Abstract
Background and Purpose
Histotripsy is a noninvasive, pulsed ultrasound technology that produces mechanically homogenized tissue within targeted volumes. Previous work has demonstrated prostatic tissue debulking in a canine model. The aim was to establish safety thresholds by evaluating histologic changes of urinary sphincter, neurovascular bundle (NVB), and rectum after targeted histotripsy treatment of these critical structures.
Materials and Methods
Rectum, urinary sphincter, and NVB in five anesthetized canines were targeted for histotripsy treatment (50 total points). Locations received 1k, 10k, or 100k acoustic pulses (4 microsecond, 1 MHz) at a repetition frequency of 500 Hz. Canine subjects were euthanized immediately (2), survived 3 days (1), or 2 weeks (3) after treatment. Prostates, periprostatic tissue, and rectum were harvested and processed for histology.
Results
The sphincter was structurally intact with minimal muscle fiber disruption even after 100k pulses (n=10). Undamaged nerves, arteries, and veins of the NVB were seen despite mechanical homogenization of surrounding loose connective tissue (n=19). The rectum, however, exhibited dose-dependent damage (n=20). 1k pulses yielded mild submucosal hemorrhage. 10k pulses resulted in moderate collagen disruption and focal mucosal homogenization. 100k pulses produced damage to the mucosa and muscularis propria with extensive hemorrhage and collagen disruption. One canine treated with 100k pulses needed early euthanasia (day 3) because of complications from a urine leak.
Conclusions
Histotripsy histologic tissue effect varied based on targeted structure with substantial structural preservation of NVB and sphincter. Rectal subclinical damage was apparent after 1k pulses and increased in extent and severity with escalating doses. Future work will include assessment of functional outcomes and refinement of these initial safety thresholds.
Introduction
Histotripsy is a noninvasive, pulsed ultrasound (US) technology in which short bursts of intense acoustic energy are applied at low duty cycle (<1%) to induce cavitation within a targeted tissue volume.1 Cavitation is a process in which extreme pressure changes lead to formation of microbubbles that oscillate and collapse. Within solid organs (ie, prostate parenchyma, renal cortex), this process mechanically disrupts tissue structures such that the cumulative effect of multiple histotripsy bursts is conversion of the targeted tissue into a liquefied homogenate of subcellular debris.2,3 Interestingly, some structures, such as the collecting system of the kidney, appear to be resistant to cavitational damage, while others, such as the renal medulla, necessitate greater numbers of histotripsy pulses to induce histologic injury, suggesting a varying threshold of cavitational effect based on tissue characteristics.4
Previously, we have demonstrated that histotripsy is an effective noninvasive technology for prostate tissue ablation and debulking in the canine model.5 To further explore potential applications of histotripsy for treatment of patients with benign prostatic hyperplasia (BPH) and prostate cancer, it is necessary to understand the susceptibility or resilience of critical periprostatic structures to cavitation-induced damage.
In this study, we sought to characterize the threshold histotripsy dose (number of pulses) that would produce clinically significant damage when applied directly to the rectum, urinary sphincter, and neurovascular bundle (NVB).
Materials and Methods
Five mongrel canine subjects weighing 20 to 30 kg were included in the investigation after approval from the University of Michigan Committee on Use and Care of Animals. Subjects were anesthetized using sodium thiopentothal (4.5 mg/kg) and acepromazine (0.1 mg/kg), intubated, and maintained with forced ventilation using 1% to 2% isoflurane. Tap water enema and rectal disimpaction were performed to ensure quality transrectal ultrasonography and 40,000 IU penicillin-G benzathine was administered intramuscularly. Once secured in a supine position on the procedural table, fur was removed with clippers from the suprapubic region and lower abdomen. Real-time transrectal imaging of the prostate was performed using a Logiq 6 US scanner and ERB probe (GE Healthcare, Waukesha, WI) secured in a custom rectal sheath to prevent prostate movement during imaging.
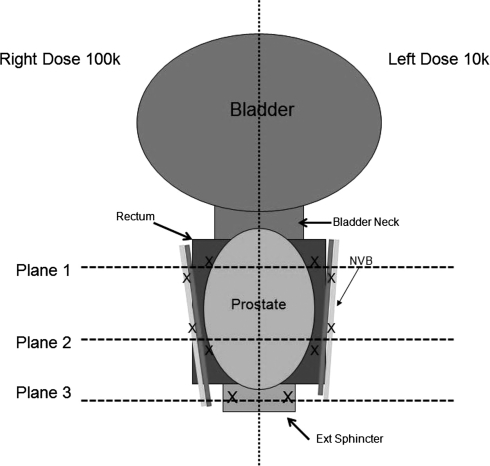
Treatment was performed using a 16-element piezocomposite array therapeutic transducer (1 MHz; focal length 11 cm; focal volume 3×3×8 mm; Imasonic, Voray sur l'Ognon, France), attached to a 3-axis positioning system (MATLAB, MathWorks, Natick, MA) and coupled to the abdomen via a water bolus contained in a plastic membrane supported by a metal frame above the abdomen. Histotripsy pulses comprised 3 cycles bursts of acoustic energy (3 microseconds) delivered at a pulse repetition frequency of 500 Hz. Within each prostate, 10 points within the critical structures (4 NVB, 4 rectum, 2 urinary sphincter) were targeted and treated with a predetermined histotripsy dose (1k, 10k, or 100k pulses) as detailed in Figure 1 and Table 1. Before treatment, the NVB was easily identified using color-flow ultrasonography. During treatment of the urinary sphincter, muscle contraction was identified in real-time to confirm structure targeting.
FIG. 1.
Diagram representing treatment planning. The prostate, rectum, and neurovascular bundle are divided into left and right segments. Each side received either 1k, 10k, or 100k pulses. Three treatment planes are used: Two for rectum/neurovascular bundle and one for the external urinary sphincter.
Table 1.
Number of Histotripsy Targets Stratified by Location and Histotripsy Dose
| |
Histotripsy Dose |
|
||
|---|---|---|---|---|
| Targeted structure | 1k pulses | 10k pulses | 100k pulses | Total |
| Sphincter | 2 [1 – 1] | 5 [2 1 2] | 3 [1 1 1] | 10 [4 2 4] |
| Rectum | 4 [2 – 2] | 10 [4 2 4] | 6 [2 2 2] | 20 [8 4 8] |
| Neurovascular bundle | 4 [2 – 2] | 9 [3 2 4] | 6 [2 2 2] | 19 [7 4 8] |
The number of targeted points harvested at each time point is indicated in brackets [acute, day 3, day 14]. One subject intended to survive 14 days was euthanized on day 3 secondary to peritonitis from a urine leak.
At the completion of histotripsy treatment, two subjects were euthanized while three subjects survived for 2 weeks and were monitored twice daily for hematuria, hematochezia, urinary retention, urinary incontinence, and abdominal discomfort. At the time of euthanasia, the prostate, external urinary sphincter, and rectum were harvested en bloc and grossly inspected. Tissue was placed in formalin for 1 week, dehydrated using 50% ethanol, paraffin embedded, sectioned intact with the rectum using a microtome at 1 mm intervals to generate hematoxylin and eosin (H&E) stained slides. All slides were reviewed by two authors (NRS and WWR) in unblinded fashion. Left and right targeted structures were compared for differential damage based on applied dose. The urinary sphincter was inspected for muscle and surrounding damage, NVBs were inspected for signs of vessel or nerve structural damage, and the rectum was inspected from mucosa to serosa for signs of damage within each layer and evidence of mucosal perforation.
Results
Within the five subjects, 49 critical structures were able to be identified and treated with histotripsy. One NVB was unable to be treated with 10k pulses because of difficulty targeting (Table 1). One canine subject treated with 10k (right) and 100k (left) pulses to corresponding target points needed early euthanasia on post-treatment day 3. Decreased urinary output and poor oral intake prompted abdominal ultrasonography that revealed free fluid within the abdomen. After euthanasia, gross inspection revealed a urethral perforation adjacent to the bladder neck.
Histologic evaluation of the targeted points within the urinary sphincter revealed structurally intact muscle fibers even after delivery of 100k histotripsy pulses. Scant amounts of surrounding hemorrhage were identified with 10k and 100k pulses acutely (Fig. 2). A similar appearance was seen on day 3 with a mild, inflammatory response. By day 14, partial resolution of hemorrhage was noted. Urinary incontinence did not develop in any subject after treatment.
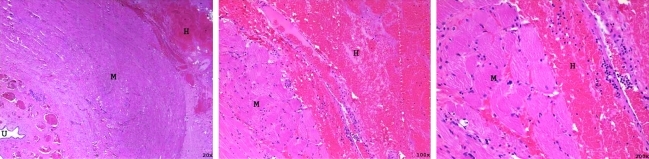
FIG. 2.
Hematoxylin and eosin stained section of the urinary sphincter that was treated with 100k pulses (immediate euthanasia) with increasing magnification (left to right). Muscle (M) can be seen structurally intact with scant hemorrhage (H) surrounding the sphincter. The urethra (U) is seen under low magnification.
Nineteen target points incorporating the NVBs were treated and evaluated histologically. After receiving up to 100k pulses, undamaged nerves, arteries, and veins were identified in all 19 targets (Fig. 3). Surrounding loose areolar tissue was homogenized with small foci of hemorrhage and hematoma seen in proportion to the applied dose (Fig. 4) in the prostates harvested immediately. No NVB hemorrhage or hematoma was present in the two prostates harvested 2 weeks after histotripsy treatment, suggesting resolution of any small hemorrhage that occurred.
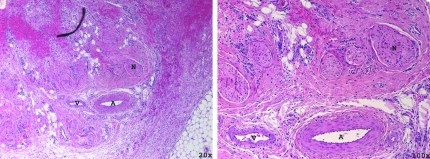
FIG. 3.
Hematoxylin and eosin stained section of the neurovascular bundle treated with 100k pulses (subject survived 2 weeks) with increasing magnification (left to right). Nerve (N), artery (A), and vein (V) can be seen intact with surrounding disruption of loose perineural tissues.
FIG. 4.
High-power magnification of an artery (A, left) and nerve (N, right) stained with hematoxylin and eosin 2 weeks after treatment with 100k histotripsy pulses. Structurally, the artery and nerve are intact with surrounding hemorrhage and collagen disruption.
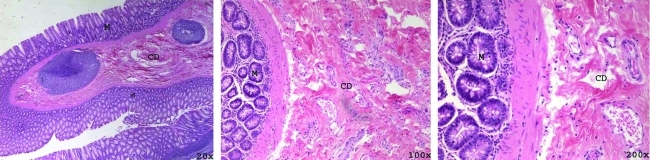
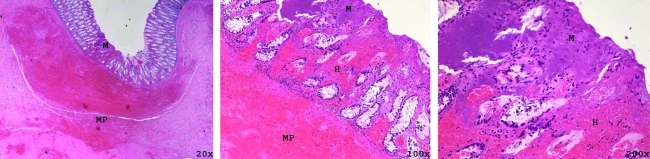
Dose dependent tissue damage was observed in the 20 target points within rectal tissue. Delivery of 1k histotripsy pulses produced mild submucosal hemorrhage. Treatment with 10k histotripsy pulses caused moderate collagen disruption (Fig. 5) with focal mucosal homogenization. Rectum that was treated with 100k pulses demonstrated damage predominantly in the mucosa and muscularis propria (rather than the muscle layers) along with extensive hemorrhage and collagen disruption (Fig. 6). The initial hemorrhage and tissue destruction was followed by an inflammatory response on day 3 and partial resolution and healing by day 14.
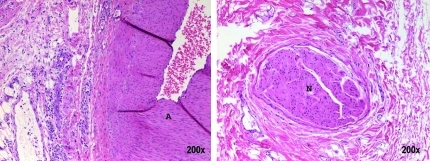
FIG. 5.
Hematoxylin and eosin stained rectum after treatment with 10k pulses (immediate euthanasia) with increasing magnification (left to right). Tissue disruption (CD) is seen within the lamina propria along with scant hemorrhage. No mucosal (M) damage is identified.
FIG. 6.
Hematoxylin and eosin stained rectum 2 weeks after treatment with 100k pulses shown with increasing magnification (left to right). Mechanical homogenization of the mucosa (M) is seen along with hemorrhage (H) extending through all layers of the rectum. Mechanical homogenization of the muscularis propria (MP) is seen at low magnification.
Discussion
Histotripsy is distinct from thermal based high intensity focused US (HIFU) and, as such, has several interesting and beneficial properties that may facilitate its usefulness as an ablative tissue modality. The cavitation bubble cloud—the actual acoustic process responsible for tissue disruption—is easily visible with ultrasonographic imaging and provides immediate feedback of accurate targeting and localization of the site of energy deposition. Furthermore, the ultrasonographic appearance of the targeted tissue changes as it is progressively subdivided, ultimately becoming hypoechoic on conventional diagnostic ultrasonography. This is believed to be the result of breakdown of tissue elements that scatter energy back to the transducer to produce a diagnostic ultrasonographic image.
HIFU and other thermal modalities are limited by concerns of thermal spread to unintended targets, including tissues overlying the target zone and heat sink effects from blood vessels that prevent homogenous heating and can lead to irregular ablation volumes or skip zones.6–9 Histotripsy uses cavitation, which is a threshold phenomenon. When acoustic pressure perturbations within the focal volume of the transducer are large enough (ie, negative pressure swings sufficiently low), cavitation will occur. Once above the threshold for cavitation, increasing the amplitude further does not change the effect within the focus and only minimally increases the size of the bubble cloud because of the highly focused geometry of the therapeutic transducer. These properties provide an element of inherent safety. Structures adjacent to the targeted volume should not be damaged by prolonged exposures, so long as the bubble cloud is correctly localized within the target volume.
The rectum, NVB, and urethral sphincter are critical structures directly adjacent to the prostate. Consequences of damaging these structures—rectal perforation, fistulae formation, impotence, and incontinence—are severe and can have a significant negative impact on quality of life.10,11 Therefore, development of efficient strategies for tissue ablation that avoid damage to these structures is essential. Although spread of energy from the targeted volume to adjacent structures is not a concern, the consequences of mistargeting the focal volume must be considered.
Results of this canine study demonstrate that the urinary sphincter and NVB are structurally resilient to damage, even at histotripsy doses that are much higher than needed to homogenize parenchymal prostate tissue. Thus, the consequence of inadvertently cavitating these structures even after 200 seconds of direct energy delivery appears insignificant. The rectum, however, appears more susceptible to cavitation damage. Doses of 10k and 100k pulses produced hemorrhage and collagen disruption that could potentially lead to delayed fistula formation, although this was not seen in the two dogs that survived for 2 weeks. A similar pattern of differential histotripsy damage was seen in an ex-vivo porcine rabbit model (cortex>medulla>wall of collecting system tissue fractionation).4 It has also been established that a greater histotripsy dose is needed to fractionate prostate urethra and periurethral stroma than prostate glandular tissue.12
These findings have led to the hypothesis that the extent of damage from histotripsy is inversely related to the collagen and connective tissue content of the targeted structures. The architectural structure may also play a role in how resistant a tissue is to histotripsy damage. Structures that are composed of closely packed fibers or bundles (muscle, nerves, renal medulla) may provide fewer suitable foci in which cavitation bubble activity can initiate and evolve. Glandular and epithelial structures, as well as the loose supportive layers of the rectum, such as muscularis propria and loose perineural tissues, may provide a more favorable environment for cavitation bubble activity to occur, and hence this may be the reason the damage is greater in these locations.
One subject needed euthanasia on day 3 secondary to intra-abdominal leakage of urine from an erosion at the junction of the prostate and bladder neck identified on necropsy. We believe this resulted from mistargeting and initial difficulty in localizing the bubble cloud.
Results from this work must be considered in light of the study limitations. First, outcomes and assessment of damage were based primarily on histologic evaluation at various time intervals. Although incontinence was assessed and not seen in the subjects that survived after treatment, no functional evaluation was performed of the NVB or sphincter. Although it is reassuring to see undamaged nerves, arteries, and veins within a field of disrupted debris, further assessment of nerve and sphincter function is also necessary to ensure that there is no functional impairment and is currently under way. As histologic analysis of canine prostates 2 weeks after histotripsy prostate treatment demonstrated tissue damage, additional time points further out from treatment should also be assessed to more fully understand the possibility of delayed rectal fistula formation.
Conclusions
Histotripsy applied directly to periprostatic structures resulted in a varied histologic response based on the targeted structure. NVB and urinary sphincter were largely structurally intact even after delivery of 100k histotripsy pulses. Subclinical rectal damage, however, was apparent after 1k pulses and increased in extent and severity with escalating doses. These findings will be critical to development of safe and efficient strategies for histotripsy prostate ablation for BPH and prostate cancer applications.
Abbreviations Used
- BPH
benign prostatic hyperplasia
- H&E
hematoxylin and eosin
- HIFU
high intensity focused ultrasound
- NVB
neurovascular bundle
- US
ultrasound
Acknowledgments
This work was funded in part by grants from the NIH (K08 DK081656 and R01 DK087871), the American Urological Association Foundation, and Astellas Pharma US, Inc.
Disclosure Statement
Nicholas R. Styn has no competing financial interests. Timothy L. Hall, J. Brian Fowlkes, Charles A. Cain, and William W. Roberts have royalty, equity, and consulting interests in HistoSonics, Inc.
References
- 1.Tran BC. Seo J. Hall TL, et al. Microbubble-enhanced cavitation for noninvasive ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Control. 2003;50:1296–1304. doi: 10.1109/tuffc.2003.1244746. [DOI] [PubMed] [Google Scholar]
- 2.Xu Z. Fowlkes JB. Rothman ED, et al. Controlled ultrasound tissue erosion: The role of dynamic interaction between insonation and microbubble activity. J Acoust Soc Am. 2005;117:424–435. doi: 10.1121/1.1828551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lake AM. Hall TL. Kieran K, et al. Histotripsy: Minimally invasive technology for prostate tissue ablation in an in vivo canine model. Urology. 2008;72:682–686. doi: 10.1016/j.urology.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lake AM. Xu Z. Wilkinson JE, et al. Renal ablation by histotripsy—does it spare the collecting system? J Urol. 2008;179:1150–1154. doi: 10.1016/j.juro.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 5.Hempel CR. Hall TL. Cain CA, et al. Histotripsy fractionation of prostate tissue: Local effects and systemic response in a canine model. J Urol. 2011;185:1484–1489. doi: 10.1016/j.juro.2010.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed M. Liu Z. Afzal KS, et al. Radiofrequency ablation: Effect of surrounding tissue composition on coagulation necrosis in a canine tumor model. Radiology. 2004;185:761–767. doi: 10.1148/radiol.2303021801. [DOI] [PubMed] [Google Scholar]
- 7.Chang I. Mikityansky I. Wray-Cahen D, et al. Effects of perfusion on radiofrequency ablation in swine kidneys. Radiology. 2004;230:500–505. doi: 10.1148/radiol.2312021248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rebillard X. Soulié M. Chartier-Kastler M, et al. High-intensity focused ultrasound in prostate cancer; a systematic literature review of French Association of Urology. BJU Int. 2008;101:1205–1213. doi: 10.1111/j.1464-410X.2008.07504.x. [DOI] [PubMed] [Google Scholar]
- 9.Jung SE. Cho SH. Jang JH. Han J-Y. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: Complications. Abd Imag. 2011;36:185–195. doi: 10.1007/s00261-010-9628-2. [DOI] [PubMed] [Google Scholar]
- 10.Xylinas E. Ploussard G. Durand X, et al. Evaluation of combined oncological and functional outcomes after radical prostatectomy: Trifecta rate of achieving continence, potency and cancer control—a literature review. Urology. 2010;76:1194–1198. doi: 10.1016/j.urology.2010.03.096. [DOI] [PubMed] [Google Scholar]
- 11.Minimally invasive and endoscopic management of benign prostatic hyperplasia. In: Wein AJ, editor; Kavoussi LR, editor; Novick AC, editor; Partin AW, editor; Peters CA, editor. Campbell-Walsh Urology. 9th. Philadelphia: Saunders Elsevier; 2007. pp. 2803–2844. [Google Scholar]
- 12.Hall TL. Hempel CR. Wojno K, et al. Histotripsy of the prostate: Dose effects in a chronic canine model. Urology. 2009;74:932–937. doi: 10.1016/j.urology.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]