Abstract
Thanks to the coordinated efforts of four major scientific organizations, this report describes the “novel cellular therapy” activity in Europe for the year 2009. Fifty teams from 22 countries reported data on 814 patients using a dedicated survey, which were combined to additional 328 records reported by 55 teams to the standard European Blood and Marrow Transplantation (EBMT) database. Indications were cardiovascular (37%; 64% autologous), graft-vs.-host disease (27%; 7% autologous), musculoskeletal (17%; 98% autologous), epithelial/parenchymal (8%; 73% autologous), autoimmune (9%; 84% autologous), or neurological diseases (3%; 50% autologous). Autologous cells were used predominantly for cardiovascular (42%) and musculoskeletal (30%) disorders, whereas allogeneic cells were used mainly for graft-vs.-host disease (58%) and cardiovascular (30%) indications. Reported cell types were mesenchymal stem/stromal cells (MSC) (46%), hematopoietic stem cells (27%), chondrocytes (7%), keratinocytes (5%), dermal fibroblast (13%), and others (2%). In 59% of the grafts, cells were delivered after expansion; in 2% of the cases, cells were transduced. Cells were delivered intraorgan (46%), on a membrane or gel (29%), intravenously (16%) or using 3D scaffolds (8%). As compared to last year, the number of teams adopting the dedicated survey was 1.7-fold higher, and, with few exceptions, the collected data confirmed the captured trends. This year's edition specifically describes and discusses the use of MSC for the treatment of autoimmune diseases, due to the scientific, clinical, and economical implications of this topic.
Introduction
In 2008, the European sections of the Tissue Engineering and Regenerative Medicine International Society (TERMIS-EU), of the International Society of Cellular Therapy (ISCT-Europe), and of the International Cartilage Repair Society (ICRS) have for the first time coordinated a joint initiative with the European Group for Blood and Marrow Transplantation (EBMT) and the European League Against Rheumatism (EULAR) to establish a comprehensive, quantitative map of patients being treated in Europe by the so-called “novel cellular therapies,” namely, the use of nonhematopoietic stem cells (HSC) or of HSC for nonhematological indications. The first activity report, published last year,1 was an extension of the well-established EBMT annual report, an instrument to observe trends and to monitor changes in the use of HSC transplants for the treatment of hematologic disorders in Europe.2,3 The activity survey does not provide any data on specific protocols, outcome, age, or sex of patients or their pre- and post-transplant therapy. The goal of the data collection is the rapid dissemination of the status in the field of novel cellular therapies, identifying trends related to covered indications, as well as specific cell types, cell processes, and delivery modes used. Long-term analyses of the EBMT survey provided evidence that the instrument can foresee trends with high predictability and very rapidly (e.g., the increasing use of cord blood as a stem cell source, the change from bone marrow to peripheral blood or the utilization and integration of unrelated donor transplants4,5) and can thus provide a formal basis for patient counseling and healthcare planning.
In this article, we report the results of the second survey edition for the activity in 2009, with a direct comparison to the 2008 data reported last year and specifically discussing the use of mesenchymal stem/stromal cells (MSC) for the treatment of autoimmune diseases.
Patients and Methods
Data collection and validation
Participating teams were requested to report their data for 2009 by indication, cell type and source, donor type, processing method, and delivery mode. The survey followed the traditional principles of the EBMT, concentrating on numbers of patients with a first cellular therapy. For EBMT teams not using the full questionnaire, information on cellular therapies was limited to numbers of HSC for nonhematopoietic use, MSC-based therapies (later identified to be almost exclusively related to treatment of graft-vs.-host-disease), and donor type. Questionnaires were collected by paper forms or electronically. Quality control measures, for EBMT members only, included several established independent systems: confirmation of validity of the entered data by the reporting team, selective comparison of the survey data with MED-A data sets in the EBMT ProMISE data system, cross-checking with the National Registries and onsite visits of selected teams. No quality control system could be applied for the non-EBMT reporting teams yet.
Teams
Members of the four participating societies from 47 countries (39 European and 8 affiliated countries) were contacted for the 2009 report (EBMT survey). The non-European countries affiliated with the EBMT were Algeria, Iran, Israel, Jordan, Lebanon, Saudi Arabia, South Africa, and Tunisia. Eighty teams in 22 countries (20 European and 2 affiliated countries) reported novel cellular therapies using the survey form, with detailed information on indication, cell source and type, donor type, processing, and delivery mode. Thirty of the 80 teams reported that no cellular therapies had been performed in 2009. An additional 84 teams from 16 countries (14 European and 2 affiliated country) reported using the standard EBMT transplant activity survey, allowing to include only limited information. Of these 84 teams, 29 reported that no cellular therapies were performed in 2009. Teams that responded with activity are listed in the Appendix in alphabetical order by country, city, and EBMT centre code (if applicable), along with the total numbers of reported cellular therapies. There were no cellular therapies (including HSC transplants) reported to the survey from Albania, Algeria, Andorra, Armenia, Azerbaijan, Bosnia-Herzegovina, Bulgaria, Croatia, Cyprus, Czech Republic, Estonia, Georgia, Iceland, Ireland, Jordan, Latvia, Liechtenstein, Lithuania, Luxembourg, Macedonia, Malta, Moldavia, Monaco, Montenegro, Romania, San Marino, Saudi Arabia, South Africa, Tunisia, and the Ukraine in 2009.
Transplant rates
Transplant rates, defined as numbers of cellular therapies per 10 million inhabitants, were computed for each country, without adjustments for patients who crossed borders or received treatment in a foreign country. Population numbers were obtained from the U.S. census office database (www.census.gov).
Results
Number of novel cellular therapies and disease indications
According to the received reports, a total of 1142 patients were treated with novel cellular therapies, 496 (43%) with allogeneic and 646 (57%) with autologous cells (Table 1). Main indications were cardiovascular disorders (37%; 64% autologous), graft-vs.-host disease (27%; 7% autologous), musculoskeletal disorders (17%; 98% autologous), autoimmune diseases (9%; 84% autologous), and epithelial (8%; 73% autologous) and neurological disorders (3%; 50% autologous).
Table 1.
Number of Cellular Therapy Transplants for Novel Cellular Therapies (i.e., for Nonhematopoietic Indications or Using Nonhematopoietic Cells) in Europe 2009 Sorted by Indication, Cell Source, and Donor Type
| |
Cell type and source |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Autologous |
Allogeneic |
|
|
|
|||||||
| Indications | HSC | MSC | Chondrocyte | Keratinocyte | Other | HSC | MSC | Fibroblast | Other | Autologous | Allogeneic | Total |
| Cardiovascular | ||||||||||||
| Peripheral artery disease | 36 | 20 | 56 | 0 | 56 | |||||||
| Cardiomyopathy | 11 | 1 | 12 | 0 | 12 | |||||||
| Heart failure | 21 | 12 | 33 | 0 | 33 | |||||||
| Myocardial ischemia | 109 | 2 | 111 | 0 | 111 | |||||||
| Bypass graft | 14 | 2 | 16 | 0 | 16 | |||||||
| Ulcer | 100 | 0 | 100 | 100 | ||||||||
| Other/unspecified | 38a | 50 | 38 | 50 | 88 | |||||||
| Musculoskeletal | 0 | 0 | 0 | |||||||||
| Bone repair (maxillofacial) | 1 | 1 | 1 | 1 | 2 | |||||||
| Bone repair (orthopedics) | 23 | 43 | 2 | 66 | 2 | 68 | ||||||
| Osteogenesis imperfecta | 1 | 1 | 0 | 1 | ||||||||
| Cartilage repair | 9 | 82 | 91 | 0 | 91 | |||||||
| Muscle repair | 0 | 0 | 0 | |||||||||
| Tendon/ligament | 5 | 5 | 0 | 5 | ||||||||
| Reconstructive surgery | 1 | 1 | 0 | 1 | ||||||||
| Other/unspecified | 7a | 17 | 24 | 0 | 24 | |||||||
| Neurological | 0 | 0 | 0 | |||||||||
| Parkinson's | 0 | 0 | 0 | |||||||||
| Peripheral nerve regeneration (trauma) | 0 | 0 | 0 | |||||||||
| Other/unspecified | 9a | 8 | 14a | 3 | 17 | 17 | 34 | |||||
| Epithelial | 0 | 0 | 0 | |||||||||
| Skin reconstruction | 51 | 51 | 0 | 51 | ||||||||
| Cornea repair | 3 | 24 | 3 | 24 | 27 | |||||||
| Organ failure | 10 | 10 | 0 | 10 | ||||||||
| Diabetes | 0 | 0 | 0 | |||||||||
| Liver insufficiency | 2 | 2 | 0 | 2 | ||||||||
| Other | 0 | 0 | 0 | |||||||||
| Autoimmune | 0 | 0 | 0 | |||||||||
| Neurological | 80 | 80 | 0 | 80 | ||||||||
| Rheumatological | 1 | 1 | 1 | 1 | 2 | |||||||
| Gastrointestinal | 3 | 2 | 3 | 2 | 5 | |||||||
| Other | 3 | 13 | 3 | 13 | 16 | |||||||
| Graft vs. host disease | 21a | 286a | 21 | 286 | 307 | |||||||
| Total | 288 | 204 | 82 | 51 | 11 | 16 | 306 | 150 | 24 | 646 | 496 | 1142 |
These numbers were imported from the limited questionnaire included in the standard EBMT survey sheet.
HSC, hematopoietic stem cells; MSC, mesenchymal stromal/stem cells; EBMT European group for Blood and Marrow Transplantation.
From 814 patients, more detailed information was obtained concerning indications. Among the cardiovascular disorders, decubitus and leg ulcers (n=150), myocardial ischemia (n=111), peripheral artery disease (n=56), and heart failure (n=33) were the most frequently reported indications. Among the musculoskeletal disorders, cartilage repair (n=91) and bone repair (n=68) were the main reason for a cellular therapy. Skin reconstruction (n=51) and cornea repair (n=27) were the two main reported indications for epithelial/parenchymal disorders. Neurological indications were only performed for disorders not included in the form (n=16, 8 of which for glioblastoma). Among autoimmune disorders, neurological diseases (n=70), including multiple sclerosis, represented the predominant indication. The number of reports of patients treated for graft versus host disease (n=19) needs to be combined with that reported in the EBMT standard form, for a total of 307 cases (Table 1).
Cell type, source, and donor type
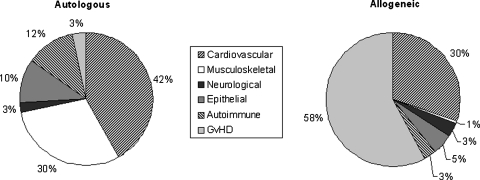
Of the 304 HSC treatments, 95% were autologous transplants and 80% were used to treat cardiovascular diseases (Table 1). All 82 chondrocyte and 51 keratinocytes transplants were autologous and the 150 dermal (100 of which of fetal origin) fibroblasts transplants were allogeneic. Of the 520 MSC based therapies, 59% were allogeneic. The donor type was associated with the disease indication: autologous cells were used predominantly for cardiovascular (42%) and musculoskeletal (30%) disorders, whereas allogeneic cells were used mainly for graft-vs.-host disease (58%) and cardiovascular (30%) indications (Fig. 1). In the detailed survey, MSC were obtained from bone marrow in 219 (94%) cases and mostly used to treat autoimmune (53%), musculoskeletal (31%), and cardiovascular disorders (14%). For the 264 HSC treatments, cells were derived from the bone marrow (85%), peripheral blood (8%), placenta (5%), or unspecified cell source (2%).
FIG. 1.
Percentage of indications for novel cellular therapies in Europe in 2009, sorted by donor type. Data used for this chart were derived from the extended questionnaire and the standard European group for Blood and Marrow Transplantation (EBMT) survey sheet. GvHD, graft-vs.-host disease.
Cell processing and delivery mode
Of all the grafted products reported in detailed form, 59% were based on expanded cells and in 2% of the cases cells were transduced (Table 2). About one half (48%) of the products was given intraorgan, 29% on a membrane or gel, 14% intravenously, and 8% using a 3D scaffold. Nonexpanded cells were used to treat 55% of cardiovascular, 38% of musculoskeletal, 38% of epithelial/parenchymal, and 31% of neurological disorders, whereas autoimmune diseases were exclusively treated with expanded cells. Cell sorting was operated for 69% of neurological, 11% of epithelial, 11% of cardiovascular, and 1% of musculoskeletal disorders. Beyond a few sporadic exceptions, reported for cardiovascular, musculoskeletal, and neurological diseases, transplanted cells were not genetically transduced.
Table 2.
Number of Cellular Therapy Transplants for Novel Cellular Therapies in Europe 2009 Sorted by Cell Processing Mode
| |
Cell processing |
|||||
|---|---|---|---|---|---|---|
| Indications | Nonexpanded | Expanded | Untransduced | Transduced | Unsorted | Sorted |
| Cardiovascular | ||||||
| Peripheral artery disease | 42 | 14 | 56 | 50 | 6 | |
| Cardiomyopathy | 11 | 1 | 12 | 12 | ||
| Heart failure | 21 | 12 | 33 | 17 | 16 | |
| Myocardial ischemia | 109 | 2 | 104 | 7 | 102 | 9 |
| Bypass graft | 14 | 2 | 16 | 4 | 12 | |
| Valve replacement | ||||||
| Ulcer | 150 | 150 | 150 | |||
| Other | 23 | 23 | 23 | |||
| Musculoskeletal | ||||||
| Bone repair (maxillofacial) | 1 | 1 | 2 | 2 | ||
| Bone repair (orthopedics) | 57 | 11 | 67 | 1 | 67 | 1 |
| Osteogenesis imperfecta | 1 | 1 | 1 | |||
| Cartilage repair | 6 | 85 | 91 | 91 | ||
| Muscle repair | ||||||
| Tendon/ligament | 5 | 5 | 5 | |||
| Reconstructive surgery | 1 | 1 | 1 | |||
| Other | 17 | 17 | 17 | |||
| Neurological | ||||||
| Parkinson's | ||||||
| Peripheral nerve regeneration | ||||||
| Other | 5 | 11 | 13 | 3 | 5 | 11 |
| Epithelial | ||||||
| Skin reconstruction | 51 | 51 | 51 | |||
| Cornea repair | 24 | 3 | 27 | 27 | ||
| Organ failure | 10 | 10 | 10 | |||
| Diabetes | ||||||
| Liver insufficiency | 2 | 2 | 2 | |||
| Other | ||||||
| Autoimmune | ||||||
| Neurological | 80 | 80 | 80 | |||
| Rheumatological | 2 | 2 | 2 | |||
| Gastrointestinal | 5 | 5 | 5 | |||
| Other | 16 | 16 | 16 | |||
| Graft vs. host disease | 19 | 19 | 19 | |||
| Total | 330 | 484 | 801 | 13 | 748 | 66 |
Data only from extended questionnaire.
For neurological and autoimmune applications, cells were delivered exclusively intravenous or intraorgan (Table 3). The use of a membrane or a gel for cell delivery was reported mainly for cardiovascular treatments (63%), epithelial/parenchymal treatments (33%), or for cartilage repair (5%). For the group of musculoskeletal indications, all possible cell delivery modes were reported, with a predominant tendency for intraorgan (57%) and for the use a 3D scaffold (36%).
Table 3.
Number of Cellular Therapy Transplants for Novel Cellular Therapies in Europe 2009 Sorted by Delivery Mode
| |
Cell delivery mode |
||||
|---|---|---|---|---|---|
| Indications | i.v. | Intraorgan | Membrane/gel | 3D | Total |
| Cardiovascular | |||||
| Peripheral artery disease | 3 | 53 | 56 | ||
| Cardiomyopathy | 12 | 12 | |||
| Heart failure | 33 | 33 | |||
| Myocardial ischemia | 47 | 64 | 111 | ||
| Bypass graft | 16 | 16 | |||
| Valve replacement | |||||
| Ulcer | 150 | 150 | |||
| Other | 23 | 23 | |||
| Musculoskeletal | |||||
| Bone repair (maxillofacial) | 1 | 1 | 2 | ||
| Bone repair (orthopedics) | 22 | 46 | 68 | ||
| Osteogenesis imperfecta | 1 | 1 | |||
| Cartilage repair | 60 | 11 | 20 | 91 | |
| Muscle repair | |||||
| Tendon/ligament | 5 | 5 | |||
| Reconstructive surgery | 1 | 1 | |||
| Other | 17 | 17 | |||
| Neurological | |||||
| Parkinson's | |||||
| Peripheral nerve regeneration | |||||
| Other | 3 | 13 | 16 | ||
| Epithelial | |||||
| Skin reconstruction | 51 | ||||
| Cornea repair | 27 | 27 | |||
| Organ failure | 10 | 10 | |||
| Diabetes | |||||
| Liver insufficiency | 2 | 2 | |||
| Other | |||||
| Autoimmune | |||||
| Neurological | 80 | 80 | |||
| Rheumatological | 2 | 2 | |||
| Gastrointestinal | 5 | ||||
| Other | 13 | 3 | 16 | ||
| Graft vs. host disease | 19 | 19 | |||
| Total | 118 | 390 | 239 | 67 | 814 |
Data only from extended questionnaire.
i.v., intravenous.
Transplant rates and active teams
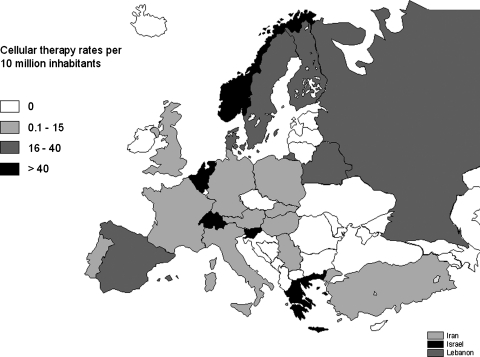
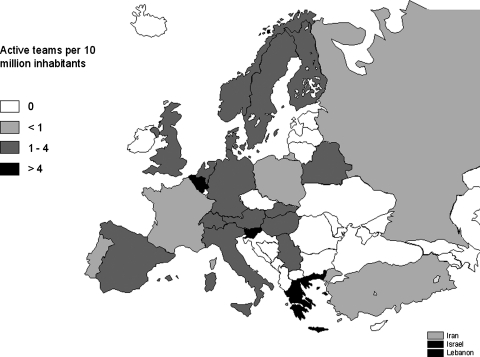
Reported cellular therapies were performed in a limited number of countries and with different intensity. Figure 2 displays the cellular therapy transplant rates per 10 million inhabitants in the different European & EBMT-associated countries. High transplants rates (i.e., 40 per 10 million population) were reported in Belgium, Greece, Israel, the Netherlands, Norway, Slovenia, and Switzerland. The number of teams reporting cellular therapies was also mapped in the different European & EBMT-associated countries after normalization to the inhabitant numbers (Fig. 3). The number of active teams per 10 million inhabitants was higher than 4 in Belgium, Greece, Israel, Lebanon, and Slovenia.
FIG. 2.
Cellular therapy rates (number of cellular therapies per 10 million inhabitants) for novel cellular therapies in Europe in 2009. Data used for this chart were derived from the extended questionnaire and the standard EBMT survey sheet.
FIG. 3.
Number of teams reporting novel cellular therapies in Europe in 2009 per 10 million inhabitants. Data used for this chart were derived from the extended questionnaire and the standard EBMT survey sheet.
Discussion
The intersociety program of the survey on novel cellular therapies represents a unique opportunity for early access to information that integrates that available in public databases (e.g., www.clinicaltrial.gov) and that will only partially and at later times be published in the scientific literature. The 1.7-fold increase in the number of participating teams within Europe and associated countries as compared to the past year demonstrates the raising interest in the program and is expected to stimulate the contribution by other groups and societies in the next years. In this regard, the list of reporting centers in the manuscript Appendix is far from being exhaustive of the effective status, but has the potential to become a key reference for the field.
As compared to the data collected for patients treated in the previous year,1 the present report confirms the main identified trends, though with few remarkable differences, especially related to the cardiovascular diseases. For the latter indications, the number of total treated cases increased by 1.4-fold, with a 7.0-fold higher percentage for peripheral artery disease and a 1.7-fold lower percentage for myocardial ischemia. In the same category of cardiovascular disorders, the cell source shifted from entirely autologous to allogeneic in 36% of the cases, with an increase from 8% to 45% in the use of expanded cells and the introduction of a membrane or gel delivery vehicle in 37% of the treatments. The changing variety of approaches tested may be considered as a sign that several key issues still need to be settled in that field, including the optimal cell type, source and dosing, the most effective route for cell transfer, and methods for enhancing survival of the cellular graft.6
The most uniform category within those analyzed was the one targeting autoimmune disorders. The cells used were uniquely expanded MSC, untransduced, unsorted, and delivered intravenously (19%) or intraorgan (80%). The use of MSC in modulating autoimmune diseases is an emerging area of activity in cellular therapy.7 Based on positive animal model data and in vitro demonstration of an antiproliferative effect of MSC on immune competent cells, many groups have launched clinical trials (14 currently registered on clinical trials.gov), but no large prospective randomized data have been published. Small phase I studies and single case reports have been published (Table 4), most showing acceptable feasibility and safety and some early efficacy. No reports of acute toxicity have occurred, which makes MSC an attractive option as a therapeutic agent capable of homing to damaged or distressed tissue. The positive therapeutic effect seems to be exerted via multiple paracrine factors, since transdifferentiation into healthy regenerated tissue is not considered biologically relevant. The data collected in the survey confirm that sources of MSC are autologous (requiring local GMP facilities and associated quality management systems) or allogeneic (requiring dedicated companies offering screened MSC batches derived under tightly regulated processes from bone marrow, adipose tissue, placenta, or umbilical cord). Despite the source dependent phenotypic and functional MSC variations, all have been demonstrated to exert in vitro immunomodulatory effects. More data are urgently required concerning clinical efficacy, MSC fate after transplantation, and long-term safety, especially with regard to tumor surveillance. The generation of these data will be critical to make the transition from a correctly prudent and still scattered use of MSC for autoimmune diseases to the routine clinical implementation of consolidated and standardized protocols, compliant to the regulatory framework for Advanced Therapy Medicinal Products by the European Medicines Agency (www.ema.europa.eu).
Table 4.
References to the Worldwide Published Clinical Studies on the Treatment of Autoimmune Diseases Using Mesenchymal Stem/Stromal Cells
| Autoimmune disease | No. of patients | MSC source | Delivery route | Clinical outcome | Reference |
|---|---|---|---|---|---|
| Multiple sclerosis | 10 | Allogeneic bone marrow | Intrathecal | Mixed | Mohyeddin et al.9 |
| Scleroderma | 1 | Allogeneic bone marrow | i.v. | Improved | Christopeit et al.10 |
| Multiple sclerosis | 1 | Allogeneic umbilical cord | i.v. | Improved | Liang et al.11 |
| Multiple sclerosis | 3 | Mixed allo & autologous fat | Mixed i.v. & Intrathecal | Improved clinic MRI unchanged | Riordan et al.12 |
| Crohns fistulae | 14 | Autologous fat | Intrafistula | 71 % closure | Garcia-Olmo et al.13 |
| Scleroderma digital ulcus | 2 | Autologous blood & bone marrow | Local | Improved | Nevskaya et al.14 |
| Lupus nephritis | 15 | Allogeneic bone marrow | i.v. | Improved SLEDAI and proteinuria | Liang et al.15 |
| Lupus nephritis | 16 | Allogeneic umbilical cord | i.v. | Improved SLEDAI and proteinuria | Sun et al.16 |
| SLE | 2 | Autologous bone marrow | i.v. | No change | Carrion et al.17 |
| SLE alveolar hemorrhage | 1 | Allogeneic umbilical cord | i.v. | Improved | Liang et al.18 |
| Crohns disease | 10 | Autologous bone marrow | IVI | Improved | Duijvestein et al.19 |
| Multiple sclerosis | 15 | Autologous bone marrow | Intrathecal (all) plus IVI (5) | Some stabilized | Karussis et al.20 |
| Amyothrophic lateral sclerosis | 19 | Autologous bone marrow | Intrathecal (all) plus i.v. (9) | No change | Karussis et al.20 |
| Multiple sclerosis | 10 | Autologous bone marrow | Intrathecal | Mixed | Yamout et al.21 |
SLE, systemic lupus erythematosus.
The European program on novel cellular therapies is paralleled by similar initiatives in the United States, capturing the activity of 18 centers for a total of 115 treatments.8 Despite the inherent difficulties associated with the collection and management of data across different geographical areas, for the years to come efforts should be directed at coordinated initiatives toward the establishment of a global survey and report.
Appendix
Appendix: List of Reporting Novel Cellular Therapy Centers in Europe in 2009
Format: City, Hospital, Center Identification Code number (if data were imported from the standard EBMT survey sheet), Physicians (total treatments; allogeneic/autologous)
Austria
Graz, University of Graz, CIC 308, W. Linkesch (1; 1/0)
Vienna, Medical University Hospital, S. Marlovitis, Ch. Albrecht (5; 0/5)
Belarus
Minsk, Belorussian Center, CIC 591, O Aleinikova (15; 15/0)
Minsk, Hospital No. 9, N. Milanovich (2; 1/1)
Belgium
Antwerpen, Uiversity Antwerpen, CIC 996, W. Schroyens (6; 0/6)
Brugge, A.Z. St. Jan, CIC 506, D. Selleslag, A.v. Hoof, J.v. Droogenbroeck, K.v. Eygen (4; 4/0)
Brussels, Military Hospital Queen Astrid, Gilbert Verbeken (47; 0/47)
Leuven, University Hospital Gasthuisberg, CIC 209, G. Verhoef, M. Delforge,J. Maertens (7; 7/0)
Liège, University Hospital Sart-Tilman, CIC 726, Y. Béguin, B de Prijck (12; 12/0)
Brussels, U.L.B. Hôpital Erasme, CIC 596, B. Bailly, A. Kentos, M. Lambermont (2; 0/2)
Gent, University Hospital, CIC 744, L.A. Noens (2; 2/0)
Denmark
Aarhus, Amtssygehus, E. Segel, Dr. Moeller (2; 0/2)
Copenhagen, The Heart Centre Rigshospitalet, J. Kastrup (13; 0/13)
Finland
Helsinki, Helsinki University Central Hospital, CIC 515, L. Volin (12; 0/12)
France
Clermont Ferrand, CRCTCP, CHU Estaing, J.-O. Bay, F. Deméocq, P. Travade (37; 24/13)
Grenoble, Hospitalier A. Michallon, CIC 270, J.Y. Cahn, F.Garban, P. Drillat, D. Plantaz (2; 0/2)
Germany
Berlin, Universitäts-Klinik Benjamin Franklin, CIC 590, E. Thiel, L. Uharek (2; 2/0)
Dresden, Universitätsklinikum Carl Gustav Carus, Med. Poliklinik, G. Ehninger, H. Bornhäuser (4; 3/1)
Dresden, Universitätsklinikum Carl Gustav Carus, Hematology, CIC 808, G. Ehninger, H. Bornhäuser (22; 21/1)
Frankfurt, Universitätsklinikum d. J. W. Goethe, CIC 138, T. Klingebiel, P. Bader (3; 3/0)
Halle, Clinic Bergmannstrost, H.J. Meisel (14; 0/14)
Hannover, Medizinische Hochschule, CIC 295, A. Ganser, M. Eder (1; 1/0)
Köln, Universitäts-Klinik, CIC 534, M. Hallek, Ch. Scheid, F. Berthold, T. Simon (6; 0/6)
Tübingen, Medizinische Universitäts-Klinik (peds), CIC 535, R. Handgretinger, P. Lang (13; 12/1)
Ulm, Kinderklinik der Universität, CIC 204, W. Friedrich, K. Debatin (1; 1/0)
München, KK Munchen Schwabing, CIC 189, S. Burdach, A. Wawer, M. Nathrath (1; 1/0)
Mainz, Medizinische Klinik der Universität, CIC 786, K. Kolbe, D. Wehler (1; 1/0)
Heidelberg, Angelika Lautenschläger-Klinik, CIC 524, J. Greil (1; 1/0)
Ratingen, DRK Blutspendedienst West (6; 0/6)
Würzburg, Universitätsklinikum, P. Speer, P. Schlegel (1; 1/0)
Greece
Athens, Academy of Athens, A. Papassavas (37; 0/37)
Athens, Evanghelismos Hospital, CIC 622, D. Karakasis, N. Harhalakis (2; 2/0)
Athens, Aghia Sophia Children's Hospital, CIC 752, S. Graphakos (2; 1/1)
Thessaloniki, Sports Clinic, Emanuel T. Papacostas (3; 0/3)
Athens, Aghia Sophia Children's Hospital, S. Graphakos (1; 0/1)
Hungary
Debrecen, University of Debrecen, Z. Boda, E. Rajnavolgyi (2; 0/2)
Iran, Islamic Rep.
Teheran, Shariati Hospital, CIC 633, M. Jahani (44; 36/8)
Teheran, Teheran University of Medical Sciences, M. Mohyeddin (49; 0/49)
Israel
Jerusalem, Hadassah University Hospital, CIC 258, R. Or, S. Slavin (34; 34/0)
Petach-Tikva, Beilinson Hospital, CIC 409, M. Yeshurun (1; 1/0)
Tel Hashomer, Chaim Sheba Medical Cebter, CIC 572, A. Toren (2; 2/0)
Tel Hashomer, Sheba Medical Center, CIC 754, A. Nagler, A. Shimoni, (6; 6/0)
Italy
Bologna, 6th div Rizzoli Orthopedic Institute, S. Giannini, R. Buda, E. Kon (5; 0/5)
Firenze, Policlinico di Careggi, CIC 304, A. Bosi, S. Guidi (2; 0/2)
Monza, Ospedale S. Gerardo, CIC 279, C. Uderzo (8; 8/0)
Piacenza, Ospedale Civile, CIC 163, L. Cavanna (14; 0/14)
Reggio di Calabria, Azienda Ospedale “Riuniti e Morelli,” Bianchi- Melacrino, CIC 587 P. Iacopino (3; 0/3)
Udine, Policlinico Universitario & General Hospital, CIC 705, A. Sperotto, R. Fanin (1; 0/1)
Brescia, Universitá degli Studi di Brescia, CIC 741, F. Porta (1; 1/0)
Genova, Istituto Giannina Gaslini, CIC 274, G. Dini, E. Lanino (1; 1/0)
Bergamo, Ospedale Riuniti, CIC 658, A. Rambaldi (2; 2/0)
Pavia, Policlinico IRCCS St. Matteo, CIC 557, M. Zecca (2; 2/0)
Bologna, Instituto Oropedico Rizzoli, L. Roseti (9; 0/9)
Milano, IRCCS Galeazzi Orthopedic Institute, M. Moretti, P. Volpi, H. Schoenhuber (5; 0/5)
Piemonte, Ospedale degli infermi di Biella, A. Siclari (34; 0/34)
Lebanon
Beirut, American University of Beirut, A. Bazarbachi (4; 0/4)
Beirut, American University Medical Centre, B. Yamout (10; 0/10)
Netherlands
Leiden, University Hospital, CIC 203, R. Willemze, M. Egeler (70; 24/46)
Utrecht, University Hospital, E. Petersen, N.M. Wulffraat, I. Slaper (24; 0/24)
Rotterdam, Erasmus University Medical Center, Wim J. van der Giessen (6; 0/6)
Groningen, University Hospital, CIC 546, G. van Imhoff, (1; 1/0)
Maastricht, University Hospital, CIC 565, H.C. Schouten, J. Wagstaff (1; 1/0)
Amsterdam, Academic Med Centre, CIC 247, M.J. Kersten, J. Zsiros (3; 3/0)
Norway
Oslo, University Hospital Rikshospitalet, J. Brinchmann (22; 0/22)
Poland
Cracow, University Children's Hospital JUMC, CIC 507, J. Gozdzik (9; 0/9)
Wroclaw, Lower Silesian Cent./BM Donor Registry, A. Lange (2; 0/2)
Portugal
Lisbon, Instituto Portugues de Oncologia, CIC 300, M. Abecasis (3; 3/0)
Russian Fed.
Moscow, Russian Children's Hospital, CIC 694, A. Maschan, E. Skorobogato, E. Pachanov (15; 15/0)
Moscow, Cancer Research centre, G. Mentrevich (14; 14/0)
Moscow, Research Haematology Center of RAS, V.G. Savtchenko (9; 9/0)
Novosibirsk, Inst. Clinical Immunology, CIC 376, I. Lisukov (20; 2/18)
St. Petersburg, Pavlov Medical University, B.V. Afanassiev, L. Zubarovskaya (180; 150/30)
St. Petersburg, Pavlov Medical University, CIC 725, B.V. Afanassiev, L. Zubarovskaya (2; 2/0)
Serbia
Belgrade, Military Medical Academy, D. Stamatovic (7; 0/7)
Slovak Republic
Bratislava, National Cancer Institute, CIC 560, J. Lakota (1;1/0)
Slovenia
Ljublijana, University Medical Centre, CIC 640, J. Pretnar (10; 0/10)
Spain
Barcelona, Hospital Clinic, CIC 214, E. Carreras (4; 4/0)
Cordoba, Hospital Reina Sofia, A. Torres-Gomez (32; 0/32)
Madrid, Hospital La Paz, CIC 734, A. Martinez, A. Sastre, R. Arrieta (2; 0/2)
Murcia, Hospital Virgen de la Arrixaca, JM. Moraleda, A. Morales Lazaro (9; 3/6)
Palma de Mallorca, Hospital Son Dureta, CIC 722, J. Besalduch, M. Canaro (4; 0/4)
Pamplona, Clinica Universitaria de Navarra, CIC 737, J. Rifon (1; 1/0)
Salamanca, Complejo Hospital, CIC 727, D. Caballero (9; 9/0)
Murcia, Hospital General Universitario Morales Meseguer, CIC 735, V. Vicente-Garcia, I. Heras (3; 0/3)
Barcelona, ITRT Inst. de Terapia Regenerativa Tissular, C.M. Teknon (15; 0/15)
Madrid, Hospital Universitario San Carlos, J. Diaz-Mediavilla, L. Llorente, R. Martinez (5; 0/5)
Madrid, Hospital General La Paz (adults), R. Arrieta (3; 0/3)
Sweden
Lund, University Hospital, CIC 283, S. Lenhoff (5; 5/0)
Stockholm, Karolinska University Hospital, Huddinge, CIC 212, P. Ljungman (14; 12/2)
Switzerland
Geneva, Hôpital Cantonal Universitarie, CIC 261, J. Passweg, Y. Chalandon, M. Ansari (10; 0/10)
Lugano, Cardiocentro Ticino, G. Astori (23; 0/23)
Turkey
Adana, Baskent University Adana, H. Ozdogu, C. Boga, S. Asma, S. Yuce (1; 1/0)
Ankara, Ihsan Dogramaci Children's Hospital (Hacettepe), CIC 399, A. Tuncer, D. Uckan (6; 5/1)
Istanbul, University of Istanbul, CIC 760, D. Sargin, S. Kalayoglu-Besisik (1; 1/0)
Antalya, Medical Park Hospitals, CIC 919, Y. Koc (3; 3/0)
Ankara, Gazi University, Besevler, CIC 169, G. Sucak (3; 3/0)
United Kingdom
Manchester, Royal Children's Hospital, CIC 521, R. Wynn (1; 1/0)
London, University College Hospital, CIC 224, K. Thomson, (1; 1/0)
London, Hammersmith Hospitals NHS Trust, CIC 205, J. Apperley, E. Olavarria, E. Kanfer, A. Rahemtulla, R. Szydlo (3; 3/0)
London, Great Ormond Street Hospital, CIC 243, P. Veys, (7; 7/0)
London, St. Bartholomew's and the Royal London Hospital, CIC 768, J. Gribben, J. Cavenagh, S. Agrawal, T. Lister (14; 0/14)
Oswestry, Oswestry Orthopaedic Hospital, P. Harrison (33; 0/33)
Acknowledgments
We greatly appreciate the cooperation of all participating teams and their staff (listed in the Appendix) and the engagement of the different working groups and their highly committed representatives, namely TERMIS-EU (Sarah Wilburn), ISCT-Europe (Francesco Lanza), ICRS (Daniel Saris and Stephan Seiler), EBMT (Alejandro Madrigal), and EULAR. We are grateful to Alois Gratwohl for the initiation and continuous support of this program.
Funding
The work was supported in part by the European Leukaemia Net LSH-2002-2.2.0-3.
EBMT is supported by grants from the corporate members: Amgen Europe, Gilead Sciences UK, Miltenyl Biotec GmbH, Schering-Plough, Celegene International SARL, Genzyme, Fresenius Biotech GmbH, CaridianBCT Europe NV, Therakos, Cephalon, F. Hoffmann-La Roche Ltd., Gentium SpA, Pierre Fabre Médicament, Alexion Europe, Pfizer, Merck Sharp and Dohme, Chugai Sanofi–Aventis, Novartis, Hospira, and MacroPharma.
Disclosure Statement
There are no conflicts of interest to declare. Writing of the article was the sole responsibility of the authors.
References
- 1.Martin I. Baldomero H. Tyndall A. Niederwieser D. Gratwohl A. A survey on cellular and engineered tissue therapies in Europe in 2008. Tissue Eng A. 2010;16:2419. doi: 10.1089/ten.TEA.2010.0056. [DOI] [PubMed] [Google Scholar]
- 2.Gratwohl A. Bone marrow transplantation activity in Europe 1990. Report from the European Group for Bone Marrow Transplantation (EBMT) Bone Marrow Transplant. 1991;8:197. [PubMed] [Google Scholar]
- 3.Gratwohl A. Baldomero H. Horisberger B. Schmid C. Passweg J. Urbano-Ispizua A. Accreditation Committee of the European Group for Blood and Marrow Transplantation (EBMT). Current trends in haematopoietic stem cell transplantation in Europe. Blood. 2002;100:2374. doi: 10.1182/blood-2002-03-0675. [DOI] [PubMed] [Google Scholar]
- 4.Gratwohl A. Baldomero H. Schwendener A. Gratwohl M. Apperley J. Niederwieser D. Frauendorfer K. Joint Accreditation Committee of the International Society for Cellular Therapy; European Group for Blood and Marrow Transplantation; European Leukemia Net. Predictability of hematopoietic stem cell transplantation rates. Haematologica. 2007;92:1679. doi: 10.3324/haematol.11260. [DOI] [PubMed] [Google Scholar]
- 5.Gratwohl A. Baldomero H. Schwendener A. Rocha V. Apperley J. Frauendorfer K. Niederwieser D. The EBMT activity survey 2007 with focus on allogeneic HSCT for AML and novel cellular therapies. Bone Marrow Transplant. 2009;43:275. doi: 10.1038/bmt.2009.7. [DOI] [PubMed] [Google Scholar]
- 6.Menasché P. Cell therapy for peripheral arterial disease. Curr Opin Mol Ther. 2010;12:538. [PubMed] [Google Scholar]
- 7.Tyndall A. Uccelli A. Multipotent mesenchymal stromal cells for autoimmune diseases: teaching new dogs old tricks. Bone Marrow Transplant. 2009;43:821. doi: 10.1038/bmt.2009.63. [DOI] [PubMed] [Google Scholar]
- 8.Pasquini M.C. Pirog S. Baldomero H. Sobocinski K. Keating A. Hare J. Loper K. Laughlin M. Horwitz E. Heslop H. Cellular therapy for regenerative medicine: activity in the US during 2008. ISCT 2010 meeting in Philadelphia. www.cibmtr.org/Meetings/Materials/WorkingCommittees/Documents/Cellular%20Therapies/2011/2011CellularTherapiesAgenda.pdf www.cibmtr.org/Meetings/Materials/WorkingCommittees/Documents/Cellular%20Therapies/2011/2011CellularTherapiesAgenda.pdf
- 9.Mohyeddin Bonab M. Yazdanbakhsh S. Lotfi J. Alimoghaddom K. Talebian F. Hooshmand F. Ghavamzadeh A. Nikbin B. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol. 2007;4:50. [PubMed] [Google Scholar]
- 10.Christopeit M. Schendel M. Föll J. Müller L.P. Keysser G. Behre G. Marked improvement of severe progressive systemic sclerosis after transplantation of mesenchymal stem cells from an allogeneic haploidentical-related donor mediated by ligation of CD137L. Leukemia. 2008;22:1062. doi: 10.1038/sj.leu.2404996. [DOI] [PubMed] [Google Scholar]
- 11.Liang J. Zhang H. Hua B. Wang H. Wang J. Han Z. Sun L. Allogeneic mesenchymal stem cells transplantation in treatment of multiple sclerosis. Mult Scler. 2009;15:644. doi: 10.1177/1352458509104590. [DOI] [PubMed] [Google Scholar]
- 12.Riordan N.H. Ichim T.E. Min W.P. Wang H. Solano F. Lara F. Alfaro M. Rodriguez J.P. Harman R.J. Patel A.N. Murphy M.P. Lee R.R. Minev B. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med. 2009;7:29. doi: 10.1186/1479-5876-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Olmo D. Herreros D. Pascual I. Pascual J.A. Del-Valle E. Zorrilla J. De-La-Quintana P. Garcia-Arranz M. Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 14.Nevskaya T. Ananieva L. Bykovskaia S. Eremin I. Karandashov E. Khrennikov J. Mach E. Zaprjagaeva M. Guseva N. Nassonov E. Autologous progenitor cell implantation as a novel therapeutic intervention for ischaemic digits in systemic sclerosis. Rheumatology. 2009;48:61. doi: 10.1093/rheumatology/ken407. [DOI] [PubMed] [Google Scholar]
- 15.Liang J. Zhang H. Hua B. Wang H. Lu L. Shi S. Hou Y. Zeng X. Gilkeson G.S. Sun L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69:1423. doi: 10.1136/ard.2009.123463. [DOI] [PubMed] [Google Scholar]
- 16.Sun L. Wang D. Liang J. Zhang H. Feng X. Wang H. Hua B. Liu B. Ye S. Hu X. Xu W. Zeng X. Hou Y. Gilkeson G.S. Silver R.M. Lu L. Shi S. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 17.Carrion F. Nova E. Ruiz C. Diaz F. Inostroza C. Rojo D. Mönckeberg G. Figueroa F.E. Autologous mesenchymal stem cell treatment increased T regulatory cells with no effect on disease activity in two systemic lupus erythematosus patients. Lupus. 2010;19:317. doi: 10.1177/0961203309348983. [DOI] [PubMed] [Google Scholar]
- 18.Liang J. Gu F. Wang H. Hua B. Hou Y. Shi S. Lu L. Sun L. Mesenchymal stem cell transplantation for diffuse alveolar hemorrhage in SLE. Nat Rev Rheumatol. 2010;6:486. doi: 10.1038/nrrheum.2010.80. [DOI] [PubMed] [Google Scholar]
- 19.Duijvestein M. Vos A.C. Roelofs H. Wildenberg M.E. Wendrich B.B. Verspaget H.W. Kooy-Winkelaar E.M. Koning F. Zwaginga J.J. Fidder H.H. Verhaar A.P. Fibbe W.E. van den Brink G.R. Hommes D.W. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut. 2010;59:1662. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 20.Karussis D. Karageorgiou C. Vaknin-Dembinsky A. Gowda-Kurkalli B. Gomori J.M. Kassis I. Bulte J.W. Petrou P. Ben-Hur T. Abramsky O. Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamout B. Hourani R. Salti H. Barada W. El-Hajj T. Al-Kutoubi A. Herlopian A. Baz E.K. Mahfouz R. Khalil-Hamdan R. Kreidieh N.M. El-Sabban M. Bazarbachi A. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol. 2010;227:185. doi: 10.1016/j.jneuroim.2010.07.013. [DOI] [PubMed] [Google Scholar]