Abstract
Acetaminophen (N-acetyl-para-aminophenol (APAP), paracetamol) is a commonly used analgesic and antipyretic agent. Although considered safe at therapeutic doses, accidental or intentional overdose causes acute liver failure characterized by centrilobular hepatic necrosis with high morbidity and mortality. Although many molecular aspects of APAP-induced cell death have been described, no conclusive mechanism has been proposed. We recently identified TNF-related apoptosis-inducing ligand (TRAIL) and c-Jun kinase (JNK)-dependent activation of the pro-apoptotic Bcl-2 homolog Bim as an important apoptosis amplification pathway in hepatocytes. In this study, we, thus, investigated the role of TRAIL, c-JNK and Bim in APAP-induced liver damage. Our results demonstrate that TRAIL strongly synergizes with APAP in inducing cell death in hepatocyte-like cells lines and primary hepatocyte. Furthermore, we found that APAP strongly induces the expression of Bim in a c-JNK-dependent manner. Consequently, TRAIL- or Bim-deficient mice were substantially protected from APAP-induced liver damage. This study identifies the TRAIL-JNK-Bim axis as a novel target in the treatment of APAP-induced liver damage and substantiates its general role in hepatocyte death.
Keywords: hepatocytes, Jun kinase, death receptor signaling, Bcl-2 family, paracetamol
Acetaminophen (N-acetyl-para-aminophenol (APAP), paracetamol) is a commonly used analgesic and antipyretic agent.1 Although considered safe at therapeutic doses, accidental or intentional overdose causes acute liver failure characterized by centrilobular hepatic necrosis with high morbidity and mortality.2 Despite substantial effort in the past to investigate the processes involved in APAP-induced liver toxicity, the exact molecular and biochemical mechanisms remain incompletely understood. There is a general consensus that the formation of a toxic electrophilic metabolite by the P450 system, N-acetyl-p-benzoquinone imine (NAPQI), is a prerequisite for hepatocyte injury.3 NAPQI is usually detoxified by glutathione in the liver. However, APAP overdose depletes hepatic glutathione so much that NAPQI covalently binds to cellular proteins leading to mitochondrial dysfunction. Thus, APAP inhibits mitochondrial respiration and causes a decrease in ATP levels in liver cells.4 More recently, in vivo study provided evidence for the formation of peroxynitrite in the mitochondria and the induction of mitochondrial damage.5 It is still under debate whether covalent binding to critical protein targets or oxidative stress as a consequence of mitochondrial glutathione depletion is the promoting factor of hepatocyte death. Most probably, however, liver cell death after APAP overdose is the consequence of interplay between multiple events. Furthermore, glutathione depletion and covalent binding to cellular proteins seems not to be sufficient to induce mitochondrial dysfunction resulting in liver failure. In experimental animal models, the progression and severity of APAP-induced liver toxicity is not only associated with the triggering of the membrane permeability transition and the collapse of mitochondrial membrane potential, but depends on the interplay of other death-inducing signals, both within and outside the hepatocytes.
The death mechanism(s) of APAP-induced liver damage is still a matter of debate. Clearly, typical signs of both necrotic and apoptotic cell death can be observed. There is also a significant overlap between events normally associated with apoptosis and APAP-induced cell death. For instance, mitochondrial events generally linked to apoptosis have been observed in APAP-induced hepatic cell death, including the proteolytic processing of the pro-apoptotic Bcl-2 family member Bid, the translocation of Bid and Bax to the mitochondria and the release of cytochrome c.6 In agreement with the role of Bcl-2 family members in APAP-induced hepatocyte death is that mitochondrial Bax translocation accelerates DNA fragmentation and cell death in APAP-induced liver damage.7 Moreover, a critical role of the stress kinase c-Jun N-terminal kinase (Jun kinase, JNK) has been demonstrated in both APAP-induced liver damage8 and other forms of hepatocyte apoptosis.9, 10, 11 Thus, APAP treatment leads to JNK activation, and administration of JNK inhibitors have a protective effect on APAP-induced hepatocyte death and associated liver damage. Similarly, a role of JNK has been postulated in TNFα12 and Fas ligand-mediated hepatocyte apoptosis and hepatitis.9
JNK is likely involved in death ligand-induced hepatocyte apoptosis by the phosphorylation and activation of downstream apoptosis effector molecules. In agreement with this notion, we have recently shown that JNK phosphorylates the pro-apoptotic Bcl-2 homolog Bim, and that JNK-activated Bim critically regulates Fas-induced hepatocyte apoptosis.9 Interestingly, we have seen that Fas-induced hepatocyte apoptosis is synergistically enhanced by the TNF-related apoptosis-inducing ligand (TRAIL) receptor signaling pathway via TRAIL-induced JNK activation and Bim phosphorylation. Similarly, Kaufmann et al.13 demonstrated that -galactosamine/lipopolysaccharide-induced hepatitis in mice, proceeding via TNF-α-mediated hepatocyte apoptosis, also depends on JNK and Bim. Finally, a beneficial effect of JNK inhibition has also been observed in hepatic ischemia-reperfusion injury.14
Given the critical role of JNK and Bim in various forms of hepatocyte apoptosis, and the similarity between APAP-induced liver damage and hepatitis mediated by the apoptosis pathway, we explored in this study the role of death receptor-induced JNK and associated Bim activation in APAP-induced liver damage. We here demonstrate that APAP-induced hepatocyte death is enhanced by the TRAIL signaling pathway. Similarly, APAP-induced liver damage is reduced in TRAIL-deficient mice. Treatment of mice, isolated hepatocytes or hepatoma cell lines with APAP results in an increase in Bim expression, which is dependent on APAP-induced JNK activation. Finally, we demonstrate that APAP-induced liver damage is significantly attenuated in Bim-deficient mice. These findings demonstrate a novel role of the BH3-only protein Bim in APAP-induced liver damage, and further accentuate the role of JNK and Bim in hepatocyte cell death.
Results
APAP-induced death is synergistically enhanced by TRAIL
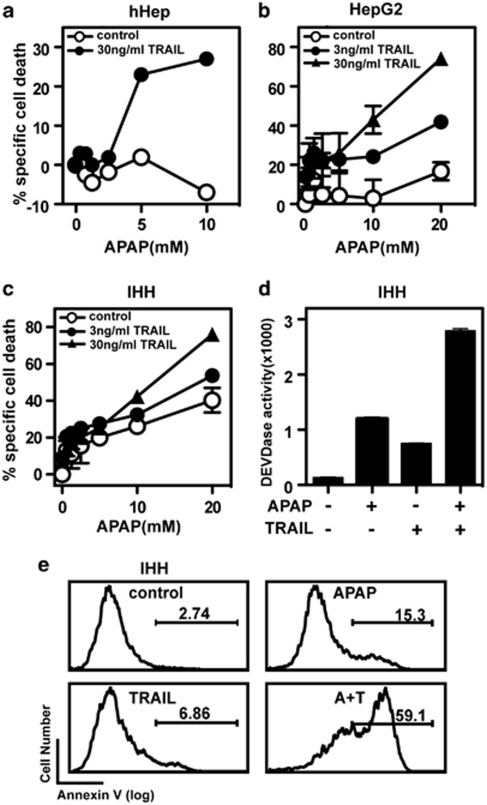
We have previously shown that TRAIL synergistically enhances Fas-induced apoptosis in hepatocytes in a JNK-Bim-dependent manner.9 We thus tested whether APAP-induced hepatocyte cell death is also enhanced by treatment the cells with TRAIL. Isolated primary human hepatocytes (hHep) were stimulated with increasing doses of APAP, in the presence or absence of low doses of TRAIL. Figure 1a illustrates that APAP alone failed to induce significant cell death in primary hHeps. Similarly, TRAIL alone, at 3 and 30 ng/ml, did not promote any death-inducing activities. However, combined treatment of cells with TRAIL and increasing doses of APAP synergistically induced cell death in primary hepatocytes. Similar results were also confirmed in the hepatoma cell line HepG2 and immortalized human hepatocytes (IHHs) (Figures 1b and c). The synergistic induction of cell death in IHH was paralleled by increased caspase 3 activity (DEVDase activity) and phosphatidyl serine externalization. These data confirm that TRAIL synergistically enhances APAP-induced cell death in hepatocytes and hepatocyte-like cells in vitro.
Figure 1.
TRAIL synergizes with APAP in inducing cell death. Human hepatocytes (hHep) (a), the hepatoma cell line HepG2 (b) or immortalized human hepatocytes (IHH) (c) were preincubated with buffer control or increasing concentrations of TRAIL, prior stimulation with different doses of APAP. Cell death was assessed by MTT assay (a–c) in primary hepatocytes, HepG2 and IHH cells, by DEVD cleavage assay (d) or Annexin V staining (e) in control IHH cells or IHH cells treated with 10 mM APAP, 30 ng TRAIL or the combination thereof, respectively. Mean values±S.D. of triplicates are shown for MTT and DEVDase assays, which were repeated three times, yielding similar results. A typical experiment out of two is shown for Annexin V staining
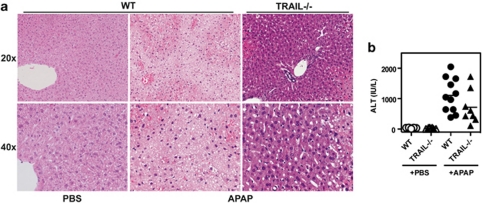
We next investigated the relevance of TRAIL-mediated enhancement of APAP-induced cell death also in vivo. Wild-type (WT) C57Bl/6 mice or TRAIL-deficient mice were treated with overdose of APAP (400 mg/kg body weight) and liver damage was assessed 5 h later by tissue histology and serum transaminases. Figure 2a illustrates that APAP induced severe tissue destruction with large areas of damaged and necrotic hepatocytes in WT animals. In contrast, histological alterations were reduced in APAP-treated TRAIL-deficient mice. Reduced liver damage of TRAIL-deficient mice compared with WT animals after APAP treatment was also confirmed by transaminase activity in the sera. Although treatment of WT animals with APAP resulted in a strong increase in serum ALT activity, reduced levels were seen in APAP-treated TRAIL-deficient mice. These data indicate that endogenous TRAIL contributes to APAP-induced hepatocyte death and liver damage also in vivo.
Figure 2.
TRAIL is required for efficient APAP-induced liver damage in vivo. Wild type (WT) or TRAIL-deficient mice (TRAIL−/−) were treated with PBS as control or APAP (400 mg/kg body weight), and liver damage was analyzed by histology (a) and transaminase levels (AST) in serum (b) after 5 h. Pooled experimental data from 8 to 12 mice per group are shown
APAP induces expression of Bim
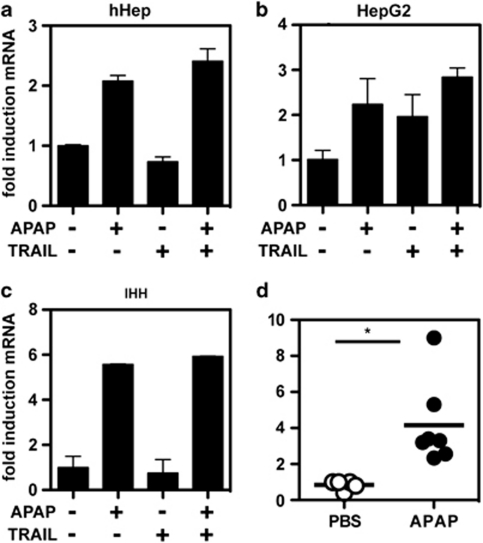
Our previous studies have demonstrated an important role of Bim in hepatocyte apoptosis.9 We thus investigated whether APAP or TRAIL would affect Bim expression in primary hepatocytes and hepatocyte-like cell lines. Analysis of Bim expression by western blot revealed that treatment of primary hHep (Figure 3a), HepG2 (Figure 3b) and IHH cells (Figure 3c) with APAP resulted in a strong induction of Bim expression. In contrast, no increase in Bim levels were seen in primary hHeps and HepG2 cells after treatment with TRAIL, and only a slight induction was observed in IHH cells. Combined treatment of cells (HepG2 and IHH), with TRAIL and APAP did not further increase expression of Bim. In agreement with these in vitro data, increased Bim protein levels were also observed in liver samples from APAP-treated mice (Figure 3d).
Figure 3.
APAP induces Bim protein expression. Human hepatocytes (a), HepG2 (b) or IHH (c) cells were treated with APAP, TRAIL or both for 6 h, and Bim protein expression was analyzed by western blot. (d) Liver samples of PBS- or APAP-treated wild-type mice were analyzed for the expression of Bim by western blot. JNK or tubulin was used to normalize protein loadings
To define in more detail whether APAP induces an increased Bim protein levels either by stabilization of the protein or by promoting Bim gene expression, we analyzed Bim mRNA expression after treatment with APAP and/or TRAIL by quantitative RT-PCR. TRAIL was found to be an inefficient inducer of Bim mRNA expression in primary hepatocytes and IHH cells, and moderately induced Bim expression in HepG2 cells. In contrast, APAP strongly promoted Bim mRNA expression in all cells tested (Figures 4a–c). No synergy between APAP and TRAIL in promoting Bim mRNA expression was detected. Bim mRNA levels were also significantly increased in the liver of APAP-treated WT mice compared with PBS-treated control mice (Figure 4d).
Figure 4.
APAP induces Bim transcription. Human hepatocytes (a), HepG2 (b) or IHH (c) cells were treated with with 10 mM APAP, 30 ng TRAIL or the combination thereof for 6 h, and Bim expression was analyzed by quantitative RT-PCR. (d) Wild-type mice were treated with PBS or APAP, and Bim expression was analyzed in liver samples by quantitative RT-PCR after 5 h. GAPDH was used to normalize Bim expression levels. Mean values±S.D. of triplicates are shown for fold induction of mRNA levels. Pooled data from 6 to 7 mice per group are shown. *P=0.011 (unpaired Student's t-test)
APAP induces Bim promoter activity in a JNK-dependent manner
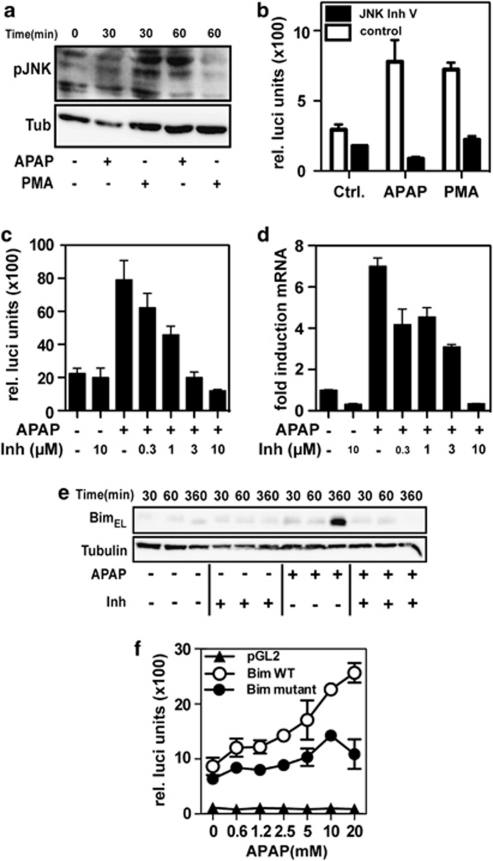
JNK has been previously implicated in the transcriptional control of Bim expression via an AP-1-dependent pathway. Furthermore, JNK activity has been shown to participate in APAP-induced liver disease.8, 15 In accordance with these reports, we demonstrated that APAP alone induced a robust JNK activation as measured by the detection of phospho-JNK by western blot (Figure 5a). APAP-induced JNK activation was comparable to that induced by phorbol 12-myristate 13-acetate used as positive control. To investigate the role of JNK in APAP-induced Bim gene expression we used a luciferase reporter construct containing 0.8 kb of the Bim proximal promoter.16 Although this Bim promoter construct was constitutively active in control-treated cells, relatively low concentrations of APAP (2.5 mM) induced the Bim reporter construct (Figure 5b). Higher concentrations further induced Bim promoter activity by about fourfold above levels of unstimulated cells (Figures 5b and c). Inhibition of JNK, by the pharmacological inhibitor, resulted in a strong inhibition of APAP-induced Bim promoter activity (Figures 5b and c), Bim mRNA expression (Figure 5d) and Bim protein expression (Figure 5e).
Figure 5.
APAP-induced Bim transcription is JNK- and Foxo3a-dependent. (a) IHH cells were stimulated with APAP (10 mM) or, as positive control, phorbol 12-myristate 13-acetate (30 ng/ml) and phospo-JNK and tubulin protein levels were analyzed at different time points by western blot. (b) IHH cells were transfected with the wild-type (WT) Bim reporter construct, pre-treated with JNK V inhibitor (10 μM) and stimulated with APAP (10 mM) or phorbol 12-myristate 13-acetate (30 ng/ml). (c) Similarly, IHH cells were transfected with the WT Bim reporter construct, pretreated with different concentrations of the JNK inhibitor V and stimulated with APAP (10 mM). Luciferase activity was measured and normalized to β-galactosidase. (d) IHH were pretreated with JNK inhibitor V and stimulated with APAP (10 mM). Bim mRNA expression was measured by quantitative RT-PCR (6 h). (e) IHH cells were treated as described above and Bim were analyzed at different time points by western blot. (f) IHH cells were transfected with empty vector (pGL2), the wild-type (Bim WT) or the Foxo3a mutant Bim reporter constructs (Bim Foxo3a), and stimulated with APAP (10 mM) for 6 h. Luciferase activity was measured and normalized to β-galactosidase activity. Mean values±S.D. of triplicates are shown for relative luciferase units and fold induction of mRNA levels. The experiments have been repeated three times, yielding similar results
These findings indicate that JNK has a role in APAP-induced Bim expression in liver cells. Foxo3a has also been reported to be involved in the regulation of Bim expression and associated apoptosis in various forms of liver disease.17 In agreement with this proposed role of Foxo3a, we also found that APAP-induced Bim promoter activity in IHH cells was reduced when Foxo3a binding sites were mutated in the Bim promoter, indicating that APAP-induced Bim expression is also regulated in part by the transcription factor Foxo3a (Figure 5f).
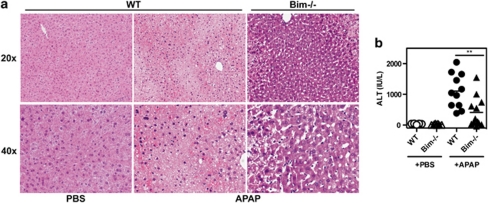
APAP-induced liver damage is Bim-dependent
Given the potent Bim-inducing activity of APAP in vivo and in vitro, we analyzed the role of Bim in APAP-induced liver damage in vivo. Wild-type and Bim-deficient C57Bl/6 mice were treated with an overdose of APAP (400 mg/kg body weight) and liver damage was analyzed by histological alterations and serum transaminase levels 5 h later. While in WT animals APAP treatment resulted in the extensive hepatic cell death and large necrotic lesions described earlier (Figure 6a), tissue damage was substantially reduced in Bim-deficient mice, indicating that Bim is an important determinant of APAP-induced liver damage. These findings were confirmed when analyzing serum transaminase levels. While APAP treatment of WT mice resulted in a strong increase of ALT levels, significantly reduced levels were seen in drug-treated Bim-deficient mice. These findings support a critical role of Bim in APAP-induced hepatocyte death and liver damage.
Figure 6.
Bim is required for efficient APAP-induced liver damage in vivo. Wild-type (WT) or Bim-deficient mice (Bim−/−) were treated with PBS as control or 400 mg/kg body weight APAP, and liver damage was analyzed by histology (a) and transaminase levels (AST) in serum (b). Pooled data from 10 to 12 mice per group are shown. **P=0.0047 (unpaired Student's t-test)
Discussion
Owing to its unique position, the liver is a frequent target of damage induced by drugs, xenobiotics and oxidative stress. Although the liver has a sophisticated detoxification system, overdoses of hepatotoxic agents may eventually lead to liver cell death, acute liver damage and liver failure. APAP is a well-characterized liver-damaging substance, whose hepatotoxic activities have been widely studied. Nevertheless, so far investigations have failed to identify a single critical pathway involved in APAP-induced liver damage, and rather suggest a multitude of factors and signaling events leading to the observed effects in vitro and in vivo. Thus, APAP-induced liver damage is the interplay of several distinct death mechanisms.
Our study, identifying TRAIL, JNK and Bim as critical elements in APAP-induced hepatocyte cell death in vitro and in vivo, has added new elements into this scheme of underlying mechanisms of APAP-induced liver damage. It also provides interesting new links between known pathways of APAP-induced hepatocyte death and other forms of hepatitis. In particular, JNK has been previously associated with APAP-induced and other forms of hepatocyte death.8, 10 Our findings that APAP induces JNK activation, and that JNK activity is critical for APAP-induced Bim expression further supports an important role of this stress kinase pathway in the regulation of liver cell death. Recent findings suggest that APAP-induced JNK activation may be mediated via an ASK1-dependent manner.18 Interestingly, we have also previously demonstrated a regulatory role of JNK activation in death ligand-induced hepatocyte apoptosis. JNK was found to be critical for the phosphorylation and activation of Bim in response to Fas and TNF receptor activation.9, 13 Similarly, we describe here a critical role of JNK in APAP-induced Bim induction. In contrast to our previous results on death receptor-induced hepatitis, our present data suggest that APAP-induced JNK activation may be rather involved in activation of the Bim promoter and induction of Bim expression. APAP treatment induced a strong increase in Bim promoter activity, mRNA and protein expression, which was significantly inhibited by a JNK inhibitor. Although phosphorylation of Bim has been observed in some circumstances, the induction of Bim expression appears to be the main pathway by which JNK is involved in APAP-induced hepatocyte death.
Our study also substantiates a relevant role for Bim in APAP-induced liver damage, and further supports a major function of this Bcl-2 homolog protein in hepatocyte cell death in general. Although Fas- and TNF receptor-induced hepatocyte cell death, a prototypical type II cell, has been suggested to depend on caspase 8-mediated cleavage of the BH3-only protein Bid to tBid and subsequent amplification of the apoptosis signal via the mitochondrial pathway, our own results and those by others demonstrate that Bim is also an essential component of this pathway. Absence of Bim almost completely abrogated anti-Fas antibody-induced hepatitis, and synergized with lack of Bid in protecting from TNF-α-mediated liver damage.9, 13 Similarly, we find here that Bim is an important component of the APAP-initiated hepatotoxic signaling pathway.
Although we demonstrate here also an important amplifying role of TRAIL in APAP-induced hepatocyte death in vitro and liver damage in vivo, our results are not fully conclusive as how TRAIL is involved in these processes. Clearly, we failed to find strong evidence that TRAIL signaling may be involved in the induction of Bim expression. In general, TRAIL did either not induce Bim expression or was only a weak trigger. Thus, TRAIL may directly or indirectly contribute to APAP-induced Bim expression depending on varying circumstances. Amplification of the death signal in vivo and associated hepatocyte death by TRAIL may enhance inflammatory responses, which could further contribute to induction of Bim expression. Our previous results on Fas-induced hepatocyte death rather suggest, however, that TRAIL-induced signaling pathways may be more critical for the activation of Bim by phosphorylation. For instance, absence of TRAIL led to reduced JNK activation and Bim phosphorylation. Similarly, TRAIL may be involved in activating APAP-induced Bim by JNK-mediated phosphorylation.9 Disturbing in this hypothesis is the fact that strong JNK activation, as measured by detection of phospho-JNK, was much stronger in response to APAP than TRAIL treatment. Clearly, though, TRAIL signaling can activate the JNK pathway, and differential activation of JNK1 or JNK2 by APAP and TRAIL may result in APAP-mediated and JNK-dependent Bim expression, or TRAIL-induced and JNK-mediated Bim phosphorylation. Although our results are not fully conclusive in this respect, they support a synergistic role of TRAIL, JNK and Bim in APAP-induced liver damage.
An open question is the cellular source of TRAIL in vivo. TRAIL expression has been previously demonstrated in various liver-homing leukocytes, including Kupffer cells and NK cells and NKT cells.19, 20 Interestingly, immune cells and inflammation have been implicated in APAP-induced liver damage, particularly as an amplifying element in this form of liver disease. APAP-induced macrophage activation and subsequent amplification of tissue damage has been shown to involve toll-like receptor 9 and NALP3 inflammasome activation.21 Similar processes may also result in TRAIL expression in Kupffer cells and amplification of APAP-induced liver damage.
Although our data support a role of the TRAIL-JNK-Bim axis in a rather apoptotic form of cell death in response to APAP, APAP-induced hepatocyte cell death clearly shows also more necrosis-like features. Currently it is unknown whether necrosis and apoptosis represent two independent events, whether apoptosis occurs in some cells and necrosis in others, or whether the two pathway are intimately linked. Although the absence of TRAIL and Bim likely has a strong inhibitory effect on the apoptosis pathway, it should not affect APAP-induced necrosis if occurring independently. Histology of APAP-treated liver tissue from both TRAIL and Bim deficient mice revealed strongly reduced liver damage and necrotic areas, suggesting that APAP-induced apoptosis and necrosis depend on each other. Detailed examination, however, also reveals that surviving hepatocytes still show features of stressed cells, for example, strong vacuolization. Thus, blocking apoptosis may strongly reduce tissue damage, however, may not be sufficient to block APAP-induced cellular damage.
Nevertheless, our present data illustrate that inhibition of either TRAIL or Bim results in a substantial amelioration of disease pathology. Thus, selective inhibition of this signal transduction pathway may represent a novel and successful therapy in the treatment of APAP overdose-associated liver damage. Current treatment of APAP poisoning involved administration of N-acetylcysteine, which helps to build up cellular glutathione levels and thus to detoxify NAPQI. This treatment targets, however, not the cell death-inducing pathway per se but seeks to remove the trigger of tissue destruction, which may be often too late. Targeting critical signaling elements in APAP-induced liver cell death may thus represent a more efficient strategy.
Materials and Methods
Cell and cell lines
HepG2 (a hepatoma cell line) and IHH were kindly provided by JF Dufour (Institute for Clinical Pharmacology, Inselspital, University of Bern). Cells were cultured in IMDM medium, containing 5% FCS, 1% -glutamine and 0.1% gentamicin. Primary hHeps were isolated as described previously from the liver tissue of consented patients undergoing liver surgery. Human hepatocytes were enzymatically dissociated from human liver samples using a two-step enzymatic microperfusion technique with collagenase and kept on ice in suspension.22 Human hepatocytes were cultured in Williams E medium containing 10% FCS, 1% -glutamine, 0.1% gentamicin, 0.01% insulin/aprotinin and 0.05% transferrin.
Cell death assay (MTT)
HepG2 and IHH were grown in logarithmic phase, harvested and plated in 96-well flat bottom plates. Primary hHeps were directly seeded into collagen-coated 96-well flat bottom plates. After overnight adherence, medium was changed and cells were treated with increasing concentrations of recombinant human TRAIL (untagged form, R&D Systems, Schonenbuch/Basel, Switzerland) and APAP (paracetamol, Sigma-Aldrich, St. Louis, MO, USA) for 16 h. Cell viability was then assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide MTT assay (Sigma-Aldrich) and normalized to untreated controls.9
Annexin V staining
After different treatments, HepG2 and IHH cell death was monitored by Annexin V-FITC staining (eBioscience, San Diego, CA, USA) at different time points depending on the experiment. Briefly, the cells were harvested, centrifuged and resuspended in 50 ml Annexin V binding buffer (10 mM Hepes pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2 and 1.5 mM CaCl2) containing Annexin V-FITC. The cells were stained for 10 min in the dark, washed two times with Annexin V binding buffer, resuspended into 200 ml Annexin V binding buffer and analyzed on a FACScan flow cytometer using Cell Quest software (Becton Dickinson, Heidelberg, Germany). The percentage of Annexin V-FITC-positive cells was then calculated using the Flow Jo software 8.7.3 (FlowJo LLC Software package, Ashland, OR, USA).
Caspase activity assay (DEVDase activity)
Cells were treated with TRAIL and/or APAP as indicated, and harvested after 6 h. Cells were then washed and lysed for 10 min on ice with 200 ml PBS containing 1% Triton X-100. After centrifugation for 5 min at 16 000 × g at 4 °C, supernatant was harvested and 50 ml were mixed with 150 ml Hepes buffer (100 mM Hepes, pH 7.5, 20% glycerol, 0.5 mM EDTA, 5 mM DTT) containing 100 mM Ac-DEVD-AFC (Alexis Biochemicals, San Diego, CA, USA). Reactions were incubated for 1 h at 37°C, and enzymatic activity was measured on a spectrofluorometer (400 nm excitation, 505 nm emission). Background fluorescence was measured by incubating caspase substrate with lysis buffer.
Western blot analysis
Cells were treated as indicated, before lysis in cell lysis buffer containing 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS and 50 mM Tris, pH 7.4. Cell lysates were separated by electrophoresis and transferred to nitrocellulose. JNK, phosphorylated JNK and Bim were detected by incubation of membranes with the respective primary antibodies (anti-JNK and anti-phospho JNK, Cell Signaling Technology, Allschwil, Switzerland; anti-Bim antibody dilution, Sigma-Aldrich) and corresponding horseradish peroxidase–labelled secondary antibodies. Signals were visualized by enhanced chemiluminescence and detected in a Fujifilm LAS 4000 imaging system (Fujifilm Corporation, Akasaka, Japan).
Quantitative PCR
Human and mouse Bim mRNA expression was detected by quantitative RT-PCR as described earlier.16 Briefly, cells were lysed in TRI reagent (Sigma-Aldrich) and RNA was isolated. RNA was DNase-treated (Promega, Madison, WI, USA) and 2 mg of RNA were reverse transcribed using a high capacity kit (Applied Biosystems, Rotkreuz Zug, Switzerland). Real-time PCR was performed in an Applied Biosystems Real-time PCR 7500 machine using SYBR green and Quantitec primer assays (QIAGEN, Hilden, Germany). GAPDH was used to normalize Bim expression levels.
Bim promoter assay
The murine bim p0.8 reporter was generated by cloning 0.8 kb upstream of the transcription start site of the murine bim gene (Genbank AL805950) into the pGL2-basic plasmid (Promega). The two FOXO sites present in bim p0.8 promoter construct (FOXO binding site 1 ggaaacaac → ggttcgaag; position −618 to −626 bp upstream of bim exon1; FOXO binding site 2 gtaaacac → gtagtggc; position −168 to −175 bp) were modified by site-directed mutagenesis to generate bim p0.8 (mut1, 2)-pGL2-basic luciferase reporter construct. Wild-type and mutated bim reporter constructs, and β-galactosidase expression vector for transfection control, were co-transfected into IHH cells using Amaxa Nuleofection kit (Amaxa, Cologne, Germany). Cells were plated on a 10 cm plate for 1 day, harvested and distributed on a 96-well flat bottom plate. Cells were then incubated with increasing concentrations of buffer control, APAP (10 mM) or TRAIL (30 ng/ml) for 6 h. In some experiments, cells were preincubated with different concentrations of the JNK V Inhibitor (Calbiochem, Darmstadt, Germany). Cells were washed once with PBS, and then cell lysates were prepared by adding lysis buffer directly to the cells for 20 min at RT on a rocking table. Lysates were transferred to a V-bottom plate and centrifuged for 10 min with 2000 × g at RT. A total volume of 40 ml was used for the reporter assay and 40ml for the β-galactosidase assay. Luciferase activity was quantified using Orion Microplate Luminometer (Pforzheim, Germany).
In vivo experiments
After a 24 h fasting period, adult TRAIL-deficient (TRAIL−/−), Bim-deficient (Bim−/−) mice and WT C57BL/6 mice were injected i.p. with 400 ml PBS or 10 mg APAP (400 mg/kg body weight) dissolved in warm PBS. Mice were killed at the indicated time points, and serum and liver samples were collected. Liver samples were either snap-frozen in liquid nitrogen for subsequent isolation of mRNA and proteins, or fixed in 4% paraformaldehyde in PBS for paraffin embedding and histology. Serum AST levels were measured using a commercially available kit (TECO Diagnostics, Anaheim, CA, USA). All animal experiments were reviewed and approved by the Animal Experimentation Review Board of the State of Bern.
Statistical analysis
Statistical differences were analyzed using unpaired two-tailed Student's t-test. P-values<0.05 were considered significant.
Acknowledgments
We thank Immunex/Genetech for providing the TRAIL-deficient mice, Andreas Strasser and Georg Häcker for the Bim-deficient mice and Jean-François Dufour for IHH cells. This work was supported by grants received from the Swiss National Science Foundation to TB and NC, and the Ettore and Valeria Rossi Foundation to NC.
Glossary
- APAP
N-acetyl-para-aminophenol, acetaminophen, paracetamol
- ATP
adenosine triphosphate
- Bax
Bcl-2-associated X protein
- Bcl-2
B cell lymphoma gene 2
- BH3
Bcl-2 homology domain 3
- Bid
BH3-interacting domain death agonist
- Bim
Bcl-2-interacting mediator of cell death
- DISC
death-inducing signaling complex
- FADD
Fas-associated death domain
- FasL
Fas ligand
- JNK
c-Jun N-terminal kinase
- FoxO3a
forkhead box O3a
- MPT
membrane permeability transition
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NAPQI
N-acetyl-p-benzoquinone imine
- NK cell
natural killer cell
- NKT cell
natural killer T cell
- ROS
reactive oxygen species
- TNF-α
tumor necrosis factor-α
- TRAIL
TNF-related apoptosis-inducing ligand
The authors declare no conflict of interest.
Footnotes
Edited by G Melino
References
- Rumack BH. Acetaminophen misconceptions. Hepatology. 2004;40:10–15. doi: 10.1002/hep.20300. [DOI] [PubMed] [Google Scholar]
- Lee WM. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology. 2004;40:6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42:110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Harman AW. Acetaminophen toxicity results in site-specific mitochondrial damage in isolated mouse hepatocytes. J Biol Chem. 1991;266:5049–5054. [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, et al. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, et al. Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: roles of cytochrome c, Bax, Bid, and caspases. Toxicol Appl Pharmacol. 2003;191:118–129. doi: 10.1016/s0041-008x(03)00240-0. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Corazza N, Jakob S, Schaer C, Frese S, Keogh A, Stroka D, et al. TRAIL receptor-mediated JNK activation and Bim phosphorylation critically regulate Fas-mediated liver damage and lethality. J Clin Invest. 2006;116:2493–2499. doi: 10.1172/JCI27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- Takamura M, Matsuda Y, Yamagiwa S, Tamura Y, Honda Y, Suzuki K, et al. An inhibitor of c-Jun NH2-terminal kinase, SP600125, protects mice from D-galactosamine/lipopolysaccharide-induced hepatic failure by modulating BH3-only proteins. Life Sci. 2007;80:1335–1344. doi: 10.1016/j.lfs.2006.12.034. [DOI] [PubMed] [Google Scholar]
- Das M, Sabio G, Jiang F, Rincon M, Flavell RA, Davis RJ. Induction of hepatitis by JNK-mediated expression of TNF-alpha. Cell. 2009;136:249–260. doi: 10.1016/j.cell.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Jost PJ, Pellegrini M, Puthalakath H, Gugasyan R, Gerondakis S, et al. Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity. 2009;30:56–66. doi: 10.1016/j.immuni.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llacuna L, Mari M, Garcia-Ruiz C, Fernandez-Checa JC, Morales A. Critical role of acidic sphingomyelinase in murine hepatic ischemia-reperfusion injury. Hepatology. 2006;44:561–572. doi: 10.1002/hep.21285. [DOI] [PubMed] [Google Scholar]
- Kiessling MK, Linke B, Brechmann M, Suss D, Krammer PH, Gulow K. Inhibition of NF-kappaB induces a switch from CD95L-dependent to CD95L-independent and JNK-mediated apoptosis in T cells. FEBS Lett. 2010;584:4679–4688. doi: 10.1016/j.febslet.2010.10.047. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Zhang LC, Huang DC, Webb GC, Bottema CD, Shore P, et al. Gene structure alternative splicing, and chromosomal localization of pro-apoptotic Bcl-2 relative Bim. Mamm Genome. 2001;12:163–168. doi: 10.1007/s003350010242. [DOI] [PubMed] [Google Scholar]
- Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ. Transcriptional regulation of bim by FOXO3A mediates hepatocyte lipoapoptosis. J Biol Chem. 2007;11:11. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, et al. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner Gastroenterology 2010138325–335.e321–e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraza N, Malato Y, Sander LE, Al-Masaoudi M, Freimuth J, Riethmacher D, et al. Hepatocyte-specific NEMO deletion promotes NK/NKT cell- and TRAIL-dependent liver damage. J Exp Med. 2009;206:1727–1737. doi: 10.1084/jem.20082152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rencurel F, Foretz M, Kaufmann MR, Stroka D, Looser R, Leclerc I, et al. Stimulation of AMP-activated protein kinase is essential for the induction of drug metabolizing enzymes by phenobarbital in human and mouse liver. Mol Pharmacol. 2006;70:1925–1934. doi: 10.1124/mol.106.029421. [DOI] [PubMed] [Google Scholar]