2000.278.6.C1088. [DOI] [PubMed] [Google Scholar]
- Clark K.A. McElhinny A.S., et al. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol. 2002;18:637–706. doi: 10.1146/annurev.cellbio.18.012502.105840. [DOI] [PubMed] [Google Scholar]
- Eisenberg E. Greene L.E. The relation of muscle biochemistry to muscle physiology. Annu Rev Physio. 1980;42:293–309. doi: 10.1146/annurev.ph.42.030180.001453. [DOI] [PubMed] [Google Scholar]
- Fraser I.D. Marston S.B. In vitro motility analysis of actin-tropomyosin regulation by troponin and calcium. The thin filament is switched as a single cooperative unit. J Biol Chem. 1995;270:7836–7841. doi: 10.1074/jbc.270.14.7836. [DOI] [PubMed] [Google Scholar]
- Fujita H. Sasaki D., et al. Elementary steps of the cross-bridge cycle in bovine myocardium with and without regulatory proteins. Biophys J. 2002;82:915–928. doi: 10.1016/S0006-3495(02)75453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A.V. Potter J.D. Cellular and molecular aspects of familial hypertrophic cardiomyopathy caused by mutations in the cardiac troponin I gene. Mol Cell Biochem. 2004;263:99–114. doi: 10.1023/B:MCBI.0000041852.42291.aa. [DOI] [PubMed] [Google Scholar]
- Gordon A.M. Chen Y., et al. Skeletal muscle regulatory proteins enhance F-actin in vitro motility. Adv Exp Med Biol. 1998;453:187–196. doi: 10.1007/978-1-4684-6039-1_22. discussion 196–197. [DOI] [PubMed] [Google Scholar]
- Gordon A.M. Homsher E., et al. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Gordon A.M. LaMadrid M.A., et al. Calcium regulation of skeletal muscle thin filament motility in vitro. Biophys J. 1997;72:1295–1307. doi: 10.1016/S0006-3495(97)78776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E. Kean C.J. Skeletal muscle energetics and metabolism. Annu Rev Physiol. 1978;40:93–131. doi: 10.1146/annurev.ph.40.030178.000521. [DOI] [PubMed] [Google Scholar]
- Homsher E. Kim B., et al. Calcium regulation of thin filament movement in an in vitro motility assay. Biophys J. 1996;70:1881–1892. doi: 10.1016/S0006-3495(96)79753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E. Lee D.M., et al. Regulation of force and unloaded sliding speed in single thin filaments: effects of regulatory proteins and calcium. J Physiol. 2000;524(Pt 1):233–243. doi: 10.1111/j.1469-7793.2000.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H.E. Faruqi A.R. Time-resolved X-ray diffraction studies on vertebrate striated muscle. Annu Rev Biophys Bioeng. 1983;12:381–417. doi: 10.1146/annurev.bb.12.060183.002121. [DOI] [PubMed] [Google Scholar]
- Köhler J. Chen Y., et al. Familial hypertrophic cardiomyopathy mutations in troponin I (K183D, G203S, K206Q) enhance filament sliding. Physiol Genomics. 2003;14:117–128. doi: 10.1152/physiolgenomics.00101.2002. [DOI] [PubMed] [Google Scholar]
- Korte F.S. McDonald K.S., et al. Loaded shortening, power output, and rate of force redevelopment are increased with knockout of cardiac myosin binding protein-C. Circ Res. 2003;93:752–758. doi: 10.1161/01.RES.0000096363.85588.9A. [DOI] [PubMed] [Google Scholar]
- Kron S.J. Toyoshima Y.Y., et al. Assays for actin sliding movement over myosin-coated surfaces. Methods Enzymol. 1991;196:399–416. doi: 10.1016/0076-6879(91)96035-p. [DOI] [PubMed] [Google Scholar]
- Lehrer S.S. Geeves M.A. The muscle thin filament as a classical cooperative/allosteric regulatory system. J Mol Biol. 1998;277:1081–1089. doi: 10.1006/jmbi.1998.1654. [DOI] [PubMed] [Google Scholar]
- Lehrer S.S. Morris E.P. Dual effects of tropomyosin and troponin-tropomyosin on actomyosin subfragment 1 ATPase. J Biol Chem. 1982;257:8073–8080. [PubMed] [Google Scholar]
- Liang B. Chen Y., et al. Ca2+ regulation of rabbit skeletal muscle thin filament sliding: role of cross-bridge number. Biophys J. 2003;85:1775–1786. doi: 10.1016/S0006-3495(03)74607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihajlović G. Brunet N.M., et al. All-electrical switching and control mechanism for actomyosin-powered nanoactuators. Appl Phys Lett. 2004;85:1060–1062. [Google Scholar]
- Murakami K. Yumoto F., et al. Structural basis for Ca2+-regulated muscle relaxation at interaction sites of troponin with actin and tropomyosin. J Mol Biol. 2005;352:178–201. doi: 10.1016/j.jmb.2005.06.067. [DOI] [PubMed] [Google Scholar]
- Pardee J.D. Spudich J.A. Purification of muscle actin. Methods Enzymol. 1982;85(Pt B):164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Pirani A. Vinogradova M.V., et al. An atomic model of the thin filament in the relaxed and Ca2+-activated states. J Mol Biol. 2006;357:707–717. doi: 10.1016/j.jmb.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Potter J.D. Gergely J. Troponin, tropomyosin, and actin interactions in the Ca2+ regulation of muscle contraction. Biochemistry. 1974;13:2697–2703. doi: 10.1021/bi00710a007. [DOI] [PubMed] [Google Scholar]
- Potter J.D. Sheng Z., et al. A direct regulatory role for troponin T and a dual role for troponin C in the Ca2+ regulation of muscle contraction. J Biol Chem. 1995;270:2557–2562. doi: 10.1074/jbc.270.6.2557. [DOI] [PubMed] [Google Scholar]
- Pyle W.G. Solaro R.J. At the crossroads of myocardial signaling: the role of Z-discs in intracellular signaling and cardiac function. Circ Res. 2004;94:296–305. doi: 10.1161/01.RES.0000116143.74830.A9. [DOI] [PubMed] [Google Scholar]
- Rarick H.M. Tu X.H., et al. The C terminus of cardiac troponin I is essential for full inhibitory activity and Ca2+ sensitivity of rat myofibrils. J Biol Chem. 1997;272:26887–26892. doi: 10.1074/jbc.272.43.26887. [DOI] [PubMed] [Google Scholar]
- Schoffstall B. Brunet N.M., et al. Ca2+ sensitivity of regulated cardiac thin filament sliding does not depend on myosin isoform. J Physiol. 2006a;577(Pt 3):935–944. doi: 10.1113/jphysiol.2006.120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffstall B. Clark A., et al. Positive inotropic effects of low dATP/ATP ratios on mechanics and kinetics of porcine cardiac muscle. Biophys J. 2006b;91:2216–2226. doi: 10.1529/biophysj.105.079061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffstall B. Kataoka A., et al. Effects of rapamycin on cardiac and skeletal muscle contraction and crossbridge cycling. J Pharmacol Exp Ther. 2005;312:12–18. doi: 10.1124/jpet.104.073445. [DOI] [PubMed] [Google Scholar]
- Stelzer J.E. Larsson L., et al. Activation dependence of stretch activation in mouse skinned myocardium: implications for ventricular function. J Gen Physiol. 2006;127:95–107. doi: 10.1085/jgp.200509432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer J.E. Patel J.R., et al. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ Res. 2007;101:503–511. doi: 10.1161/CIRCRESAHA.107.153650. [DOI] [PubMed] [Google Scholar]
- Szczesna D. Zhang R., et al. Altered regulation of cardiac muscle contraction by troponin T mutations that cause familial hypertrophic cardiomyopathy. J Biol Chem. 2000;275:624–630. doi: 10.1074/jbc.275.1.624. [DOI] [PubMed] [Google Scholar]
- Taylor E.W. Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem. 1979;6:103–164. doi: 10.3109/10409237909102562. [DOI] [PubMed] [Google Scholar]
- Trentham D.R. Eccleston J.F., et al. Kinetic analysis of ATPase mechanisms. Q Rev Biophys. 1976;9:217–281. doi: 10.1017/s0033583500002419. [DOI] [PubMed] [Google Scholar]
- Trybus K.M. Taylor E.W. Kinetic studies of the cooperative binding of subfragment 1 to regulated actin. Proc Natl Acad Sci U S A. 1980;77:7209–7213. doi: 10.1073/pnas.77.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eyk J.E. Thomas L.T., et al. Distinct regions of troponin I regulate Ca2+-dependent activation and Ca2+ sensitivity of the acto-S1-TM ATPase activity of the thin filament. J Biol Chem. 1997;272:10529–10537. doi: 10.1074/jbc.272.16.10529. [DOI] [PubMed] [Google Scholar]
- White H.D. Special instrumentation and techniques for kinetic studies of contractile systems. Methods Enzymol. 1982;85(Pt B):698–708. doi: 10.1016/0076-6879(82)85057-x. [DOI] [PubMed] [Google Scholar]
- Wu S. Liu J., et al. Methods for identifying and averaging variable molecular conformations in tomograms of actively contracting insect flight muscle. J Struct Biol. 2009;168:485–502. doi: 10.1016/j.jsb.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]