Abstract
Objective
We sought to determine if there is a correlation between D'Amico risk stratification and degree of suspicion of prostate cancer on multi-parametric MRI, based on targeted biopsies obtained with our electromagnetically (EM) tracked MRI/ultrasound (US) fusion platform.
Methods
101 patients underwent 3 Tesla multi-parametric MR imaging of the prostate which consisted of T2, DCE, DWI, and spectroscopy images in patients with a suspicion for, or diagnosis of prostate cancer. All prostate MRI lesions were then identified and graded by the number of modalities positive: low (≤2), moderate (3) and high (4) suspicion. Patients and lesions were stratified by D'Amico risk stratification. The biopsy protocol included a standard 12 core biopsy followed by real-time MRI/US fusion-targeted biopsies of the suspicious MR lesions.
Results
90.1% of men were clinical T1c with a mean age of 62.7 ± 8.3 years and the median PSA was 5.8 ng/ml. 54.5% of the patients were positive for cancer on the protocol biopsy. A Chi-squared analysis resulted in a statistically significant correlation between the MR suspicion and D'Amico risk stratification for patients (p<0.0001). Within-cluster re-sampling technique determined that there was a statistically significant correlation between MR suspicion and D'Amico risk stratification for MR ‘targeted’ core biopsies and MR lesions (p<0.01)
Conclusion
Our data supports that with multi-parametric MR prostate imaging, one may be able to quantitatively assess the degree of risk associated with MR visible lesions within the prostate.
Keywords: Prostate Cancer, Fusion Imaging, Biopsy, Magnetic Resonance Imaging, Transrectal Ultrasound
Prostate cancer is the leading cause of cancer in American men with 192,280 new cases in 2009 and it is the second most common cause of cancer-related deaths. 1 Since 1986 the landscape of prostate cancer has changed significantly with regard to screening, age of diagnosis, stage at diagnosis and incidence. The incidence of prostate cancer peaked in 1992 due to the prevalent use of prostate-specific antigen (PSA) as a new screening tool.2
Historically, prostate cancer was diagnosed by digitally guided trans-rectal prostate biopsies.3,4 However today, PSA screening and trans-rectal ultrasound guided prostate biopsy have become the standard of care to diagnose localized prostate cancer.5,6 These biopsies were performed following a random sextant scheme to sample the prostate. In order to improve the diagnostic yield and provide a more broadly representative specimen, the number of cores obtained during prostate biopsy has increased.7 In fact, an extended (standard) 12-14 core prostate biopsy is now common practice, detecting cancer in 27%8 to 44%9 of patients. Practitioners need to take into account the types of patients who have been included in these historical series when trying to decipher the impact of new biopsy techniques on these heterogeneous patients (T1c vs. ≥ T2).
Initially, prostate MR imaging was not considered for routine clinical practice.10 However, the addition of an endorectal-coil probe, functional imaging and a 3 Tesla magnet have improved its diagnostic utility dramatically.11, 12 MR-guided prostate biopsies have traditionally been performed in the MR suite.13, 14 Known as “in gantry” biopsies, these can be technically challenging to perform, time-consuming, and require specialized equipment which along with extended MR time can increase the cost significantly.
To meet this challenge of moving the biopsy out of the MR gantry, a custom platform has been developed at the National Institutes of Health that fuses real-time trans-rectal ultrasound (TRUS) imaging with previously obtained prostate MR images utilizing an electromagnetic tracking system (Philips Research, Briarcliff Manor, NY, and Philips Healthcare, Toronto, CA). The urologist can then perform image guided transrectal prostate biopsies of MR-identified targets in addition to the standard 12 core biopsies with the ease and familiarity of the real-time TRUS prostate biopsies urologists already perform. The technical aspects of this platform have been previously described,15, 16 and now we report the correlation between MR suspicion and the fusion guided biopsy results using the D'Amico risk stratification.
The D'Amico risk stratification was used because of its clinical utility. It is a confirmed and validated method to determine a patient's pretreatment prostate cancer specific mortality.17 This stratification was applied to specific biopsy data from MR visible lesions within the prostate, due to the possibility of assessing an index lesion's aggressiveness that may help guide future care.
Materials and Methods
All patients were counseled and informed consent was obtained with the supervision of the institutional review board at the National Cancer Institute which approved this prospective trial. From March 2007 to June 2009, 101 patients entered the protocol and underwent a 3T endorectal-coil (ec) MRI of the prostate and subsequent biopsy under MAC (monitored anesthesia care). An ecMRI of the prostate was performed obtaining triplane T2 weighted (T2W), dynamic contrast enhanced (DCE), diffusion-weighted images (DWI), and proton MR spectroscopy images. These images were interpreted by two radiologists with expertise in reading prostate MRI (PC, BT). Intraprostatic lesions were identified and then scored by the number of modalities positive on MR imaging in a non weighted fashion, low (≤2), moderate (3), or high suspicion (4) for prostate cancer (Figure 1).
Figure 1.
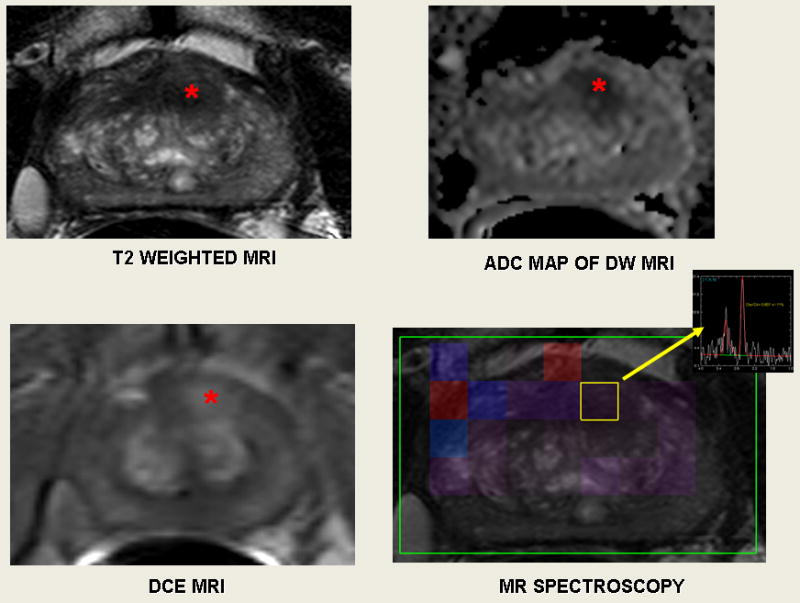
55-year-old male with a serum PSA of 3.33 ng/ml. Axial T2W MR image demonstrates a round shaped low signal intensity lesion (
 ) at the anterior mid gland. (T2 weighted MRI); lesion appears as hypointense on corresponding apparent diffusion coefficient (ADC) map (
) at the anterior mid gland. (T2 weighted MRI); lesion appears as hypointense on corresponding apparent diffusion coefficient (ADC) map (
 ); dynamic contrast enhanced MR image demonstrates increased enhancement at lesion (
); dynamic contrast enhanced MR image demonstrates increased enhancement at lesion (
 ); and MR spectroscopy demonstrates increased choline to citrate ratio within the lesion (yellow box). These four positive modalities results in a high suspicion lesion.
); and MR spectroscopy demonstrates increased choline to citrate ratio within the lesion (yellow box). These four positive modalities results in a high suspicion lesion.
Pre-biopsy, each patient was given a cleansing fleet enema and standard antibiotic prophylaxis. All patients underwent monitored anesthesia care for the procedure. The protocol required each patient to undergo a standard 12 core TRUS biopsy followed by MRI/US fusion biopsy of the suspicious lesions using a custom prototype prostate navigation system (Philips Research, Briarcliff Manor, NY), which has FDA (510K) clearance.
Details of this novel biopsy platform have been described previously.16 The pre-operative MR images are imported directly from the picture archiving and communication systems (PACS). An electromagnetic field generator (Northern Digital Inc., Waterloo, Canada) is placed above the pelvis which allows for real-time tracking of a custom biopsy needle guide (Civco Inc, Kalona IA, USA) embedded with a miniature electromagnetic tracking sensor (Philips Healthcare, Toronto, Canada). A 2D prostate sweep is performed manually to render a 3D ultrasound image that is then registered and fused to the pre-operative prostate MR images.16 The tracking also allows for motion compensation to improve image registration. The real-time ultrasound images are fused with the MR images and the selected MR lesions are labeled for tracking (Figure 2). The physician manually guides the biopsy gun to the highlighted lesion visualized on the MR and US fused images. Once aligned, two biopsies of each lesion are performed (minimum of one in the axial and sagittal planes). In order to ensure core lengths > 5mm, additional biopsies were taken (up to 4). Each specimen was sent in a separate container for pathological evaluation.
Figure 2.
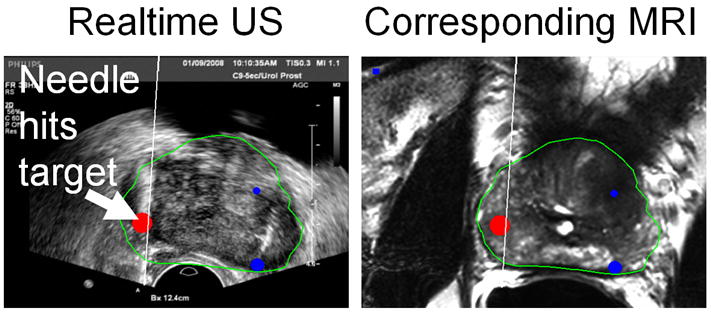
Combination of real-time ultrasound image (left) with the corresponding multi-planar reconstruction (MPR) of the co-registered pre-procedural MRI scan (right). The MRI-based prostate segmentation (green) and MRI-identified targets (red, blue) are superimposed on both images.
Descriptive statistics were used to describe the patient's characteristics: age, pre-biopsy PSA, digital rectal exam (DRE), prostate volume and previous biopsy data. A statistician (JS) performed all calculations for the study. The results of the fusion biopsies were stratified according to the pre-operative MRI scoring system (low, moderate, high) as described. The D'Amico risk stratification was calculated at the time of the biopsy (using the stage, Gleason score and PSA) as low (Gleason score ≤6 and PSA ≤ 10), intermediate (Gleason score = 7 and PSA > 10 and ≤ 20) and high risk (Gleason score ≥8 and PSA>20).18 Chi-square analysis was used to determine if there was a correlation between the degree of MRI suspicion and D'Amico risk stratification for patients. Within-cluster re-sampling technique was used to account for the correlation between repeated measures in each patient. This was done to assess the correlation between MRI suspicion and D'Amico risk stratification for both MR lesions and each MR ‘targeted’ core biopsy.
Results
One hundred and one patients with a mean age of 62.7 ± 8.3 years, a median and mean PSA of 5.8 ng/ml and 8.3 ± 11.8 respectively were included in the study. 90.1% (91/101) of patients were cT1c. The remaining patients were cT2a (Table 1). Of the 101 patients, 55 were positive for prostate cancer by either a standard biopsy or MR ‘targeted’ biopsy. Thirty-five patients were positive both on a MR ‘targeted’ core biopsy and standard biopsy. Ten patients were only positive on MR ‘targeted’ core biopsy. The mean number of MRI lesions identified per patient was 2.6 (range 1-7). Twelve hundred and fifty two standard cores were obtained, 149 of which were positive for prostate cancer (11.0 %).
Table 1. Patient characteristics.
| Total No. patients | 101 |
| Clinical T1c | 91/101 |
| Clinical T2a | 10/101 |
| Mean age, yrs (range) | 62.4 (41-82) |
| Mean PSA, ng/mL (range) | 8.3 (0.2-103) |
| Median PSA, ng/mL | 5.8 |
| Biopsy History | |
| No Prior Biopsy | 36 |
| Previous Negative Biopsy | 29 |
| Previous Positive Biopsy | 36 |
| Mean number lesions suspicious for cancer on MRI (range) | 2.6 (1-7) |
| Median number lesions suspicious for cancer on MRI | 3 |
A Chi-square analysis was used to determine if there was a correlation between the MRI suspicion and D'Amico risk stratification for patients. Within-cluster re-sampling technique was performed comparing the MR suspicion and D'Amico risk stratification for MR ‘targeted’ core biopsies and MR lesions. All tests were found to be statistically significant (p<0.01), (Table 2).
Table 2. Results of biopsies were sub-stratified by MR ‘targeted’ core biopsies, MR lesions and patients.
| D'Amico Risk Stratification | ||||||||
|---|---|---|---|---|---|---|---|---|
| MR Suspicion |
Low | Intermediate | High | Patients | Lesions | Targets | ||
| Low | Patients | 10 | 1 | 1 | 12 | 23 | ||
|
|
||||||||
| MR Lesions | 17 | 2 | 4 | |||||
|
|
||||||||
| MR Core Biopsies | 23 | 3 | 7 | 33 | ||||
|
|
||||||||
| Moderate | Patients | 10 | 15 | 1 | 26 | 29 | ||
|
|
||||||||
| MR Lesions | 10 | 15 | 4 | |||||
|
|
||||||||
| MR Core Biopsies | 13 | 25 | 8 | 46 | ||||
|
|
||||||||
| High | Patients | 2 | 6 | 9 | 17 | 24 | p < 0.0001 | |
|
|
||||||||
| MR Lesions | 2 | 6 | 16 | p < 0.01 | ||||
|
|
||||||||
| MR Core Biopsies | 3 | 12 | 34 | 49 | p < 0.01 | |||
|
|
||||||||
| Total | 55 | 76 | 128 | |||||
Multiple MR ‘targeted’ core biopsies were taken from each MR lesion and the lesions were labeled as positive for statistical analysis if at least one of the MR ‘targeted’ core biopsies were positive for cancer. This analysis was done because there was a possibility of inadequate sampling of the lesion, due to ‘missing’ the lesion on one of the targeted biopsies, limitations of manually guided biopsies, or the limitations of the spatial accuracy of the system. There were a total of 588 MR ‘targeted’ core biopsies of 264 MR lesions. This method of using MR ‘targeted’ core biopsies versus “lesions” only increased the biopsy yield an average of 7.4%, which did not alter our conclusions in this study. Averages of 2.2 MR ‘targeted’ core biopsies were performed per lesion with at least one core biopsy in the axial and sagittal planes.
Discussion
Prostate cancer is the most common cancer and the second most common cause of cancer-related mortality among American males. The diagnosis of prostate cancer has gone through significant improvements which have resulted in a 5 year relative survival of 100% in local or regional stages.1 As urologists, we have adapted our treatment paradigm using a multidisciplinary approach (urologists, diagnostic radiologists, radiation oncologists, pathologists, interventional radiologists). During the evaluation of patients with prostate cancer, practitioners need to determine prostate cancer specific mortality and tailor the treatment accordingly. Using this rational, the D'Amico risk stratification was applied to each patient in order to determine if there was a correlation with MP (ec)MR imaging.
Currently, several publications describe the initial experience with in gantry MRI-guided prostate biopsies.19 There are several limitations of this approach. First, specialized MR compatible biopsy equipment is required.14, 19 In addition, an extended biopsy time is required, which could decrease MR efficiency and throughput, as well as increasing the cost significantly. If anesthesia is required, the length of the procedure could be even longer when compared to the traditional or transrectal MRI/US fusion biopsies of the prostate. Another advantage of this system over a purely MRI-guided system is that the procedure time is very short. Typically, about (15) minutes are required to complete both the standard (12 core) biopsy and the ‘fusion guided biopsy’. After our initial experience, we have modified our technique to use local anesthesia only, which decreases costs and procedure time. This platform allows urologists to utilize this technology in the office setting with little change to the current flow, protocols, and setting for TRUS guided biopsies of the prostate.
There was a statistically significant association between the degree of MR suspicion and the D'Amico risk stratification for each patient, MR ‘targeted’ core biopsy, and MR lesion (p<0.01). Our data supports that with multi-parametric MR prostate imaging, one may be able to quantitatively assess the degree of risk associated with specific MR lesions within the prostate. This is consistent with data correlating whole mount prostate specimens to prostate MRI images.20
One of the concerns with active surveillance is under staging patients. Over the past 20 years there has been a significant decrease in the upgrading of Gleason scores on prostatectomy specimens. Historically, pathological upgrading on radical prostatectomy specimens was reported to be 54%.21 Most recently, the University of Chicago reported that 20.3% of patients were upstage after prostatectomy with regards to their Gleason score.22 We are currently investigating if our platform can further decrease the number of patients upstaged after surgery (local regional staging).
Finally, this platform may be utilized in the emerging field of focal prostate therapy. In addition to improving the quantification of prostate cancer, this platform may also guide the treatment of focal areas of the prostate and allow close follow-up of treated lesions and re-biopsy as indicated.
One of the limitations of this platform is that MR of the prostate is still not able to detect all cancerous lesions (< 3mm diameter). Recently, our histopathological correlation with multi-parametric (T2W MRI, DCE MRI, MR Spectroscopy) MR imaging for lesions within the peripheral zone demonstrated that the sensitivity is 94%, 55%, and 32% and the specificity is 84%, 97% and 99%, respectively.20
Conclusion
The multi-parametric MR assessment of patients with positive lesions for prostate cancer resulted in a statistically significant correlation with MR detected lesion suspicion and Gleason score (D'Amico risk stratification). This multiparametric MR data was used to guide prostate biopsies with a custom MRI/TRUS fusion guided biopsy platform. Interval imaging to assess the lesion(s) may obviate the need for multiple biopsies and the associated morbidity in patients undergoing “watchful waiting” long term.23 While a larger prospective trial and further evaluation is certainly needed, the multi-parametric MR assessment may give insight into which patients may be eligible for active surveillance.
Chart 1.
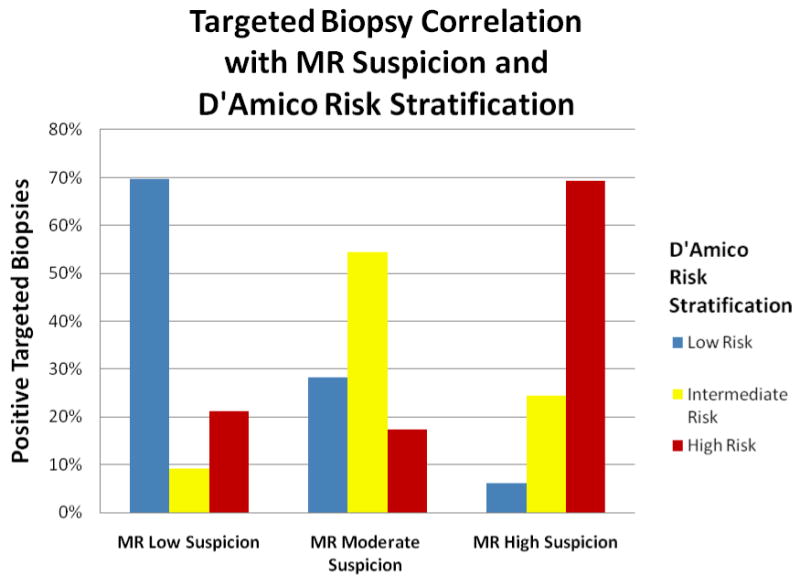
Illustrates the correlation between MR imaging suspicion of MR ‘Targeted’ core biopsies with the D'Amico risk stratification. (p<0.01) The percentage values were calculated using total number of positive targeted biopsies for each MR suspicion category then sub-stratified by D'Amico risk stratification.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and by a Cooperative Research and Development Agreement between NIH and Philips Healthcare.
Footnotes
Conflicts of Interest: Neil Glossop, Jochen Kruecker, Sheng Xu, Pingkun Yan and Samuel Kadoury are salaried employees of Philips Electronics.
NIH (BW, PC, PP) and Philips (JK, SX) have intellectual property in related fields.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009 Jul-Aug;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Welch HG, Albertsen PC. Prostate Cancer Diagnosis and Treatment After the Introduction of Prostate-Specific Antigen Screening: 1986-2005. J Natl Cancer Inst. 2009 Aug 31; doi: 10.1093/jnci/djp278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Needell MH, Slotkin GE, Mitchell FD, Friedman M. Prostatic needle biopsy. J Urol. 1955 Jul;74(1):138–41. doi: 10.1016/S0022-5347(17)67255-0. [DOI] [PubMed] [Google Scholar]
- 4.Pearlman CK. Transrectal biopsy of the prostate. J Urol. 1955;74:387. doi: 10.1016/S0022-5347(17)67295-1. [DOI] [PubMed] [Google Scholar]
- 5.Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, Petros JA, Andriole GL. Measurement of Prostate-Specific Antigen in Serum as a Screening Test for Prostate Cancer. N Engl J Med. 1991 Apr 25;324(17):1156–61. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 6.Cooner WH, Mosley BR, Rutherford CL, Jr, et al. Prostate Cancer Detection in a Clinical Urological Practice By Ultrasonography, Digital Rectal Examination and Prostate Specific Antigen. J Urol. 1990;143:1146. doi: 10.1016/s0022-5347(17)40211-4. [DOI] [PubMed] [Google Scholar]
- 7.M DP, Niemann TH, Bahnson RR. Extended Sector Biopsy For Detection of Carcinoma of the Prostate. Urol Oncol. 2001;6:91. doi: 10.1016/s1078-1439(00)00111-3. [DOI] [PubMed] [Google Scholar]
- 8.Naughton CK, Miller DC, Mager DE, Ornstein DK, Catalona WJ. A Prospective Randomized Trial Comparing 6 Versus 12 Prostate Biopsy Cores: Impact on Cancer Detection. J Urol. 2000 Aug;164(2):388–92. [PubMed] [Google Scholar]
- 9.Presti JC, Jr, O'Dowd GJ, Miller MC, Mattu R, Veltri RW. Extended Peripheral Zone Biopsy Schemes Increase Cancer Detection Rates and Minimize Variance in Prostate Specific Antigen and Age Related Cancer Rates: Results of a Community Multi-Practice Study. J Urol. 2003 Jan;169(1):125–9. doi: 10.1016/S0022-5347(05)64051-7. [DOI] [PubMed] [Google Scholar]
- 10.D'Amico AV, Whittington R, Malkowicz B, Schnall M, Schultz D, Cote K, Tomaszewski JE, Wein A. Endorectal Magnetic Resonance Imaging as a Predictor of Biochemical Outcome After Radical Prostatectomy in Men with Clinically Localized Prostate Cancer. J Urol. 2000 Sep;164(3 Pt 1):759–63. doi: 10.1097/00005392-200009010-00032. [DOI] [PubMed] [Google Scholar]
- 11.Fütterer JJ, Heijmink SW, Scheenen TW, Jager GJ, Hulsbergen-Van de Kaa CA, Witjes JA, Barentsz JO. Prostate Cancer: Local Staging at 3-T endorectal MR Imaging--Early Experience. Radiology. 2006;238:184. doi: 10.1148/radiol.2381041832. [DOI] [PubMed] [Google Scholar]
- 12.Turkbey B, Albert PS, Kurdziel K, Choyke PL. Imaging Localized Prostate Cancer: Current Approaches and New Developments. AJR Am J Roentgenol. 2009 Jun;192(6):1471–80. doi: 10.2214/AJR.09.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anastasiadis AG, Lichy MP, Nagele U, Kuczyk MA, Merseburger AS, Hennenlotter J, Corvin S, Sievert KD, Claussen CD, Stenzl A, Schlemmer HP. MRI-Guided Biopsy Of The Prostate Increases Diagnostic Performance in Men with Elevated or Increasing PSA Levels after Previous Negative TRUS Biopsies. Eur Urol. 2006;50:738. doi: 10.1016/j.eururo.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Krieger A, Susil RC, Ménard C, Coleman JA, Fichtinger G, Atalar E, Whitcomb LL. Design of a Novel MRI Compatible Manipulator for Image Guided Prostate Interventions. IEEE Trans Biomed Eng. 2005 Feb;52(2):306–13. doi: 10.1109/TBME.2004.840497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu S, Kruecker J, Turkbey B, Glossop N, Singh AK, Choyke P, Pinto P, Wood B. Real-time MRI-TRUS Fusion For Guidance of Targeted Prostate Biopsies. J Comput Aided Surg. 2008 Sep;13(5):255–64. doi: 10.1080/10929080802364645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu S, Kruecker J, Guion P, Glossop N, Neeman Z, Choyke P, Singh AK, Wood BJ. Closed-Loop Control in Fused MR-TRUS Image-Guided Prostate Biopsy. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 2007;10(Pt 1):128–3. doi: 10.1007/978-3-540-75757-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Cancer-Specific Mortality after Surgery or Radiation for Patients with Clinically Localized Prostate Cancer Managed During The Prostate-Specific Antigen Era. J Clin Oncol. 2003 Jun 1;21(11):2163–72. doi: 10.1200/JCO.2003.01.075. [DOI] [PubMed] [Google Scholar]
- 18.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A. Biochemical Outcome After Radical Prostatectomy, External Beam Radiation Therapy, or Interstitial Radiation Therapy for Clinically Localized Prostate Cancer. JAMA. 1998 Sep 16;280(11):969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 19.Pondman KM, Fütterer JJ, ten Haken B, Schultze Kool LJ, Witjes JA, Hambrock T, Macura KJ, Barentsz JO. MR-Guided Biopsy Of The Prostate: An Overview of Techniques and a Systematic Review. Eur Urol. 2008 Sep;54(3):517–27. doi: 10.1016/j.eururo.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Turkbey B, Pinto P, Mani H, Bernardo M, Pang Y, McKinney Y, Khurana K, Ravizzini G, Albert PS, Merino MJ, Choyke P. Prostate Cancer: Value of Multiparametric MR Imaging at 3 T for Detection--Histopathologic Correlation. Radiology. 2010 Apr;255(1):89–99. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cookson MS, Fleshner NE, Soloway SM, Fair WR. Correlation Between Gleason Score of Needle Biopsy and Radical Prostatectomy Specimen: Accuracy and Clinical Implications. J Urol. 1997 Feb;157(2):559–62. [PubMed] [Google Scholar]
- 22.Gofrit ON, Zorn KC, Taxy JB, Lin S, Zagaja GP, Steinberg GD, Shalhav AL. Predicting the Risk of Patients with Biopsy Gleason Score 6 to Harbor a Higher Grade Cancer. J Urol. 2007 Nov;178(5):1925–8. doi: 10.1016/j.juro.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 23.Fujita K, Landis P, McNeil BK, Pavlovich CP. Serial Prostate Biopsies are Associated with an Increased Risk of Erectile Dysfunction in Men with Prostate Cancer on Active Surveillance. J Urol. 2009 Dec;182(6):2664–9. doi: 10.1016/j.juro.2009.08.044. [DOI] [PubMed] [Google Scholar]


