Abstract
INTRODUCTION
Elevated clotting factors and thrombin generation have been reported to occur in patients with heart failure (HF). Circulating activated factor XI (FXIa) and active tissue factor (TF) can be detected in acute coronary syndromes and stable angina.
OBJECTIVES
We investigated circulating FXIa and active TF and their associations in patients with systolic HF due to ischemic cardiomyopathy.
PATIENTS AND METHODS
In an observational study, we assessed 53 consecutive patients, aged below 75 years, with stable HF associated with documented coronary artery disease (CAD). Atrial fibrillation (LA), recent thromboembolic events, and current anticoagulant therapy were the exclusion criteria. Plasma TF and FXIa activity was determined in clotting assays by measuring the response to inhibitory monoclonal antibodies.
RESULTS
Coagulant TF activity was detected in 20 patients (37.7%), and FXIa in 22 patients (41.5%). Patients with detectable TF activity and/or FXIa were younger, had a history of myocardial infarction more frequently, significantly higher F1+2 prothrombin fragments, larger LA and right ventricular diastolic diameter, and higher right ventricular systolic pressure than the remaining subjects (P ≤0.01 for all). Circulating FXIa was positively correlated with F1+2 levels (r = 0.69; P <0.001).
CONCLUSIONS
Circulating active TF and FXIa occurred in about 40% of patients with systolic HF due to ischemic cardiomyopathy. The presence of these factors was associated with enhanced thrombin formation. Associations between both factors and LA diameter and right ventricular parameters might suggest that TF and FXIa predispose to thromboembolic complications of HF.
Keywords: activated factor XI, coronary artery disease, systolic heart failure, thrombin generation, tissue factor
INTRODUCTION
The predominant cause of systolic dysfunction is coronary artery disease (CAD), which commonly leads to ischemic cardiomyopathy due to inadequate oxygen delivery to the myocardium. Elevated levels of clotting factors, together with high levels of circulating tissue factor (TF) have been found in patients with CAD.1,2 Circulating activated factor XI (FXIa) and active TF can be detected in acute coronary syndromes and stable angina.3-5
It is known that there is an increased risk of thromboembolic events in HF patients with or without concomitant atrial fibrillation.4-7 Left ventricular ejection fraction (LVEF) appears to be independently associated with thromboembolic risk.5
TF, a membrane-bound glycoprotein, is exposed through the rupture or erosion of an atherosclerotic plaque in the coronary artery6 and is the primary initiator of the extrinsic coagulation cascade. Coagulation is initiated when TF is exposed to FVIIa circulating in blood. The TF/FVIIa complex activates FIX and FX, ultimately resulting in prothrombin activation to thrombin.7,8 Thrombin catalyzes the conversion of soluble fibrinogen to fibrin, potently activates platelets, and amplifies its own generation by activating plasma pro-cofactors FV and FVIII and zymogens FVII and FXI.
FXI is a homodimeric coagulation protein produced in the liver and assigned with a role in the intrinsic coagulation pathway. In vitro, activated FXII on negatively charged surfaces activates FXI to FXIa, and FXIa activates FIX, leading to thrombin generation. However, under physiologic conditions, FXI is activated by thrombin bypassing the contact activation pathway.9 It has been suggested that in vivo FXI and FXII can be activated in the presence of extracellular RNA.10 The pro-coagulant activity of thrombin is regulated by dynamic and stoichiometric inhibitors, including antithrombin that forms thrombin-antithrombin complex. Thrombin-mediated FXI activation contributes to the impairment of fibrinolysis via enhanced activation of thrombin-activatable fibrinolysis inhibitor.11 The regulation of thrombin is central to maintaining the balance between hemorrhage and thrombosis.
Coagulant TF activity detectable in circulating blood has been postulated to be a marker for dysregulated coagulation, which may predict the atherothrombotic risk.12 Plasma TF antigen levels are increased in patients with acute myocardial infarction.13 Moreover, TF expression on macrophages is upregulated in coronary atherosclerotic plaques in patients with unstable angina.14
Increased thrombotic risk is also associated with high plasma concentrations of FXI and may be explained by an increase in endogenous thrombin potential.15 FXI levels are known to confer a higher risk of venous thromboembolism (VTE).16 Elevated levels of FXI clotting activity were also observed in patients with CAD.17
Therefore, the aim of this study was to investigate associations between the presence of active TF and FXIa in circulating blood and both clinical and echocardiographic variables in patients with systolic HF due to ischemic cardiomyopathy.
PATIENTS AND METHODS
Patients
We enrolled 53 consecutive patients with stable systolic HF (20 with New York Heart Association [NYHA] class I, 17 with NYHA class II, and 16 with NYHA class III) due to ischemic cardiomyopathy. Patients were eligible if they fulfilled the following criteria: diagnosis of HF,18 LVEF <50% on transthoracic echocardiography, hemodynamic stability with unaltered medication dosage within 3 months preceding the study. Ischemic etiology of HF was assumed in patients with history of angiographically confirmed CAD, myocardial infarction, and/or revascularization. All patients following myocardial infarction and/or revascularization were eligible if the time since the event was at least 6 months. Subjects with history and tests (virus and parasite screening, antinuclear antibodies, rheumatoid factor, magnetic resonance imaging, and thyroid gland function) negative for secondary cause of HF were defined as having idiopathic cardiomyopathy and were excluded. Additional exclusion criteria were anticoagulation therapy, documented persistent or paroxysmal atrial fibrillation, any acute illness, cancer, liver injury (alanine transaminase 1.5-fold above the upper limit of the reference range), serum creatinine >221 μM, oral anticoagulant or heparin administration, and recent thromboembolic events (within the previous 6 months). No patient had the prior diagnosis of pulmonary arterial hypertension. The Jagiellonian University Ethical Committee approved the study, and patients provided written informed consent.
Methods
Echocardiography
Standard transthoracic echocardiography was performed with the Acuson Sequoia C512 machine. The following parameters were evaluated: right ventricular diastolic diameter (RVDD), right ventricular systolic pressure (RVSP) with the baseline estimated RVSP of 10 mmHg, left ventricular diastolic diameter, left ventricular systolic diameter, LVEF, interventricular septum diastolic diameter, left ventricular posterior wall diameter in diastole, left atrial (LA) systolic diameter, and ascending aorta diameter.
Laboratory procedures
Fasting blood samples were drawn between 8 and 10 a.m. from an antecubital vein with minimal stasis. Routine blood tests, including lipid profile, blood cell count, glucose, and serum creatinine, were conducted by automated laboratory techniques. Plasma samples (9:1 of 3.2% trisodium citrate) were centrifuged (20 min at 2500 g) within 30 minutes of collection, immediately frozen and stored in aliquots at −80°C. Fibrinogen and high-sensitivity C-reactive protein were measured by latex nephelometry (Siemens, Marburg, Germany). Commercially available immunoenzymatic assays were used to determine tissue-type plasminogen activator antigen (t-PA:Ag) (Biopool, Ventura, CA, United States), plasminogen activator inhibitor-1 antigen (PAI-1:Ag) (Biopool), and prothrombin fragment F1+2 (Siemens).
Determination of active tissue factor and activated factor FXI
Plasma clotting assays were performed as described previously.1 Briefly, citrated plasma was thawed at 37°C in the presence of corn trypsin inhibitor (an inhibitor of the contact pathway of blood coagulation, which blocks FXI activation by FXIIa) and either buffer or inhibitory monoclonal anti-FXI (αFXI-2) or anti-TF (αTF-5) antibody (both produced in house) at a final 0.1 mg/ml concentration. CaCl2 at a final 15 mM concentration was added and plasma clotting was initiated by the addition of 2 μM phospholipid vesicles composed of 25% dioleoyl-sn-glycero-3-phospho-L-serine and 75% 1,2-dioleoyl-sn-glycero-3-phosphocholine (both from Avanti Polar Lipids, Inc; Alabaster, AL, United States). Clotting times were determined using the ST8 clotting instrument (Diagnostica Stago, Parsippany, NJ, United States).
TF and FXIa activity was calculated from calibration curves built by sequential dilutions of human FXIa (a gift from Dr. R. Jenny, Haematologic Technologies, Inc., Essex Junction, VT, United States) or relipidated TF1-242 (a gift from Dr. R. Lundblad, Baxter Healthcare Corp., Duarte, CA, United States) in pooled 10-donor plasma. Quantifiable amounts of plasma TF and FXIa activity were from 0.5 pM and 10 pM, respectively. Laboratory personnel were “blinded” to the status of samples. Age- and sex-matched healthy individuals (n = 12) recruited from the hospital staff showed no detectable TF or FXIa activity.
Statistical analysis
Data were presented as mean ± standard deviation or median and interquartile range as appropriate. Continuous variables were checked for normal distribution by the Shapiro-Wilk statistics and compared by the Student’s t-test when normally distributed or by the χ2, Mann-Whitney, or Wilcoxon test for non-normally distributed variables. To assess linear dependence between variables the Pearson correlation coefficient (Pearson’s r) for normally distributed variables or Spearman’s rank correlation coefficient (Spearman’s rho) for non-normally distributed variables were calculated. P <0.05 was considered statistically significant.
RESULTS
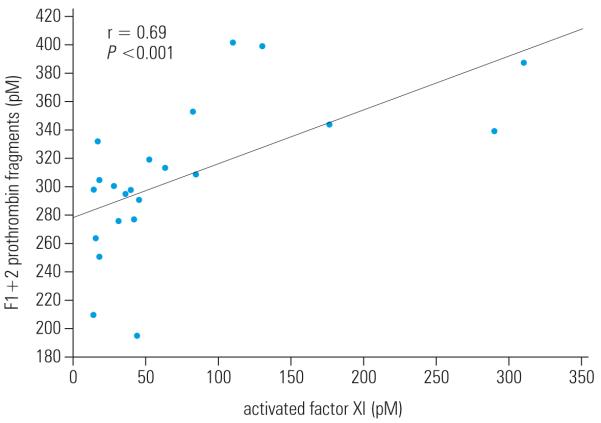
A total of 53 patients were studied (TABLE 1). The time since myocardial infarction to blood collection ranged from 1 to 12 years with a median of 7 years. The coagulant TF activity was detectable in 20 patients (37.7%) with systolic HF due to ischemic cardiomyopathy, while FXIa was found in 22 individuals (41.5%). Both parameters were detectable in 20 patients (37.7%), and all of the patients with TF activity had also circulating FXIa. The demographic, clinical, and routine laboratory data in the subgroups of patients with and without TF and FXIa are summarized in TABLE 1. Subjects without circulating active TF and FXIa had higher frequency of arterial hypertension, whereas patients with detectable TF and FXIa activity were younger and had elevated frequency of previous myocardial infarction. Additionally, the latter had higher F1+2 prothrombin fragments than the remaining subjects (TABLE 1). Moreover, circulating FXIa was positively associated with F1+2 (r = 0.69, P <0.001, FIGURE). No other laboratory variables showed associations with FXIa (data not shown).
TABLE 1.
Characteristics of patients with systolic heart failure due to ischemic cardiomyopathy
| Variable | The whole study group n = 53 (100%) |
TF+, FXIa+ n = 20 (37.7%) |
TF−, FXIa− n = 31 (58.5%) |
P |
|---|---|---|---|---|
| age, yrs | 61.5 ±8.3 | 57.9 ±7.1 | 63.7 ±8.5 | 0.01 |
| male sex, n (%) | 40 (75.5) | 13 (24.5) | 25 (47.2) | NS |
| BMI, kg/m2 | 28.8 ±3.7 | 28.7 ±3.4 | 28.6 ±3.9 | NS |
| myocardial infarction, n (%) | 18 (34.0) | 15 (28.3) | 1 (1.9) | <0.0001 |
| arterial hypertension, n (%) | 38 (71.7) | 11 (20.8) | 26 (49.1) | 0.02 |
| current smoking, n (%) | 17 (32.1) | 9 (17.0) | 7 (13.2) | NS |
| diabetes mellitus, n (%) | 11 (20.8) | 3 (5.7) | 7 (13.2) | NS |
| medication | ||||
| statins, n (%) | 44 (83.0) | 17 (32.1) | 27 (50.9) | NS |
| aspirin, n (%) | 46 (86.8) | 16 (30.2) | 28 (52.8) | NS |
| ACEI, n (%) | 35 (66.0) | 10 (18.9) | 23 (43.4) | NS |
| ARA, n (%) | 9 (17.0) | 3 (5.7) | 6 (11.3) | NS |
| β-blockers, n (%) | 30 (56.6) | 9 (17.0) | 19 (35.8) | NS |
| digoxin, n (%) | 7 (13.2) | 5 (9.4) | 2 (3.8) | NS |
| laboratory parameters | ||||
| TC, mM | 4.5 [4.0–5.0] | 4.5 [4.1–5.1] | 4.4 [3.8–5.0] | NS |
| LDL-C, mM | 2.7 [2.4–3.1] | 2.7 [2.3–3.3] | 2.7 [2.4–3.1] | NS |
| HDL-C, mM | 1.2 [1.0–1.3] | 1.2 [1.0–1.3] | 1.1 [1.0–1.3] | NS |
| triglycerides, mM | 1.5 [1.1–2.1] | 1.6 ±0.7 | 1.6 ±0.6 | NS |
| creatinine, μM | 96.6 ±28.3 | 94.0 [81.5–112.5] | 97 [78–120] | NS |
| hs-CRP, mg/l | 2.6 [1.6–3.8] | 2.6 [1.4–3.7] | 2.2 [1.6–4.1] | NS |
| glucose, mM | 5.3 [4.8–5.9] | 5.2 [4.8–5.6] | 5.3 [4.8–6.3] | NS |
| platelets, ×1000/μl | 226 [200–261] | 235 [210–286] | 220 [187–258] | NS |
| fibrinogen, g/l | 3.7 [3.0–4.8] | 3.4 [2.9–4.2] | 3.9 [3.1–4.9] | NS |
| PAI-1:Ag, ng/ml | 15.0 [11.4–20.7] | 17.3 [10.5–22.0] | 14.9 [11.9–20.7] | NS |
| tPA:Ag, ng/ml | 10.8 [9.2–13.2] | 11.0 [8.7–13.3] | 10.7 [9.6–12.6] | NS |
| F1+2, pM | 221.3 [185.4–297.4] | 300.7 [275.5–339.0] | 191.8 [171.2–221.1] | <0.0001 |
Values are mean ± SD or median [25–75%]
Abbreviations: ACEI – angiotensin-converting enzyme inhibitors, ARA – angiotensin II receptor subtype 1 antagonists, BMI – body mass index, FXIa – activated factor XI, FXIa+ – patients with detectable FXIa, FXIa− – patients with undetectable FXIa, F1+2 – prothrombin fragments 1+2, HDL-C – high-density lipoprotein cholesterol, hs-CRP – high-sensitivity C-reactive protein, LDL-C – low-density lipoprotein cholesterol, NS – nonsignificant (P >0.05), PAI-1:Ag – plasminogen activator inhibitor-1 antigen, SD – standard deviation, TC – total cholesterol, TF – tissue factor, TF+ – patients with detectable TF, TF− – patients with undetectable TF, tPA – tissue plasminogen activator
Figure.
Linear correlation between activated factor XI and F1+2 prothrombin fragments
Among the patients with active TF in plasma, 15 subjects (28.3%) had TF below the quantitation limit (<0.5 pM), and 5 subjects (9.4%) had higher quantifiable TF levels (a median of 0.5; interquartile range, 0.7 pM), including only 1 subject with 1.2 pM TF. Twenty-two patients (41.5%) had detectable FXIa levels, which ranged from 14 to 310 pM with a median of 43 (interquartile range, 65) pM; 17 of 22 FXIa-positive patients had FXIa below 100 pM.
The time since myocardial infarction was similar in both TF-positive and -negative patients (medians, 6 vs. 7 months). This held true also for patients positive toward FXIa (medians, 6 vs. 7 months).
Analysis of echocardiographic parameters (TABLE 2) showed that in patients with detectable TF activity, LA and RVDD were larger than in TF-negative subjects. RVSP was also significantly higher in TF-positive patients. Similar differences were observed in patients with circulating FXIa vs. those without this factor. No differences between the groups in other echocardiographic variables, including LVEF, were observed. The medication used had no effect on TF or FXIa activity (TABLE 1).
TABLE 2.
Echocardiographic parameters in patients with systolic heart failure due to ischemic cardiomyopathy
| Variable | The whole study group n = 53 (100%) |
TF+, FXIa+ n = 20 (37.7%) |
TF−, FXIa− n = 31 (58.5%) |
P |
|---|---|---|---|---|
| LA, mm | 43 [39–47] | 47 [44–49] | 41 [39–43] | 0.004 |
| RVDD, mm | 28.5 [25–33] | 33 [29–35] | 26 [24–29] | <0.0001 |
| LVDD, mm | 62 [58–68] | 62 [59–69] | 63 [55–68] | NS |
| LVSD, mm | 51[45–55] | 51.5 [47–55] | 51 [44–5] | NS |
| LVEF, % | 35 [30–41] | 35 [30–41] | 36 [30–40] | NS |
| IVS, mm | 9.0 [7.0–11.8] | 9.0 [6.8–11.6] | 10 [7–12] | NS |
| PW, mm | 9 [8–11] | 9 [8–12] | 9 [8–11] | NS |
| Ao, mm | 27 [25–29] | 28 [26–32] | 27 [24–29] | NS |
| RVSPa, mmHg | 37.5 [32.5–43.5] | 40 [36–53] | 33 [28–42] | 0.02 |
Values are mean ± SD or median [25–75%]
means ± SD or medians [25–75%] were calculated for n = 30
Abbreviations: Ao – ascending aorta, IVS – interventricular septum, LA – left atrium, LVDD – left ventricular diastolic diameter LVEF – left ventricular ejection fraction, LVSD – left ventricular systolic diameter, PW – posterior wall, RVDD – right ventricular diastolic diameter, RVSP – right ventricular systolic pressure, others – see TABLE 1
Separate analyses of the patients who were positive toward TF and FXIa vs. those who were not yielded the same differences as shown in TABLES 1 and 2. (data not shown).
DISCUSSION
Our study shows that about 40% of stable patients with systolic HF due to ischemic cardiomyopathy and normal sinus rhythm have coagulant TF activity and FXIa in plasma. The presence of these factors showed association with some specific echocardiographic variables. Notably, HF patients with both active TF and FXIa in plasma were characterized by larger LA and RVDD and higher RVSP than the remaining subjects. This suggests that even in patients with sinus rhythm and HF, higher pulmonary pressure, combined with increased LA diameter, predisposes to blood hypercoagulability and a prothrombotic state. Additional evidence for this concept stems from the observation that circulating FXIa and TF are associated with enhanced thrombin formation, reflected by increased F1+2 concentrations. It confirms that the presence of both factors has a major role in hypercoagulability observed in HF and may result in thromboembolic manifestations, particularly ischemic stroke or transient ischemic attacks. It has been shown that LA enlargement is a negative prognostic factor for survival in patients with stroke, congestive HF, and myocardial infarction. Dilated left ventricular (LV) chamber sizes and ischemic cardiomyopathy are independently associated with LV thrombi.19
We have shown previously that 96% of the patients with acute myocardial infarction and 76% of CAD patients with a history of myocardial infarction have circulating FXIa.1 Thirty-eight per cent of acute myocardial infarction patients and only 6% of stable CAD patients showed detectable TF activity.1 In the current study, the proportions of patients with active TF and those with FXIa were almost identical. This observation, in combination with elevated F1+2 levels in those patients, suggests that FXIa was generated by thrombin via the TF-initiated pathway, and this reaction is not affected by other potent factors. One might expect that the proportion of the HF patients with CAD positive for active TF will be much lower than that of acute myocardial infarction patients, but the two numbers (38% vs. 37.7%) are quite similar.1 However, it should be stressed that the levels of TF were markedly lower in the present study than in the previous one,1 despite the fact that almost 40% of HF and acute infarction patients exhibit such activity. For thrombin formation, the levels of TF activity appear to be of utmost importance. On the other hand, FXIa was observed relatively infrequently in HF patients compared with those with acute or chronic CAD. The reason for these differences is not clear. It might by hypothesized that small circulating amounts of active TF accompanied by thrombin-induced FXIa generation is a novel characteristic of stable HF associated with CAD, in contrast to high amounts of TF and FXIa generated in acute coronary ischemia patients.
It has been reported that plasma TF activity leading to thrombin generation is associated with microparticle-bound TF,20 because soluble (non-cell-bound) forms of TF have little (if any) procoagulant activity.21 As a consequence, only those plasma samples that contain TF-bearing microparticles (probably originating from apoptotic cells, such as macrophages, smooth muscle cells, and endothelium) will show procoagulant TF activity. High levels of procoagulant endothelial microparticles are present in the circulating blood of patients with acute coronary syndromes.22 It might be speculated that microparticles are also the source of active TF in plasma collected from stable HF patients associated with CAD.
This study has several limitations. First, the size of the study group as well as that of the subgroups with detectable TF and FXIa activity is limited, and the results of such analyses should be interpreted with caution. Second, all laboratory measurements were performed on a single occasion. Third, we did not perform right-heart catheterization on asymptomatic patients with RVSP <40 mmHg, which did not allow an evaluation of potential associations of the parameters tested with mild pulmonary artery hypertension. Moreover, it has been suggested that statins and, to a lesser extent, angiotensin-converting enzyme inhibitors might suppress TF expression; however, to our knowledge, few, if any, reports have shown a reduced coagulant activity of TF in plasma following therapy with those agents.1,8 No data on such actions of statins or other medications on FXIa are available. The populations evaluated previously were limited and relatively homogenous in terms of the treatment administered,1 so we were unable to reliably assess drug-induced effects using our methodology.
In conclusion, we showed that detectable TF and FXIa activity leading to an elevated procoagulant potential can be measured in HF patients without atrial fibrillation. Enlarged LA and right ventricle are associated with the presence of these 2 coagulation factors. Clinical relevance of these observations needs to be validated in a larger prospective study. Our data suggest that TF and FXIa activity could be used as markers of enhanced thrombogenic potential in patients with systolic HF due to ischemic cardiomyopathy and thromboembolic complications such as ischemic stroke.
Acknowledgments
This study was supported by P01 HL46 703 grant from the National Institutes of Health (SB). We would like to thank Matthew Gissel for his technical support.
REFERENCES
- 1.Butenas S, Undas A, Gissel MT, et al. Factor XIa and tissue factor activity in patients with coronary artery disease. Thromb Haemost. 2008;99:142–149. doi: 10.1160/TH07-08-0499. [DOI] [PubMed] [Google Scholar]
- 2.Kim HK, Song KS, Park YS, et al. Changes of plasma tissue factor and tissue factor pathway inhibitor antigen levels and induction of tissue factor expression on the monocytes in coronary artery disease. Cardiology. 2000;93:31–36. doi: 10.1159/000006999. [DOI] [PubMed] [Google Scholar]
- 3.Minnema MC, Peters RJ, de Winter R, et al. Activation of clotting factors XI and IX in patients with acute myocardial infarction. Arterioscler Thromb Vasc Biol. 2000;20:2489–2493. doi: 10.1161/01.atv.20.11.2489. [DOI] [PubMed] [Google Scholar]
- 4.The Stroke Prevention in Atrial Fibrillation Investigators Predictors of thromboembolism in atrial fibrillation: I. Clinical features of patients at risk. Ann Intern Med. 1992;116:1–5. doi: 10.7326/0003-4819-116-1-1. [DOI] [PubMed] [Google Scholar]
- 5.Freudenberger RS, Hellkamp AS, Halperin JL, et al. SCD-HeFT Investigators Risk of Thromboembolism in Heart Failure An Analysis From the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Circulation. 2007;115:2637–2641. doi: 10.1161/CIRCULATIONAHA.106.661397. [DOI] [PubMed] [Google Scholar]
- 6.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 7.Edgington TS, Dickinson CD, Ruf W. The structural basis of function of the TF. VIIa complex in the cellular initiation of coagulation. Thromb Haemost. 1997;78:401–405. [PubMed] [Google Scholar]
- 8.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 9.Seligsohn U. Factor XI in haemostasis and thrombosis: past, present and future. Thromb Haemost. 2007;98:84–89. [PubMed] [Google Scholar]
- 10.Kannemeier C, Shibamiya A, Nakazawa F, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007;104:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von dem Borne PA, Bajzar L, Meijers JC, et al. Thrombin-mediated activation of factor XI results a thrombin-activatable fibrinolysis inhibitor-dependent inhibition of fibrinolysis. J Clin Invest. 1997;99:2323–2327. doi: 10.1172/JCI119412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steppich BA, Braun SL, Stein A, et al. TF activity predicts cardiovascular mortality in patients with acute myocardial infarction. Thromb J. 2009;7:11. doi: 10.1186/1477-9560-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 14.Kaikita K, Ogawa H, Yasue H, et al. Tissue factor expression on macrophages in coronary plaques in patients with unstable angina. Arterioscler Thromb Vasc Biol. 1997;17:2232–2237. doi: 10.1161/01.atv.17.10.2232. [DOI] [PubMed] [Google Scholar]
- 15.Siegemund A, Petros S, Siegemund T, et al. The endogenous thrombin potential and high levels of coagulation factor VIII, factor IX and factor XI. Blood Coagul Fibrinolysis. 2004;15:241–244. doi: 10.1097/00001721-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Cushman M, O’Meara ES, Folsom AR, Heckbert SR. Coagulation factors IX through XIII and the risk of future venous thrombosis: the Longitudinal Investigation of Thromboembolism Etiology. Blood. 2009;114:2878–2883. doi: 10.1182/blood-2009-05-219915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merlo C, Wuillemin WA, Redondo M, et al. Elevated levels of plasma prekallikrein, high molecular weight kininogen and factor XI in coronary heart disease. Atherosclerosis. 2002;161:261–267. doi: 10.1016/s0021-9150(01)00666-9. [DOI] [PubMed] [Google Scholar]
- 18.ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 19.Sharma ND, McCullough PA, Philbin EF, Weaver WD. Left ventricular thrombus and subsequent thromboembolism in patients with severe systolic dysfunction. Chest. 2000;117:314–320. doi: 10.1378/chest.117.2.314. [DOI] [PubMed] [Google Scholar]
- 20.Simak J, Gelderman MP. Cell membrane microparticles in blood and blood products: potentially pathogenic agents and diagnostic markers. Transfus Med Rev. 2006;20:1–26. doi: 10.1016/j.tmrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Morrissey JH, Macik BG, Neuenschwander PF, Comp PC. Quantitation of activated factor VII levels in plasma using a tissue factor mutant selectively deficient in promoting factor VII activation. Blood. 1993;81:734–744. [PubMed] [Google Scholar]
- 22.Mallat Z, Benamer H, Hugel B, et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]