Abstract
The incidence of calcium phosphate (CaP) stone disease has increased over the last three decades; specifically, brushite stones are diagnosed and treated more frequently than in previous years. Brushite is a unique form of CaP, which in certain patients can form into large symptomatic stones. Treatment of brushite stones can be difficult since the stones are resistant to shock wave and ultrasonic lithotripsy, and often require ballistic fragmentation. Patients suffering from brushite stone disease are less likely to be rendered stone-free after surgical intervention and often experience stone recurrence despite maximal medical intervention. Studies have demonstrated an association between brushite stone disease and shock wave lithotripsy (SWL) treatment. Some have theorized that many brushite stone formers started as routine calcium oxalate (CaOx) stone formers who sustained an injury to the nephron (such as SWL). The injury to the nephron leads to failure of urine acidification and eventual brushite stone formation. We explore the association between brushite stone disease and iatrogenic induced transformation of CaOx stone disease to brushite by reviewing the current literature.
Keywords: brushite, calcium phosphate, shock wave lithotripsy, Randall’s Plaque
Introduction
Although not as prevalent as in developing countries, urolithiasis is a common medical problem in the United States with an estimated prevalence of 11.7% by age 70 [1]. Renal and ureteral calculi can significantly impact the health of those suffering from the disease and are associated with considerable patient morbidity and even occasional mortality [1]. Due in great part to the use of pharmacological, radiological, and surgical resources kidney stones have a significant economic costs estimated at more than 2 billion dollars per year [2]. These numbers are likely underestimates as the incidence and patient population of stone disease continues to evolve.
The epidemiology of stone disease appears to be evolving with time. Over the past 25 years the male to female ratio for the prevalence of renal calculi has decreased from 3:1 to currently less than 2:1 [2,3]. The observed gender shift in stone disease is thought to be the result of changes in the diets of women. Some have also speculated that stone disease is increasing in the pediatric population, with one institution reporting a 5 fold increase in the diagnosis of pediatric stone patients from 1994 to 2005 [4]. Dietary changes have again been cited as a possible cause for the increasing number of pediatric stone patients. Researchers have also noted a change in stone composition over the last two to three decades, with an increase in calcium phosphate (CaP) stone disease noted [5–7].
The cause for the increase in CaP stones is not entirely understood and is most likely multifactorial. Whereas infection stones were once considered the most common stone encountered in patients with neurogenic bladders, with the advent of effective antibiotic therapy physicians now encounter less struvite stone disease and more CaP [8]. Pak and colleagues have previously reported an association between the amount of CaP composition in a stone and metabolic abnormalities, such as renal tubular acidosis and primary hyperparathyroidism [9]. Certain new medications such as topiramate-which is commonly used in the treatment of seizure disorders, migraine headaches, and now even for obesity-have been associated with the development of a type of renal tubular acidosis. Since CaP stone formation is pH dependent these patients with a high urine pH are at an increased risk of developing CaP stones [10]. Some researchers have even theorized that iatrogenic renal insult in the form of surgical and metabolic treatment of stone disease has lead to the increase in CaP stones treated over the last 20 years.
Brushite Stone Disease
Although the prevalence of CaP stones is increasing, they still only make up a minority of stone disease, with approximately 15% of all stone formers currently producing predominantly CaP stones. In general, CaP is present in one of three forms in the urinary tract: hydroxyapatite, carbonate apatite, and brushite (i.e. calcium monohydrogen phosphate, CaHPO4·2H2O). Of the urinary forms of CaP brushite is the most problematic. A quarter of CaP patients form stones containing brushite [11]. Brushite is considered the precursor phase of hydroxyapatite [12,13]. If brushite does not convert to hydroxyapatite, brushite stones will form. To date, what causes the persistence of the brushite crystal phase with development of overt stones rather than conversion to hydroxyapatite is not yet understood. Unlike hydroxyapatite, which fragments easily, brushite stones are exceptionally hard and difficult to remove surgically. The purpose of this review is to explore the epidemiology of brushite stone disease, clinical impact, and possible causes for the disease.
Increase in Incidence
The face of stone disease has changed over the past 30 years. Specifically the number of patients with CaP stones has increased. Parks and colleagues evaluated a large cohort of 1,201 stone formers over the last 30 years [5]. They found that although the predominant stone identified was CaOx, over time the incidence of CaP stones had increased. The increase in CaP coincided with an increase in urinary pH and hypercalciuria resulting in an increase in CaP supersaturation.
Similar results have been identified when stone composition laboratories evaluated their results. Mandel and colleagues studied 33,198 stones for composition from the National Veterans Administration Crystal Identification Center [6]. They also noted that the number of CaP stones had increased since 1989, but specifically observed an increase in the number of brushite stones. The percent occurrence of hydroxyapatite stones had increased 1% (26.9% to 27.9%) and brushite stones had increased 3% (1.7% to 4.14%) from the 1989 publication to the 2002 evaluation. Furthermore they found that some patients who once produced calcium oxalate stones had converted to CaP stone producers over time. Similar to Mandel’s observations, when we evaluated 82 brushite stone former patients we noted that a significant number, 17% of our cohort, had converted from another stone composition to brushite stones [14].
The underlying cause of the increasing incidence of brushite stones and conversion of some CaOx stone formers to brushite is not yet understood. Parks and colleagues in their original study noted that CaP supersaturation had increased along with an increase in the number CaP stone formers over the last 3 decades [5]. The increase in CaP supersaturation was thought to be secondary to hypercalciuria and elevated urine pH. They concluded that the hypercalciuria was genetic but the underlying cause for the increase in urine pH is yet to be determined. To follow-up their original study Parks and colleagues evaluated 62 CaP stone formers who transformed from CaOx stone formers over time [7]. They compared the 62 CaP transformers to 134 CaOx controls. The CaP transformers demonstrated higher urine pH compared to the CaOx controls at all time points. They further found that CaP transformation was associated with SWL, but that SWL was not associated with pH change since many of the patients had elevated urine pH prior to their SWL procedure.
Treatment Difficulties
Epidemiology
Previous studies have indicated that women are more likely than men to form CaP stones [5,15]. However, Parks and colleagues noted that when CaP stones were divided into individual subtypes brushite stone composition was strongly associated with male gender [5]. Similarly we studied 82 brushite stone formers requiring surgical intervention and found that the majority were male, 66% [14]. Evan and colleagues also noted a predominance of male patients in their study of brushite stone formers [16]. Since brushite is the precursor to hydroxyapatite these findings lead us to question what role hormones and/or genetics play in the conversion of brushite to hydroxyapatite.
Other finds from our cohort study of brushite stone formers demonstrated that the mean age of brushite patients is similar to idiopathic CaOx disease at 44 years (range 4 to 84) and 27% had a first degree relative with symptomatic stone disease. At the time of surgical intervention 84% of the patients had experienced at least one stone event. Mean stone size was large at 29.2 mm (range 2 to 130) with 34% having bilateral disease. The stone burdens could be quite extreme as illustrated by the (Figure 1).
Figure 1.
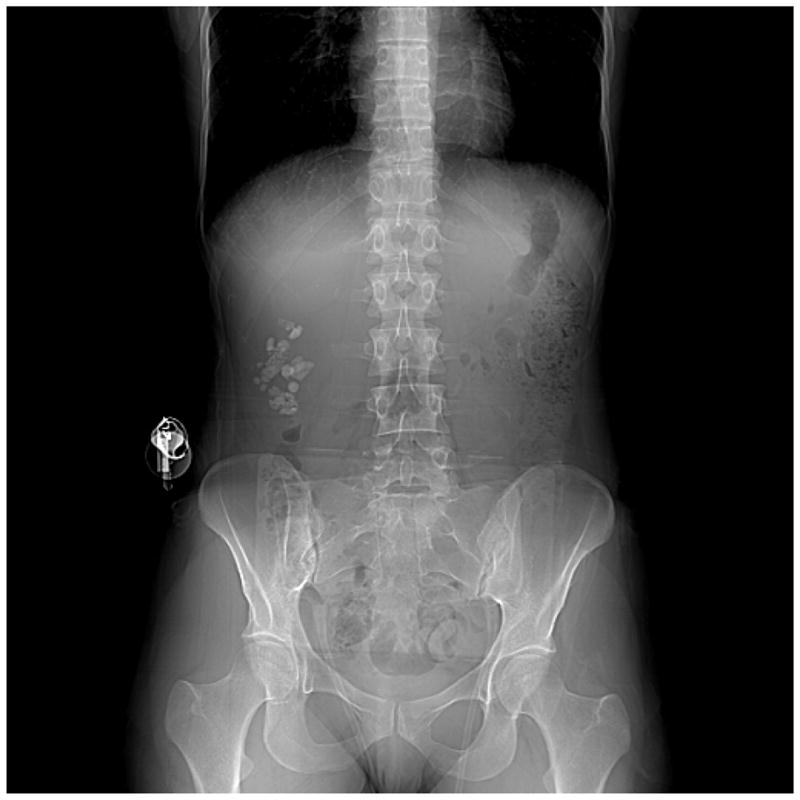
Computed tomography scout film of a typical brushite stone former. Note the multiple large stones in the right kidney.
Surgical Intervention
Brushite stones pose a difficult treatment challenge. Parks and colleagues evaluated a cohort of CaP stone formers and found that they were more likely to require surgical intervention than CaOx [5]. Brushite stones are one of the few urinary calculi that are resistant to SWL along with cystine and some CaOx monohydrate [17]. Furthermore, brushite stones are relatively resistant to ultrasonic lithotripsy, which is often used during percutaneous nephrolithotomy (PCNL), and frequently require ballistic energy to fragment [18].
Kacker and colleagues reported that stone free rates after PCNL were positively associated with the percentage of CaP found in the stone regardless of stone burden. Specifically, those patients with 60% or greater CaP stone composition were less likely to be rendered stone free than patients with less than 60% CaP [19]. However, unlike Kackers series we did not observe a decrease in stone-free rates after PCNL performed for brushite stones. In studying 82 patients with brushite stones, except for the 2 (2.4%) patients that spontaneously passed their presenting stone, all patients required surgical intervention [14]. Surgical intervention included: PCNL 63 (67.8%), ureteroscopy 8 (9.8%), SWL 3 (3.7%), and combination ureteroscopy and PCNL 6 (7.3%). The final stone-free rate was nearly 93% [14]. However, it should be noted that over a third of patients in our cohort required a secondary PCNL procedure to attain such high stone-free results. Thus, a high stone-free rate is possible for patients with brushite stone disease; however, it may require multiple surgical procedures such as PCNL or ureteroscopy to attain that goal.
Due to the stone’s resistance to SWL it is not surprising that patients with brushite stones have been found to receive a greater number of SWL treatments than idiopathic CaOx stone formers even after adjustment for sex, stone number, and stone duration [5].
Metabolic Profile
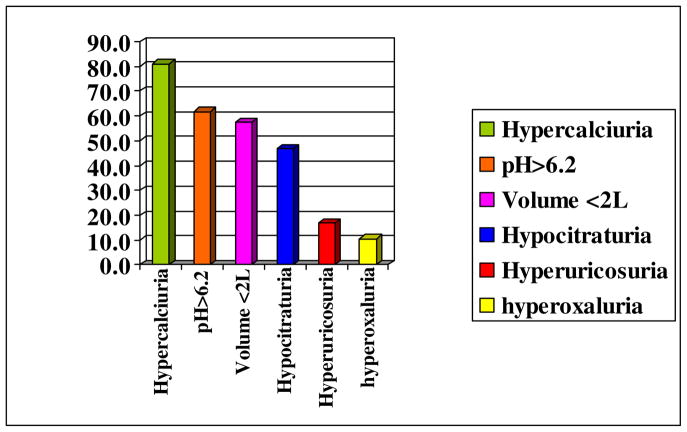
Metabolic abnormalities are common in patients suffering from brushite stone disease. Pak and colleagues have previously reported an association between the amount of CaP composition in a stone and metabolic abnormalities, such as renal tubular acidosis and primary hyperparathyroidism [9]. When analyzing a cohort of ten brushite stone formers Evan and colleagues found that the brushite stone formers had more metabolic abnormalities on 24 hour urine studies than a group of control non-stone formers [16]. Furthermore, the brushite stone-formers’ 24 hour urine studies were similar to those of a cohort of idiopathic calcium oxalate stone formers. When we analyzed our cohort of 82 brushite stone formers we found that all patients had at least one abnormality on their 24 hour urine study and many patients had multiple abnormalities [14]. Figure 2 illustrates the abnormalities noted on 24 hour urine studies. Hypercalciuria was so common in our cohort that based on these studies we now consider patients with brushite stone disease to by hypercalciuric until proven otherwise. Other findings included elevated urine pH, low urine volume, hypocitraturia, hyperuricosuria, and hyperoxaluria.
Figure 2.
Metabolic abnormalities noted in 82 brushite stone patients treated at Methodist Hospital, Indianapolis, Indiana [14]. All patients demonstrated at least one abnormality on their metabolic stone evaluation. Hypercalciuria was present in over 80% of the patients.
Recurrence and Long-term outcomes
Recurrent stone disease is problematic for brushite stone formers. In our cohort of 82 patients, at time of presentation 84.1% reported a prior stone event, of which 78.3% were previously treated with SWL [14]. The mean time from their previous stone to the presenting stone was less than 2 years (21 months). Of the 65% of patients with follow-up after their surgical intervention, recurrent stone disease occurred in 58.5% patients at a mean of 33 months (2 to 118 months) from date of our initial treatment. This recurrence rate is staggering considering that over 90% of the patients were rendered stone-free and all were treated for their underlying metabolic abnormality. Based on these results we now recommend that all patients with brushite stone disease adhere to a strict surveillance schedule with long-term follow-up.
One would assume that with frequent stone recurrences and the need for multiple surgical interventions, patients with brushite stone disease would be at risk for the development of renal insufficiency. However, data on long-term renal function for patients with brushite stone disease is limited. In the presence of documented nephropathy small cohort studies demonstrate brushite stone formers do in fact have a higher serum creatinine and lower 24-hour urine creatinine clearance than other stone formers [16]; however, large cohort studies have not identified an increased risk of renal insufficiency in patients with brushite stones [5]. When evaluating histopathologic papillary samples from brushite stone forming kidneys researchers have noted significant tubular death and fibrosis [17]. Whether the degree of cell death and fibrosis is significant enough to affect long-term overall renal function remains to be seen.
Etiology and Pathophysiology
Most of what we know about the histopathology of brushite stone disease is derived from the work of Evan and colleagues and herein we will review their work [16]. Over a 3 year period 10 brushite stone formers were treated with PCNL. To be considered a brushite stone former the patients were required to have a stone analysis of 65% or more brushite. At time of PCNL the treated kidney was mapped – a digital flexible nephroscope was used to digitally photograph and document the endoscopic appearance of each papillae. A biopsy of one papilla and the outer cortex was taken from each treated kidney.
Demographically, the researchers found that all but one patient had experienced a prior SWL, and 50% of the patients had converted to brushite from another stone type. Endoscopically, three distinct papillary lesions were noted in all studied brushite patients. The first pattern consisted of type I plaque, also known as Randall’s Plaque (RP). The second pattern was dilated ducts of Bellini plugged with crystal material. Some ducts were found to be massively dilated. Some, but not all, patients also demonstrated pits within the papillae that were not associated with ducts of Bellini or stone material. The third pattern was of suburothelial medullary collecting duct plugging that was either diffuse or focal and was present in all patients.
Unlike other forms of stone disease previously studied, the brushite stone patients also demonstrated cellular damage that varied in severity. In some kidneys there were dilated medullary collecting ducts and loops of Henle, but the surrounding tubules were normal. In other patients the inner medullary collecting ducts appeared to be damaged or dead with surrounding interstitial fibrosis, which was significant enough to entrap the vasa recta in some areas.
Comparison of Brushite Stone Formers to CaOx and Gastric Bypass
When compared to other types of stone disease previously studied by Evan and colleagues brushite stone disease appeared to be have features of both CaOx stone disease and stone disease that results from gastric bypass surgery (i.e. intestinal hyperoxaluria) (Table 1) [16]. Interstitial apatite, also known as RP or type I plaque, was present in the brushite stone formers with or without attached stones similar to the idiopathic CaOx patients; however, the GI bypass patients lack RP and never demonstrate attached stones. Tubular apatite (ductal plugs), collecting duct cell injury, and interstitial fibrosis were present all in the brushite stone formers and to some degree in the GI bypass patients as well; however, these findings are never noted in idiopathic CaOx stone formers.
Table 1.
Comparison of clinical and pathologic features of idiopathic calcium oxalate (iCaOx), brushite (BR), and gastric bypass (Bypass) stone formers.
| Idiopathic CaOx | Brushite | Gastric Bypass | |
|---|---|---|---|
| Interstitial Apatite | Yes | Yes | No |
| Tubule Apatite | No | Yes | Yes |
| Collecting Duct Cell Injury | No | Yes | Yes |
| Interstitial Fibrosis | 0 | 2++ | 1+ |
| Urinary pH | 6 | 6.5 | 5.5 |
| Stone Type | CaOx | CaOx/Brushite/Apatite | Ca Ox |
| Anchored Stone | Usually | Usually | Uncommon |
All patients with brushite stone disease demonstrated tubular atrophy, but when CaOx and GI bypass patients were studied minimal tubular atrophy was noted. Glomerulosclerosis was moderate to global in the brushite patients and was only mild to absent in the GI bypass and CaOx patients. None of the GI bypass or CaOx patients demonstrated cortical interstitial changes; however, all of the brushite stone formers had moderate to severe cortical fibrosis. Thus, it appears that although brushite stone formers have several similarities with CaOx and gastric bypass stone formers their disease appears to be more severe with greater damage to the renal parenchyma.
Statistical comparison of the brushite stone formers to the CaOx and GI bypass confirms that the degree of tissue alterations were significantly higher in the brushite patients. When assigned a quantitative score the brushite stone formers had a higher degree of glomerular abnormalities (45 ± 11) compared to the CaOx (5 ± 4), GI bypass (11 ± 11) and controls 0. The brushite patients (4.1 ± 0.3) also had a higher degree of atrophy and fibrosis compared to the CaOx (1.4 ± 0.3), GI bypass (1.3 ± 0.6) and controls (0). Looking at demographics, the brushite stone formers ((3.7 ± 1.0) experienced a higher number of SWL procedures compared to the CaOx (0.4 ± 0.1), GI bypass (2.0 ± 2.0) and controls (0). Further statistical analysis demonstrated that the degree of atrophy/fibrosis noted in the brushite cohort was not associated with patient age (P=0.64) but neared significance when looking at associated with history of SWL (P=0.059). There was no associate between patient age or prior SWL with glomerular pathology.
Theory of Conversion to Brushite
Based on the above studies we can draw several conclusions. First, endoscopically brushite stone formers and CaOx stone formers share several similarities. They both have type I interstitial plaque (RP). In fact, in the series presented by Evan and colleagues some areas of the brushite stone former kidney appeared identical to idiopathic CaOx stone formers with only RP present, while other papillae in the same kidney demonstrated clearly different pathology unique to brushite stone formers [16]. Thus, the histopathologic changes were not global. Second, hypercalciuria is common in both types of stone disease. We know from prior studies that the degree of plaque formation correlates with high urine calcium, reduced urine volume and changes in urine pH [20]. Both brushite and idiopathic CaOx stone formers demonstrate significant hypercalciuria [14, 16]. Finally, multiple studies have demonstrated that idiopathic CaOx stone formers can transform into brushite stone formers [6,7,14,16]. So what mechanism could account for the similarities and differences encountered between idiopathic CaOx and brushite stone formers?
Evan and colleagues found that histopathologically, cell injury was not present in brushite stone formers in the absence of crystals [16]. They proposed that some process initiates crystallization in the collecting ducts of the brushite stone patients. The crystals grow and contact the collecting duct cells and the collecting duct cells are injured and eventually die. The crystallization process progresses to enlarge the collecting duct and interstitial inflammation eventually develops. The authors theorize that some initial insult causes loss of collecting duct cell pH regulation which triggers the initial crystal formation.
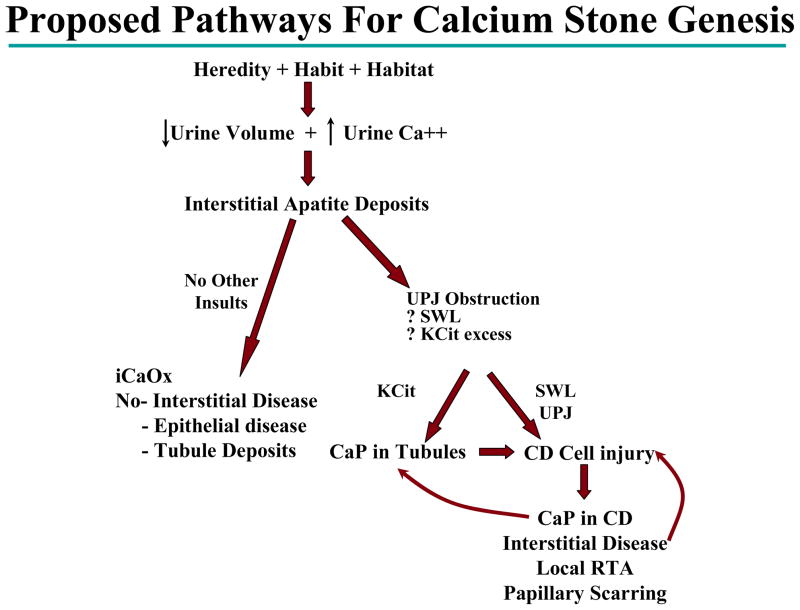
It is possible that brushite stone formers start life as an idiopathic stone former (Figure 3). The combined hereditary, habitual and habitat influences lead to a physiologic state of low urine volume and hypercalciuria. Interstitial type I plaque then develops in this ideal environment. If there is no other insult and the influences continue to produce the fruitful environment then idiopathic calcium oxalate stone disease develops. These patients have relatively “benign” disease with no interstitial fibrosis and lack tubular deposits. However; if after type I plaque develops an external insult to the kidney occurs, which can lead to dysregulation of the collecting duct cell pH, then CaP forms in the collecting duct tubules. The CaP in the collecting duct tubules lead to collecting duct injury, cell death, interstitial disease and a local RTA effect, which leads to an aggressive self propagating form of stone disease. Evan and colleagues suggested multiple possible mechanisms, which could damage the renal collecting duct leading to dysregulation of cellular pH [16]. In their study of brushite stone formers, all patients either had a history of SWL, citrate therapy, or high grade urinary obstruction – such as ureteropelvic junction obstruction.
Figure 3.
Proposed pathway for brushite and idiopathic calcium oxalate stone disease. Hereditary, environmental and dietary influences produce a state of low urine volume and hypercalciuria. This results in Type I or Randall’s plaque formation. If the state of hypercalciuria and low urine volume continues without further insults then idiopathic calcium oxalate stone disease develops. However, if the nephron sustains an injury which results in collecting duct cell death and urine alkalinization then brushite stone disease can develop.
iCaOx = idiopathic calcium oxalate
K Cit = potassium citrate
SWL = shock wave lithotripsy
UPJ = ureteropelvic junction obstruction
CD = collecting duct
RTA = renal tubular acidosis
Shock Wave Lithotripsy Induced Renal Injury
The theory that SWL could lead to nephron tubular dysfunction is not novel. A clinical dose of shock waves is known to induce an acute traumatic injury to the human kidney as observed by enlargement of the kidney, loss of cortico-medullary demarcation and the presence of peri-renal and sub-capsular hematomas [21]. In 2000 Connors and colleagues demonstrated that SWL delivered at therapeutic doses can produce significant histopathological damage to the collecting duct in the porcine model [22]. Furthermore, they found that when shock waves were delivered at 18 to 24 kV using an HM3 lithotriptor that para-aminohippurate (PAH) extraction, a measure of tubular function, was significantly decreased. Other studies have demonstrated that therapeutic doses of shock waves will produce significant damage in the area of F2, which is invariably in the region of the renal papillae [23]. Damage to the renal papillae in the animal model has been shown to impair the nephron’s ability to regulate tubular fluid pH [24]. Thus, if SWL papillary injury is significant enough to lead to increased tubular lumen pH, then luminal CaP crystallization can occur leading the eventual brushite stone disease.
The aforementioned theory linking SWL to papillary damage and subsequent brushite stone formation, although novel and logical, is based solely on animal studies and observations. To date there have been no published human studies demonstrating long-term SWL induced adverse effects on renal function. In fact, on the contrary, patients who have received SWL followed up to 19 years post procedure demonstrated no increased risk of renal insufficiency compared to urolithiasis patients not receiving SWL.25 Furthermore, although SWL has been associated with brushite stone disease, there has been no documented change in urinary pH from pre to post SWL in humans.7 Until more concrete evidence can be presented to support the role of SWL in human brushite stone disease, we are left with only theories produced in an attempt to provide explanation to observed epidemiologic associations.
Conclusions
Recent evidence indicates that CaP stone disease is increasing; specifically brushite stone disease has been shown to be on the rise. Brushite stones are associated with a large stone burden, and are technically difficult to treat due to their resistance to shock wave and ultrasonic lithotripsy. These patients often require multiple surgical interventions, have a lower stone-free rate than idiopathic calcium oxalate stone formers, and have a high risk of stone recurrence despite aggressive surgical and medical intervention. Unlike most other forms of stone disease, brushite stone formers have a significant degree of renal tissue injury on histopathologic exam. Although the etiology of brushite stone disease is not completely understood evidence exists to support the role of CaOx stone formers converting to brushite stone disease. It is possible that external injury to the nephron either by natural obstruction or iatrogenic forces, such as alkalinization therapy or SWL, is the initiating factor for this conversion. Further research in the areas of SWL induced nephron damage and brushite stone disease is necessary to draw definite conclusions.
Abbreviations
- CaP
calcium phosphate
- SWL
shock wave lithotripsy
- RP
Randall’s Plaque
- CaOx
calcium oxalate
- PCNL
percutaneous nephrolithotomy
References
- 1.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 2.Pearle MS, Calhoun EA, Curhan GC. Urologic diseases in America project: urolithiasis. J Urol. 2005;173:848–857. doi: 10.1097/01.ju.0000152082.14384.d7. [DOI] [PubMed] [Google Scholar]
- 3.Scales CD, Jr, Curtis LH, et al. Changing gender prevalence of stone disease. J Urol. 2007;177:979–982. doi: 10.1016/j.juro.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 4.VanDervoort K, Wjesen J, Frank R, Vento S, Crosby V, Chandra M, Trachtman H. Urolithiasis in pediatric patients: a single center study of incidence, clinical presentation and outcome. J Urol. 2007;177:2300–2305. doi: 10.1016/j.juro.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Parks JH, Worcester EM, Coe FL, Evan AP, Lingeman JE. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int. 2004;66:777–785. doi: 10.1111/j.1523-1755.2004.00803.x. [DOI] [PubMed] [Google Scholar]
- 6.Mandel N, Mandel I, Fryjoff K, Rejniak T, Mandel G. Conversion of calcium oxalate to calcium phosphate with recurrent stone episodes. J Urol. 2003;169:2026–2029. doi: 10.1097/01.ju.0000065592.55499.4e. [DOI] [PubMed] [Google Scholar]
- 7.Parks JH, Coe FL, Evan AP, Worcester EM. Urine pH in renal calcium stone formers who do and do not increase stone phosphate content with time. Nephrol Dial Transplant. 2009;24:130–136. doi: 10.1093/ndt/gfn420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matlaga BR, Kim SC, Watkins SL, Kuo RL, Munch LC, Lingeman JE. Changing composition of renal calculi in patients with neurogenic bladder. J Urol. 2006;175:1716–1719. doi: 10.1016/S0022-5347(05)01015-3. [DOI] [PubMed] [Google Scholar]
- 9.Pak CY, Poindexter JR, Adams-Huet B, Pearle MS. Predictive value of kidney stone composition in the detection of metabolic abnormalities. Am J Med. 2003;115:26–32. doi: 10.1016/s0002-9343(03)00201-8. [DOI] [PubMed] [Google Scholar]
- 10.Kuo RL, Moran ME, Kim DH, Abrahams HM, White MD, Lingeman JE. Topiramate-induced nephrolithiasis. J Endourol. 2002;16:229–231. doi: 10.1089/089277902753752188. [DOI] [PubMed] [Google Scholar]
- 11.Matlaga BR, Coe FL, Evan AP, Lingeman JE. The role of Randall’s plaques in the pathogenesis of calcium stones. J Urol. 2007;177:31–38. doi: 10.1016/j.juro.2006.08.088. [DOI] [PubMed] [Google Scholar]
- 12.Neman WF, Toribara TY, Mulryan BJ. Synthetic hydroxyapatite crystals, 1. Sodium and potassium fixation. Arch Biochem Biophys. 1962;98:384–390. doi: 10.1016/0003-9861(62)90202-3. [DOI] [PubMed] [Google Scholar]
- 13.Pak CY, Eanes ED, Ruskin B. Spontaneous precipitation of brushite in urine: evidence that brushite is the nidus of renal stones originating as calcium phosphate. Proc Nat Acad Sci. 1971;68:1456–1460. doi: 10.1073/pnas.68.7.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krambeck AE, Handa SE, Coe FL, Worchester EM, Evan AP, Lingeman JE. Profile of the brushite stone former. Under review. J Urol. 2010 doi: 10.1016/j.juro.2010.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gault MH, Parfrey PS, Robertson WG. Idiopathic calcium phosphate nephrolithiasis. Nephron. 1988;48:265–273. doi: 10.1159/000184940. [DOI] [PubMed] [Google Scholar]
- 16.Evan AP, Lingeman JE, Coe FL, Shao Y, Parks JH, Bledsoe SB, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005;67:576–591. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 17.Klee LW, Brito CG, Lingeman JE. The clinical implications of brushite calculi. J Urol. 1991;145:715–718. doi: 10.1016/s0022-5347(17)38432-x. [DOI] [PubMed] [Google Scholar]
- 18.Heimbach D, Jacobs D, Hesse A, Muller SC, Zhong P, Preminger GM. How to improve lithotripsy and chemolitholysis of brushite-stones: an in vitro study. Urol Res. 1999;27:266–271. doi: 10.1007/s002400050121. [DOI] [PubMed] [Google Scholar]
- 19.Kacker R, Meeks JJ, Zhao L, Nadler RB. Decreased stone-free rates after percutaneous nephrolithotomy for high calcium phosphate composition kidney stones. J Urol. 2008;180:958–960. doi: 10.1016/j.juro.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 20.Kuo RL, Lingeman JE, Evan AP, Paterson RF, Parks JH, Bledsoe SB, et al. Urine calcium and volume predict coverage of renal papilla by Randall’s plaque. Kidney Int. 2003;64:2150–2155. doi: 10.1046/j.1523-1755.2003.00316.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaude JV, Williams CM, Millner MR, Scott KN, Finlayson B. Renal morphology and function immediately after extracorporeal shock wave lithotripsy. Am J Roentgenol. 1985;145:305–313. doi: 10.2214/ajr.145.2.305. [DOI] [PubMed] [Google Scholar]
- 22.Connors BA, Evan AP, Willis LR, Blomgren PM, Lingeman JE, Fineberg NS. The effect of discharge voltage on renal injury and impairment caused by lithotripsy in the pig. J Am Soc Nephrol. 2000;11:310–318. doi: 10.1681/ASN.V112310. [DOI] [PubMed] [Google Scholar]
- 23.Evan AP, Willis LR, Lingeman JE, McAteer JA. Renal trauma and the risk of long-term complications in shock wave lithotripsy. 1998;78:1–8. doi: 10.1159/000044874. [DOI] [PubMed] [Google Scholar]
- 24.Hamm LL, Alpern R. Cellular mechanisms of renal tubular acidification. In: Seldin DW, Giebisch G, editors. The Kidney: Physiology and Pathophysiology. 2. New York: Raven Press; 1980. p. 2581. [Google Scholar]
- 25.Krambeck AE, Gettman MT, Rohlinger AL, Lohse CM, Patterson DE, Segura JW. Diabetes mellitus and hypertension associated with shock wave lithotripsy at 19 years follow-up. J Urol. 2006;175:1742–1747. doi: 10.1016/S0022-5347(05)00989-4. [DOI] [PubMed] [Google Scholar]