Abstract
It has been suggested that exercise following breast cancer diagnosis is inversely associated with mortality. However, controversy exists regarding the causality of such associations. We evaluated associations of exercise after breast cancer diagnosis with total mortality and recurrence/disease-specific mortality after accounting for conditions that restrict exercise participation. The analysis included 4826 women with stage I–III breast cancer identified 6 months after diagnosis through the population-based Shanghai Cancer Registry and recruited into the study between 2002 and 2006. Exercise was assessed approximately 6, 18, and 36 months post-diagnosis and metabolic equivalent (MET) scores were derived. Information on medical history, cancer diagnosis, treatments, quality of life (QOL), anthropometrics, and lifestyles were obtained by in-person interviews at 6 months post-diagnosis. Medical charts were abstracted to verify clinical information. During the median follow-up of 4.3 years, 436 deaths and 450 recurrences/cancer-related deaths were documented. After adjustment for QOL, clinical prognostic factors, and other covariates, exercise during first 36 months post-diagnosis was inversely associated with total mortality and recurrence/disease-specific mortality with hazard ratios of 0.70 (95% confidence interval (CI): 0.56–0.88) and 0.60 (95% CI: 0.47–0.76), respectively. Significant dose-response relationships between total and recurrence/disease-specific mortality rates and exercise duration and MET scores were observed (all Ptrend<0.05). The exercise-mortality associations were not modified by menopausal status, comorbidity, QOL, or body size assessed approximately 6 months post-diagnosis. An interaction between disease stage and hormone receptor status and total mortality was noted. Our study suggests that exercise after breast cancer diagnosis may improve overall and disease-free survival.
Keywords: breast cancer, exercise, quality of life, survival
INTRODUCTION
Approximately 4.4 million women worldwide live with breast cancer (1–2). In the U.S., there are currently over two million breast cancer survivors, and the number continues to increase (3). Identifying modifiable lifestyle factors associated with prognosis could provide an additional means of improving outcomes for cancer survivors that both complement and extend the effects of pharmacological treatments.
There is a growing body of evidence suggesting a link between exercise and breast cancer prognosis (4–14). Several studies, all conducted in Western countries, have evaluated the association of exercise after cancer diagnosis with breast cancer survival and provided some positive but inconsistent evidence (5, 7–9, 11–13, 15). Most studies assessed exercise at a single time point after cancer diagnosis (5, 7–9), even though exercise levels are known to vary over time after diagnosis (16). The major predictors of mortality, including cancer stage, progression of cancer, and poor quality of life (QOL) (17), may limit exercise participation. These factors have not been adequately considered in previous studies. We have previously reported that exercise after cancer diagnosis is associated with improved QOL and decreased depression among breast cancer survivors (18–19).
We present a detailed analysis of associations of exercise participation after breast cancer diagnosis with overall and disease-free survival using data from a cohort study of 4826 women diagnosed with stage I–III breast cancer in China.
MATERIALS AND METHODS
Study population
Details of the study design have been described elsewhere (20). Briefly, through the population-based Shanghai Cancer Registry, we recruited 5042 incident breast cancer cases diagnosed between March 2002 and April 2006 aged 20–75 years (response rate: 80.0%). approximately 6 months after cancer diagnosis. Study participants are being followed through in-person interviews administered approximately 18, 36, and 60 months after cancer diagnosis (20). Information on survival status is also obtained by annual linkage with the Shanghai Vital Statistics database.
We excluded cases with stage 0 (n=156) and stage IV (n=28) breast cancer from this study. To minimize the effect of existing and potential medical conditions and cancer treatments on exercise participation after cancer diagnosis, we also excluded cases or events (death, recurrence/metastasis) that occurred within the first year of follow-up. After excluding 32 cases who died during the 12-month period following cancer diagnosis, 4826 women remained for the total mortality analysis. We further excluded from the recurrence analysis 315 cases (leaving 4511 cases) who developed an event during the 12-month period following the cancer diagnosis. The study was approved by the institutional review boards of all institutions involved, and all participants provided written informed consent prior to interview.
Survey interviews
In-person interviews were conducted to collect information on cancer diagnosis and treatment, sociodemographics, menstrual and reproductive history, diet, comorbidity, exercise, complementary and alternative medicine use, weight history, and QOL. In addition, height, weight, and waist and hip circumferences were measured at the baseline interview following a standard protocol. Body mass index (BMI) and waist-to-hip ratio (WHR) were calculated based on these measurements.
Disease- and treatment-related information including stage of tumor-node metastasis (TNM), estrogen receptor (ER) and progesterone receptor (PR) status, type of surgery, and chemotherapy, radiotherapy, immunotherapy, and tamoxifen use was collected during in-person interviews and verified by reviewing medical charts. ER and PR status were included in the analyses in the following joint categories: ER+/PR+ (receptor-positive), ER−/PR− (receptor-negative), ER−/PR+ or ER+/PR− (mixed). A Charlson comorbidity index was created based on a validated comorbidity scoring system (21) and the diagnostic codes from the International Classification of Disease, 9th revision (ICD-9) (22).
Exercise assessment
At each interview, participants were asked if they participated in exercise regularly (at least twice a week) or not. If the woman answered “Yes”, she was further asked to report up to 5 of the most common activities in which she participated. At the baseline, 6-month post-diagnosis interview, women reported activities that took place during the 6 months preceding the interview. At subsequent interviews, women reported activities since the last interview (i.e., for the preceding 12 or 18 months). No women reported participating in more than 4 types of exercise during the first 18 months after diagnosis, and only 0.1% of women engaged in 5 types of exercise at the 36-month post-diagnosis interview. The 60-month post-diagnosis interviews are still ongoing; thus, the current analyses include only information from the first 36 months after diagnosis.
Information on frequency and duration were obtained for all exercise activities. Each activity was assigned a metabolic equivalent (MET) score (3 MET-hours is equivalent to an average walking pace (2–2.5 mph) for 1 hour; 2 MET-hours is equal to moderate bicycling for half an hour) based on the method proposed by Ainsworth et al. (23). The score for MET-hours per week (MET-hours/week) for each activity was calculated from the hours per week the participant reported engaging in that activity multiplied by the assigned MET score. The values from individual activities were summed to derive a total exercise-MET score. The exercise questionnaire has been validated (24). Significant correlations between exercise measurements derived from the exercise questionnaire and criterion measures, e.g., physical activity log (r = 0.74) and 7-day physical activity questionnaire survey, were observed. The reproducibility of exercise participation (κ = 0.64) was reasonably high (24).
Assessment of other lifestyle factors and QOL
Habitual dietary intakes, tea and alcohol consumption, and smoking habits, were also obtained through in-person interviews using validated questionnaires at baseline (19, 25).
The Shanghai Breast Cancer Survival Study was originally designed to recruit 2250 breast cancer patients and was expanded to include about 5000 patients. Two QOL instruments, the General Quality of Life Inventory-74 (GQOLI-74) and the 36-item Short Form Health Survey (SF-36), were administered as part of the 6-month post-diagnosis interview. The GQOLI-74 was administered first (44.2% cases) and the SF-36, second (55.8% cases). Women’s responses on the two QOL instruments were converted to scores on a 0–100 scale, with higher scores reflecting better QOL. The GQOLI-74 has demonstrated a satisfactory level of reliability and validity (26). Details about the GQOLI-74 have been described in our previous reports (27). The SF-36 has been used in epidemiologic studies of breast cancer patients and survivors (28–29) and has been validated in the Chinese population (30). The instrument-specific QOL distribution of general health was used to categorize the QOL scores. The mean QOL score for general health on the GQOLI-74 was comparable with that on the SF-36 (score: 56.2 vs. 56.0) in this study.
Statistical analyses
Differences in sociodemographic and medical characteristics between exercisers and non-exercisers at the baseline were evaluated using Student’s t-test or the χ2 test. The endpoints for the analyses were any death for total mortality (overall survival analysis) and cancer recurrence/metastasis or death related to breast cancer for recurrence/disease-specific mortality (disease-free survival analysis). Survival rates were calculated starting at the time of cancer diagnosis to the endpoints of the study, censoringat the date of last contact or non-cancer death (for disease-freesurvival only). The Kaplan-Meier method was used to generate survival curves for a preliminary examination of the data. The log-rank tests were applied to evaluate differences in survival rates for women with different exercise levels.
Multivariable Cox proportional hazards models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (95% CIs) in association with exercise participation. Exercise was treated as a time-dependent variable. Survival was modeled as a function of age at entry into and exit from the study. Entry time was defined as age at cancer diagnosis and exit time was defined as age at death or censoring (31). The following covariates were related to exposure (exercise) or outcome (mortality) and were adjusted for: education; income; menopausal status; BMI; WHR; QOL; intakes of cruciferous vegetables and soy protein; tea consumption; use of chemotherapy, radiotherapy, or tamoxifen; TNM stage; and ER/PR status. Type of surgery and immunotherapy were not related to exercise or mortality and were not included in the models. Regular exercisers were categorized by 2.5 hours/week and 8.3 MET-hours/week, the medians for exercise duration and exercise-MET score at 6 months post-diagnosis, levels similar to recent recommendations for physical activity for Americans and for cancer patients (32). Analyses of mortality and exercise participation over the first 36 months post-diagnosis were further stratified by TNM stage, ER/PR status, menopausal status, QOL, BMI, WHR, comorbidity, and cancer-related treatments.
Tests for trend were performed by entering the categoricalvariables as continuous parameters in the corresponding models. Tests for effect modification were examined on a multiplicative scale. All tests were performed by using Statistical Analysis Software (SAS, version 9.1; SAS Institute, Inc., Cary, North Carolina). The significance levels were set at P< 0.05 for two-sided analyses.
RESULTS
During the median follow-up period of 4.3 years, 436 deaths and 450 recurrences or breast cancer-related deaths were documented. At 6 months post-diagnosis, 65% of women reported exercising regularly with a median of 8.3 MET-hours/week of exercise. The corresponding rates were 74% (median MET=15.4) and 74% (median MET=15.8) at 18 and 36 months post-diagnosis, respectively. At 6 months post-diagnosis, women engaged in an average number of 1.5 exercise activities. Walking was the most common type of regular exercise performed in this study population (52%), followed by gymnastics (14%), body building (7%), and traditional Chinese exercises (5%, including Qigong and Tai Chi). Similar results were observed at 18 and 36 months post-diagnosis.
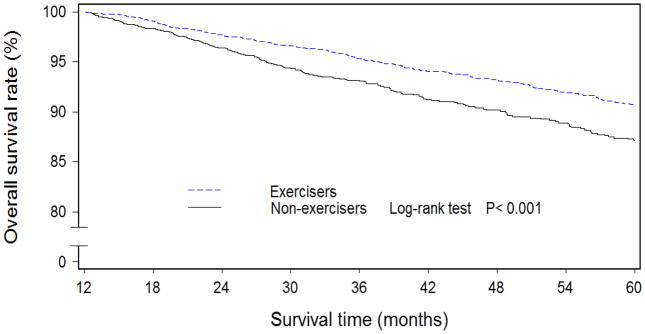
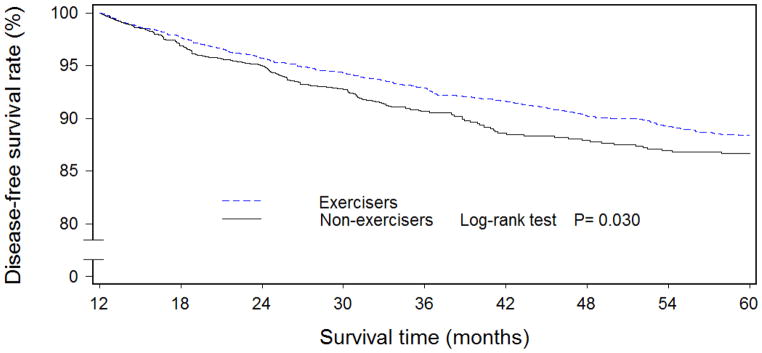
At baseline, exercisers had higher levels of education and household income, were more likely to have earlier-stage disease, to have used tamoxifen, and had higher QOL than non-exercisers (Table 1). In addition, exercisers had lower WHR and higher intakes of meats, cruciferous vegetables, soy protein, and tea. Women who engaged in exercise regularly during the first 6 months post-diagnosis had higher overall (Figure 1) and disease-free (Figure 2) survival rates than non-exercisers. After adjustment for QOL, clinical factors, and other confounders, the hazard ratios (HRs) were 0.80 (95% CI: 0.65–0.97) for total mortality and 0.91 (95% CI: 0.75–1.11) for recurrence/breast cancer-related mortality for those who exercised regularly during the first 6 months post-diagnosis compared with non-exercisers (Table 2). When exercise levels during the first 18- and 36-month post-diagnosis periods were taken into consideration, a dose-response relationship was observed for both overall and disease-free survival rates (all Ptrend:<0.05).
Table 1.
Sociodemographic and clinical characteristics of breast cancer cases at study enrollment
| Characteristic | Total (n= 4826)a | Exercisers (n= 3115)a | Non-exercisers (n= 1711)a | P valueb |
|---|---|---|---|---|
| Age at diagnosis (year) | 53.5 (10.0) | 53.4 (10.0) | 53.6 (10.1) | 0.452 |
| Education (%) | ||||
| <High school | 46.5 | 45.0 | 49.2 | 0.003 |
| High school | 37.6 | 38.0 | 37.0 | |
| >High school | 15.9 | 17.1 | 13.8 | |
| Income (yuan/month/capita) (%) | ||||
| <1000 | 57.7 | 56.5 | 59.9 | 0.008 |
| 1000–1999 | 30.4 | 30.6 | 30.0 | |
| ≥2000 | 11.9 | 12.9 | 10.1 | |
| Married/living with partner (%) | 88.0 | 88.1 | 87.8 | 0.801 |
| Interval from diagnosis to study enrollment (months) | 6.5 (0.7) | 6.5 (0.7) | 6.5 (0.8) | 0.755 |
| Charlson comorbidity index≥1 | 19.8 | 19.5 | 20.5 | 0.377 |
| Tumor-node metastasis stage (%) | ||||
| I | 34.8 | 36.4 | 31.9 | <0.001c |
| II a | 33.9 | 34.0 | 33.8 | |
| II b | 17.2 | 16.5 | 18.4 | |
| III | 9.4 | 8.4 | 11.2 | |
| Unknown | 4.7 | 4.7 | 4.9 | |
| Estrogen/progesterone receptor (ER/PR) status (%) | ||||
| Positive (ER+/PR+) | 50.3 | 50.2 | 50.3 | 0.651c |
| Negative (ER−/PR−) | 27.4 | 27.1 | 27.9 | |
| Mixed (ER+/PR− or ER−/PR+) | 20.4 | 20.8 | 19.8 | |
| Unknown | 1.9 | 1.8 | 2.0 | |
| Ever received chemotherapy (%) | 92.2 | 91.2 | 94.0 | <0.001 |
| Ever received radiotherapy (%) | 32.4 | 29.6 | 37.5 | <0.001 |
| Ever received immunotherapy (%) | 14.8 | 15.1 | 14.4 | 0.580 |
| Ever used tamoxifen (%) | 51.9 | 54.3 | 48.0 | <0.001 |
| Type of surgery (%) | ||||
| Mastectomy | 94.1 | 94.7 | 93.8 | 0.352 |
| Conservation | 2.6 | 2.5 | 2.8 | |
| Others | 3.0 | 2.8 | 3.3 | |
| Family history of breast cancer (%) | 5.6 | 5.5 | 5.7 | 0.695 |
| Body mass index (kg/m2) | 24.1 (3.4) | 24.1 (3.3) | 24.2 (3.6) | 0.174 |
| Waist-to-hip ratio | 0.83 (0.05) | 0.83 (0.05) | 0.84 (0.06) | <0.001 |
| Cigarette smoking (%) | 2.6 | 2.2 | 3.3 | 0.020 |
| Alcohol consumption (%) | 3.1 | 2.9 | 3.5 | 0.271 |
| Tea consumption (%) | 23.6 | 25.3 | 20.5 | <0.001 |
| Post-menopausal (%) | 51.1 | 51.1 | 51.2 | 0.935 |
| Meat intake (g/day) | 82.7 (57.8) | 83.6 (56.8) | 81.1 (59.0) | 0.148 |
| Cruciferous vegetable intake (g/day) | 74.6 (53.0) | 78.3 (52.1) | 68.0 (54.0) | <0.001 |
| Soy protein intake (g/day) | 11.4 (8.6) | 12.0 (8.8) | 10.1 (8.0) | <0.001 |
| Quality of life score | 58.1 (14.2) | 59.3 (13.6) | 55.9 (14.9) | <0.001 |
Unless otherwise specified, means (standard deviation) are presented.
For tests of differences between women with and without exercise participation.
For χ2 test, ‘unknown’ group was excluded.
Figure 1.
Overall survival curves among Chinese women diagnosed with breast cancer by regular exercise participation during the first 6 months after breast cancer diagnosis (n=4826)
Figure 2.
Disease-free survival curves among Chinese women diagnosed with breast cancer by regular exercise participation during the first 6 months after breast cancer diagnosis (n=4511)
Table 2.
Associations of exercise after breast cancer diagnosis with total mortality and relapse/disease-specific mortality
| Characteristics | Total | Events | First 6 months post-diagnosisa | First 18 months post-diagnosisb | First 36 months post-diagnosisb |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Total mortality | 4826 | 436 | |||
| Exercise participation | |||||
| No | 1711 | 185 | 1.00 | 1.00 | 1.00 |
| Yes | 3115 | 251 | 0.80 (0.65, 0.97) | 0.70 (0.56, 0.87) | 0.70 (0.56, 0.88) |
| Exercise participation per week (hours) | |||||
| No exercise | 1711 | 185 | 1.00 | 1.00 | 1.00 |
| <2.5 | 1654 | 126 | 0.78 (0.62, 0.98) | 0.75 (0.59, 0.96) | 0.84 (0.66, 1.08) |
| ≥2.5 | 1461 | 125 | 0.82 (0.64, 1.04) | 0.66 (0.51, 0.84) | 0.64 (0.49, 0.82) |
| P value for trend | 0.168 | 0.004 | 0.002 | ||
| Exercise energy expenditure (MET-hours/week) | |||||
| No exercise | 1711 | 185 | 1.00 | 1.00 | 1.00 |
| <8.3 | 1566 | 122 | 0.79 (0.63, 0.99) | 0.77 (0.60, 0.99) | 0.81 (0.63, 1.05) |
| ≥8.3 | 1549 | 129 | 0.80 (0.63, 1.02) | 0.65 (0.51, 0.83) | 0.65 (0.51, 0.84) |
| P value for trend | 0.198 | 0.004 | <0.001 | ||
| Relapse/disease-specific mortality | 4511 | 450 | |||
| Exercise participation | |||||
| No | 1580 | 174 | 1.00 | 1.00 | 1.00 |
| Yes | 2931 | 276 | 0.91 (0.75, 1.11) | 0.71 (0.57, 0.90) | 0.60 (0.47, 0.76) |
| Exercise participation per week (hours) | |||||
| No exercise | 1580 | 174 | 1.00 | 1.00 | 1.00 |
| <2.5 | 1552 | 133 | 0.84 (0.66, 1.05) | 0.70 (0.54, 0.90) | 0.63 (0.48, 0.82) |
| ≥2.5 | 1379 | 143 | 1.01 (0.80, 1.27) | 0.73 (0.57, 0.94) | 0.57 (0.44, 0.74) |
| P value for trend | 0.522 | 0.341 | 0.030 | ||
| Exercise energy expenditure (MET-hours/week) | |||||
| No exercise | 1580 | 174 | 1.00 | 1.00 | 1.00 |
| <8.3 | 1468 | 128 | 0.85 (0.67, 1.07) | 0.70 (0.54, 0.91) | 0.60 (0.46, 0.78) |
| ≥8.3 | 1463 | 148 | 0.98 (0.78, 1.24) | 0.72 (0.57, 0.93) | 0.59 (0.45, 0.76) |
| P value for trend | 0.471 | 0.324 | 0.006 | ||
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval; MET, metabolic equivalent.
Adjusted for date of birth, body mass index at baseline, waist-to-hip ratio at baseline, menopausal status, income, education, quality of life, cruciferous vegetable intake, soy protein intake, tea consumption, chemotherapy, radiotherapy, tamoxifen use, tumor-node metastasis status, and estrogen/progesterone receptor status.
Adjusted for the same variables as above, but exercise was treated as a time-dependent variable.
Table 3 presents associations of exercise over the first 36 months post-diagnosis with mortality stratified by TNM stage and ER/PR status. An inverse association between exercise and total mortality was observed among women with early (I–IIa) and advanced disease stage (IIb–III), although the trend test was only significant for the latter. Exercise was significantly associated with recurrence/disease-specific mortality, regardless of disease stage. Exercise was associated with reduced total mortality only among women with ER/PR-negative, but not, ER/PR-positive cancer (Pinteraction=0.004). No significant interaction was observed for recurrence/disease-specific mortality.
Table 3.
Associations of exercise over the first 36 months after breast cancer diagnosis with total mortality and relapse/disease-specific mortality, stratified by disease stage and hormone receptor status
| Total mortality | Relapse/disease-specific mortality | |||||
|---|---|---|---|---|---|---|
| Total | Events | HR (95% CI) | Total | Events | HR (95% CI) | |
| Stratified by TNMa | ||||||
| TNM I–IIa stage | 3316 | 166 | 3124 | 190 | ||
| MET-hours per weekb | ||||||
| No exercise | 1123 | 67 | 1.00 | 1044 | 69 | 1.00 |
| <8.3 | 1097 | 44 | 0.79(0.51, 1.22) | 1032 | 50 | 0.52 (0.32, 0.80) |
| ≥8.3 | 1096 | 55 | 0.89 (0.59, 1.36) | 1048 | 71 | 0.62 (0.41, 0.92) |
| P value for trend | 0.333 | 0.444 | ||||
| TNM IIb–III stage | 1282 | 249 | 1176 | 241 | ||
| MET-hours per weekb | ||||||
| No exercise | 505 | 110 | 1.00 | 461 | 102 | 1.00 |
| <8.3 | 395 | 73 | 0.83 (0.60, 1.15) | 364 | 71 | 0.57 (0.40, 0.82) |
| ≥8.3 | 382 | 66 | 0.52 (0.37, 0.72) | 351 | 68 | 0.45 (0.32, 0.64) |
| P value for trend | <0.001 | <0.002 | ||||
| P value for interaction | 0.037 | 0.218 | ||||
| Stratified by ER/PRc | ||||||
| ER+PR+ (positive) | 2426 | 161 | 2308 | 189 | ||
| MET-hours per weekb | ||||||
| No exercise | 861 | 70 | 1.00 | 815 | 80 | 1.00 |
| <8.3 | 809 | 39 | 1.41 (0.87, 2.27) | 771 | 46 | 0.72 (0.47, 1.12) |
| ≥8.3 | 756 | 52 | 1.32 (0.83, 2.12) | 722 | 63 | 0.79 (0.53, 1.19) |
| P value for trend | 0.935 | 0.540 | ||||
| ER−PR− (negative) | 1323 | 171 | 1213 | 155 | ||
| MET-hours per weekb | ||||||
| No exercise | 478 | 76 | 1.00 | 428 | 62 | 1.00 |
| <8.3 | 429 | 50 | 0.58 (0.39, 0.86) | 392 | 45 | 0.40 (0.25, 0.63) |
| ≥8.3 | 416 | 45 | 0.40 (0.29, 0.59) | 393 | 48 | 0.36 (0.24, 0.56) |
| P value for trend | <0.001 | 0.002 | ||||
| ER+PR−/ER−PR+ (Mixed) | 986 | 87 | 911 | 94 | ||
| MET-hours per weekb | ||||||
| No exercise | 338 | 31 | 1.00 | 308 | 28 | 1.00 |
| <8.3 | 299 | 28 | 0.78 (0.42, 1.43) | 282 | 32 | 0.62 (0.32, 1.23) |
| ≥8.3 | 349 | 28 | 0.67 (0.36, 1.22) | 321 | 34 | 0.51 (0.27, 1.00) |
| P value for trend | 0.051 | 0.166 | ||||
| P value for interaction | 0.004 | 0.375 | ||||
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval; MET, metabolic equivalent; TNM, tumor-node metastasis status; ER/PR, estrogen/progesterone receptor status
Adjusted for date of birth, body mass index at baseline, waist-to-hip ratio at baseline, menopausal status, income, education, quality of life, cruciferous vegetable intake, soy protein intake, tea consumption, chemotherapy, radiotherapy, tamoxifen use, and estrogen/progesterone receptor status.
Exercise was treated as a time-dependent variable.
Further adjusted for tumor-node metastasis status instead of estrogen/progesterone receptor status.
Associations of exercise with total mortality and recurrence/disease-specific mortality were not modified by menopausal status, BMI, or comorbidity (Table 4), nor were associations of mortality modified by WHR, chemotherapy, radiotherapy, or tamoxifen use (data not shown).
Table 4.
Associations of exercise over the first 36 months after breast cancer diagnosis with total mortality and relapse/disease-specific mortality, stratified by menopausal status, quality of life, and body mass index
| Total mortality | Relapse/disease-specific mortality | |||||
|---|---|---|---|---|---|---|
| Total | Events | HR (95% CI) | Total | Events | HR (95% CI) | |
| Stratified by menopausal statusa | ||||||
| Pre-menopause | 2359 | 186 | 2202 | 208 | ||
| MET-hours per weekb | ||||||
| No exercise | 835 | 76 | 1.00 | 773 | 81 | 1.00 |
| <8.3 | 764 | 49 | 0.87 (0.58, 1.10) | 714 | 55 | 0.59 (0.39, 0.89) |
| ≥8.3 | 760 | 61 | 0.86 (0.58, 1.26) | 715 | 72 | 0.69 (0.47, 1.00) |
| P value for trend | 0.317 | 0.631 | ||||
| Post-menopause | 2467 | 250 | 2309 | 242 | ||
| MET-hours per weekb | ||||||
| No exercise | 876 | 109 | 1.00 | 807 | 93 | 1.00 |
| <8.3 | 802 | 73 | 0.82 (0.59, 1.14) | 754 | 73 | 0.63 (0.44, 0.91) |
| ≥8.3 | 789 | 68 | 0.55 (0.40, 0.77) | 748 | 76 | 0.52 (0.36, 0.74) |
| P value for trend | <0.001 | 0.001 | ||||
| P value for interaction | 0.233 | 0.300 | ||||
| Stratified by QOLc | ||||||
| QOL<Median | 2278 | 205 | 2138 | 222 | ||
| MET-hours per weekb | ||||||
| No exercise | 902 | 101 | 1.00 | 827 | 99 | 1.00 |
| <8.3 | 723 | 56 | 0.63 (0.44, 0.90) | 681 | 59 | 0.45 (0.31, 0.65) |
| ≥8.3 | 653 | 48 | 0.50 (0.35, 0.71) | 630 | 64 | 0.47 (0.33, 0.66) |
| P value for trend | <0.001 | 0.006 | ||||
| QOL≥Median | 2548 | 231 | 2373 | 228 | ||
| MET-hours per weekb | ||||||
| No exercise | 809 | 84 | 1.00 | 753 | 75 | 1.00 |
| <8.3 | 843 | 66 | 1.06 (0.73, 1.55) | 787 | 69 | 0.80 (0.53, 1.21) |
| ≥8.3 | 896 | 81 | 0.83 (0.58, 1.21) | 833 | 84 | 0.74 (0.50, 1.10) |
| P value for trend | 0.111 | 0.289 | ||||
| P value for interaction | 0.294 | 0.218 | ||||
| Stratified by BMId | ||||||
| BMI<25 | 3128 | 267 | 2914 | 266 | ||
| MET-hours per weekb | ||||||
| No exercise | 1102 | 113 | 1.00 | 1015 | 98 | 1.00 |
| <8.3 | 999 | 76 | 0.76 (0.55, 1.05) | 932 | 81 | 0.70 (0.47, 0.96) |
| ≥8.3 | 1027 | 78 | 0.62 (0.45, 0.85) | 967 | 87 | 0.66 (0.47, 0.94) |
| P value for trend | 0.002 | 0.033 | ||||
| BMI≥25 | 1698 | 169 | 1597 | 184 | ||
| MET-hours per weekb | ||||||
| No exercise | 609 | 72 | 1.00 | 565 | 76 | 1.00 |
| <8.3 | 567 | 46 | 0.91 (0.60, 1.38) | 536 | 47 | 0.53 (0.35, 0.81) |
| ≥8.3 | 522 | 51 | 0.70 (0.46, 1.05) | 496 | 61 | 0.50 (0.38, 0.74) |
| P value for trend | 0.027 | 0.095 | ||||
| P value for interaction | 0.642 | 0.704 | ||||
| Stratified by Charlson comorbidity index | ||||||
| Charlson comorbidity index<1 | 3869 | 339 | 3601 | 350 | ||
| MET-hours per weekb | ||||||
| No exercise | 1360 | 138 | 1.00 | 1253 | 133 | 1.00 |
| <8.3 | 1258 | 98 | 0.89 (0.66,1.18) | 1172 | 101 | 0.65 (0.47, 0.89) |
| ≥8.3 | 1251 | 103 | 0.68 (0.50, 0.90) | 1176 | 116 | 0.66 (0.49, 0.90) |
| P value for trend | 0.003 | 0.162 | ||||
| Charlson comorbidity index≥1 | 957 | 97 | 910 | 100 | ||
| MET-hours per weekb | ||||||
| No exercise | 351 | 47 | 1.00 | 327 | 41 | 1.00 |
| <8.3 | 308 | 24 | 0.63 (0.36, 1.10) | 296 | 27 | 0.49 (0.26, 0.78) |
| ≥8.3 | 298 | 26 | 0.62 (0.37, 1.05) | 287 | 32 | 0.39 (0.23, 0.66) |
| P value for trend | 0.050 | 0.009 | ||||
| P value for interaction | 0.198 | 0.651 | ||||
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval; MET, metabolic equivalent; QOL, quality of life; BMI, body mass index.
Adjusted for date of birth, body mass index at baseline, waist-to-hip ratio at baseline, income, education, quality of life, cruciferous vegetable intake, soy protein intake, tea consumption, chemotherapy, radiotherapy, tamoxifen use, tumor-node metastasis status, and estrogen/progesterone receptor status.
Exercise was treated as a time-dependent variable.
Further adjusted for tumor-node metastasis status instead of quality of life.
Further adjusted for quality of life instead of body mass index.
DISCUSSION
Exercise may affect breast cancer prognosis by enhancing immune function and reducing levels of estrogen and growth factors, insulin resistance, and hyperinsulinemia and by decreasing depression and increasing QOL (18–19, 33–35). In this large, population-based cohort study, we found that regular exercise after breast cancer diagnosis was significantly associated with improved overall and disease-free survival following a dose-response pattern. We also observed that breast cancer patients increased their participation in exercise or their level of exercise over the first 36 months post-diagnosis. This exercise-survival association varied little by type of cancer-related treatment, BMI, QOL, comorbidity, or menopausal status and was more evident among women with advanced disease stage and with ER/PR-negative breast cancer.
The effect of post-diagnosis exercise on breast cancer survival has drawn considerable attention in recent years, because exercise is a modifiable behavior (5, 7–9, 11, 36). The Nurses’ Health Study (NHS) reported a decreased risk of breast cancer-related death among stage I–III breast cancer patients participating in physical activity at levels comparable to our study (5). The Life After Cancer Epidemiology (LACE) study and another recent US study also found that women with higher levels of recreational physical activity after diagnosis had decreased risk of mortality from breast cancer (9). However, the Women’s Healthy Eating and Living (WHEL) Study found that only when combined with higher fruit and vegetable intakes was exercise at 2 years post-diagnosis associated with favorable survival (7). A small study of 603 breast cancer patients in Canada showed no association of breast cancer survival with exercise shortly after surgery (36). Most previous studies were based on a single exercise measurement at or after cancer diagnosis (7–9, 11, 36). In our study, exercise was assessed at multiple time points after cancer diagnosis. Additionally, we accounted for the possible confounding effect of QOL and other lifestyle factors. Although we cannot completely rule out chance findings caused by other confounders, the consistency of the association and the dose-response pattern we found provide the strongest epidemiologic evidence to date that exercise after breast cancer diagnosis is associated with improved overall and disease-free survival.
In our study, walking and other moderate exercises (e.g., Tai Chi) were the most common types of exercise. Consistent with our findings, Irwin et al. reported that among 933 US women with breast cancer, exercising the recommended amounts of 2.5 hrs/wk of moderate-intensity physical activity compared with no exercise was associated with 67% decreased risk of death (11).
As with any study of exercise and mortality, there is a concern that low levels of exercise may be the result, rather than the cause, of a health condition that creates a predisposition to mortality. Breast cancer patients with late-stage or more aggressive forms of cancer may be too fatigued to participate in exercise during the period ofactive chemotherapy and radiotherapy (16, 37). To minimize this concern, we excluded women with stage IV breast cancer from the analyses. We also carefully evaluated the confounding and modifying effects of TNM stage, ER/PR status, QOL, comorbidity, and other covariates in our analyses. We observed strong associations among women with relatively advanced stage (IIb–III) and ER/PR-negative breast cancer, which supports a possible role for reverse causation. However, we found no evidence of significant interaction for QOL, comorbidity, or cancer-related treatment.
Several studies have reported a potential effect modification of hormone receptor status on the exercise-survival association (5, 7, 11). The Health, Eating, Activity, and Lifestyle (HEAL) study found a strong benefit of physical activity among women with ER-positive breast cancer (11), consistent with findings from the NHS (5). The WHEL study reported no survival advantage in ER/PR-negative breast cancer, a borderline advantage for ER−/PR+, and significant benefits for ER+/PR− and ER/PR-positive cancers (7). None of these studies had adequate statistical power to investigate ER/PR-negative breast cancer. In our study, we found stronger associations among women with ER/PR-negative breast cancer. Studies with large sample sizes are needed to address the possible differential effect of exercise on outcome of subtypes of breast cancer.
The potential modifying effect of body size on the associations between exercise and breast cancer survival has been evaluated in several studies with inconsistent findings (5–6, 8–9, 13). The California Teachers Study reported that recreational physical activity was associated with lower risk of breast cancer-related death only among overweight women (13). Holick et al. also found a stronger effect of post-diagnosis recreational physical activity on breast cancer survival for women with BMI≥25, although no interaction was detected (8). In contrast, the LACE study reported a reduced risk of all-cause mortality associated with physical activity in women with BMI<25, but not in overweight/obese women (9). In our study, we found little evidence that BMI or WHR modified the exercise-survival association, although the prevalence of overweight and obesity was relatively lower (29.5% and 5.6%, respectively) than that in Western populations.
Our study has several strengths. First, it was designed specifically to evaluate the effect of post-diagnosis exercise on breast cancer prognosis. The population-based study design and the high response rate minimized selection bias. Second, multiple exercise assessments were implemented and detailed exercise information was collected. Third, information on sociodemographics, medical and lifestyle factors, QOL, and anthropometrics were collected using structured and/or validated questionnaires by health professionals and were adjusted for in the analyses.
Our study also has limitations. First, exercise information was self-reported, which can be subject to recall bias or over-reporting. However, our validation study indicated that the self-reported exercise questionnaire has high validity (24). Self-reported physical activity has been used widely in epidemiologic studies of breast cancer patients and survivors (5, 8–9, 13). Second, two different QOL instruments were applied. However, we found that the average total QOL scores derived from these two measurements were comparable and should be sufficient for adjustment. Third, although chemotherapy and tamoxifen use rates differ from rates in the US, they are in agreement with findings from previous independent research conducted in the same population (27). Systemic adjuvant therapy is commonly used in China for women with either early or advanced disease, but the rate of hormone therapy use, usually applied after chemotherapy and radiotherapy, is low in China (38). Not being able to investigate the influence of exercise change from pre- to post-cancer diagnosis on survival outcomes is another limitation. Last, the median of our follow-up period was less than 5 years, which is relatively short. Ongoing follow-up of our cohort will allow us to evaluate the long-term effect of exercise on breast cancer prognosis.
In summary, our study provides strong epidemiologic evidence that regular exercise during the first 3 years after cancer diagnosis, particularly at the currently recommended levels for breast cancer survivors of 2.5 hours/week for exercise duration and 8.3 MET-hours/week for exercise-MET score or higher (32, 39), has a beneficial effect on breast cancer survival. Health professionals should encourage their patients to engage in regular exercise in accordance with current recommendations (32, 39) and guidelines for safe and effective exercise (39). Our finding of an increase in exercise participation or level among Chinese breast cancer survivors suggests that this goal is achievable.
Acknowledgments
Funding: This study was supported by the Department of Defense Breast Cancer Research Program (DAMD 17-02-1-0607) and by US Public Health Service grant number R01 CA118229 from the National Cancer Institute.
The authors thank Dr. Fan Jin for her support in study implementation and the participants and staff members of the SBCSS for making this study possible. The authors also thank Drs. Hui Cai and Wanqing Wen for their assistance in statistical analysis and Ms. Bethanie Rammer and Ms. Jacqueline Harbaugh for their assistance in manuscript preparation.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- 1.American Cancer Society. Breast cancer facts & figures, 2005–2006. Atlanta: American Cancer Society; 2005. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA: a cancer journal for clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Ries L, Harkins D, Krapcho M. Surveillance, Epidemiology, and End Results (SEER) cancer statistics review, 1975–2003. Bethesda (MD): National Cancer Institute; 2006. [Google Scholar]
- 4.Irwin ML, Aiello EJ, McTiernan A, Bernstein L, Gilliland FD, Baumgartner RN, et al. Physical activity, body mass index, and mammographic density in postmenopausal breast cancer survivors. J Clin Oncol. 2007;25:1061–6. doi: 10.1200/JCO.2006.07.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Jama. 2005;293:2479–86. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 6.Abrahamson PE, Gammon MD, Lund MJ, Britton JA, Marshall SW, Flagg EW, et al. Recreational physical activity and survival among young women with breast cancer. Cancer. 2006;107:1777–85. doi: 10.1002/cncr.22201. [DOI] [PubMed] [Google Scholar]
- 7.Pierce JP, Stefanick ML, Flatt SW, Natarajan L, Sternfeld B, Madlensky L, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25:2345–51. doi: 10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:379–86. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 9.Sternfeld B, Weltzien E, Quesenberry CP, Jr, Castillo AL, Kwan M, Slattery ML, et al. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomarkers Prev. 2009;18:87–95. doi: 10.1158/1055-9965.EPI-08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedenreich CM, Gregory J, Kopciuk KA, Mackey JR, Courneya KS. Prospective cohort study of lifetime physical activity and breast cancer survival. Int J Cancer. 2009;124:1954–62. doi: 10.1002/ijc.24155. [DOI] [PubMed] [Google Scholar]
- 11.Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26:3958–64. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irwin ML, Mayne ST. Impact of nutrition and exercise on cancer survival. Cancer J. 2008;14:435–41. doi: 10.1097/PPO.0b013e31818daeee. [DOI] [PubMed] [Google Scholar]
- 13.West-Wright CN, Henderson KD, Sullivan-Halley J, Ursin G, Deapen D, Neuhausen S, et al. Long-term and recent recreational physical activity and survival after breast cancer: the California Teachers Study. Cancer Epidemiol Biomarkers Prev. 2009;18:2851–9. doi: 10.1158/1055-9965.EPI-09-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emaus A, Veierod MB, Tretli S, Finstad SE, Selmer R, Furberg AS, et al. Metabolic profile, physical activity, and mortality in breast cancer patients. Breast cancer research and treatment. 2010;121:651–60. doi: 10.1007/s10549-009-0603-y. [DOI] [PubMed] [Google Scholar]
- 15.Dal Maso L, Zucchetto A, Talamini R, Serraino D, Stocco CF, Vercelli M, et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. International journal of cancer. 2008;123:2188–94. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]
- 16.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97:1746–57. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang VT, Thaler HT, Polyak TA, Kornblith AB, Lepore JM, Portenoy RK. Quality of life and survival: the role of multidimensional symptom assessment. Cancer. 1998;83:173–9. doi: 10.1002/(sici)1097-0142(19980701)83:1<173::aid-cncr23>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Zheng Y, Zheng W, Gu K, Chen Z, Lu W, et al. The effect of regular exercise on quality of life among breast cancer survivors. Am J Epidemiol. 2009;170:854–62. doi: 10.1093/aje/kwp209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Lu W, Zheng Y, Gu K, Chen Z, Zheng W, et al. Exercise, tea consumption, and depression among breast cancer survivors. J Clin Oncol. 2010;28:991–8. doi: 10.1200/JCO.2009.23.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, et al. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–43. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunau GL, Sheps S, Goldner EM, Ratner PA. Specific comorbidity risk adjustment was a better predictor of 5-year acute myocardial infarction mortality than general methods. J Clin Epidemiol. 2006;59:274–80. doi: 10.1016/j.jclinepi.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Department of Health and Human Services. Clinical modification, ICD-9-CM. 9. Washington, DC: U.S Government Printing Office; 1998. The international classification of diseases. rev. ed. [Google Scholar]
- 23.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Matthews CE, Shu XO, Yang G, Jin F, Ainsworth BE, Liu D, et al. Reproducibility and validity of the Shanghai Women’s Health Study physical activity questionnaire. Am J Epidemiol. 2003;158:1114–22. doi: 10.1093/aje/kwg255. [DOI] [PubMed] [Google Scholar]
- 25.Shu XO, Yang G, Jin F, Liu D, Kushi L, Wen W, et al. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women’s Health Study. Eur J Clin Nutr. 2004;58:17–23. doi: 10.1038/sj.ejcn.1601738. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Wei H, Young D. The development of the General Quality of Life Inventory. China Ment Health J. 1995;9:227–31. [Google Scholar]
- 27.Lu W, Cui Y, Chen X, Zheng Y, Gu K, Cai H, et al. Changes in quality of life among breast cancer patients three years post-diagnosis. Breast cancer research and treatment. 2009;114:357–69. doi: 10.1007/s10549-008-0008-3. [DOI] [PubMed] [Google Scholar]
- 28.Trentham-Dietz A, Sprague BL, Klein R, Klein BE, Cruickshanks KJ, Fryback DG, et al. Health-related quality of life before and after a breast cancer diagnosis. Breast Cancer Res Treat. 2008;109:379–87. doi: 10.1007/s10549-007-9653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buijs C, Rodenhuis S, Seynaeve CM, van Hoesel QG, van der Wall E, Smit WJ, et al. Prospective study of long-term impact of adjuvant high-dose and conventional-dose chemotherapy on health-related quality of life. J Clin Oncol. 2007;25:5403–9. doi: 10.1200/JCO.2007.11.2813. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Wang HM, Shen Y. Chinese SF-36 Health Survey: translation, cultural adaptation, validation, and normalisation. J Epidemiol Community Health. 2003;57:259–63. doi: 10.1136/jech.57.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Department of Health and Human Services. [accessed in September 2010];Physical Activity Guidelines for Americans. http://www.health.gov/paguidelines/pdf/paguide.pdf. [cited; Available from.
- 33.Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr. 2002;132:3456S–64S. doi: 10.1093/jn/132.11.3456S. [DOI] [PubMed] [Google Scholar]
- 34.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman-Goetz L, Apter D, Demark-Wahnefried W, Goran MI, McTiernan A, Reichman ME. Possible mechanisms mediating an association between physical activity and breast cancer. Cancer. 1998;83:621–8. doi: 10.1002/(sici)1097-0142(19980801)83:3+<621::aid-cncr4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 36.Borugian MJ, Sheps SB, Kim-Sing C, Van Patten C, Potter JD, Dunn B, et al. Insulin, macronutrient intake, and physical activity: are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol Biomarkers Prev. 2004;13:1163–72. [PubMed] [Google Scholar]
- 37.Demark-Wahnefried W, Hars V, Conaway MR, Havlin K, Rimer BK, McElveen G, et al. Reduced rates of metabolism and decreased physical activity in breast cancer patients receiving adjuvant chemotherapy. Am J Clin Nutr. 1997;65:1495–501. doi: 10.1093/ajcn/65.5.1495. [DOI] [PubMed] [Google Scholar]
- 38.Huang O, Chen C, Wu J, Chen S, Chen X, Liu G, et al. Retrospective analysis of 119 Chinese noninflammatory locally advanced breast cancer cases treated with intravenous combination of vinorelbine and epirubicin as a neoadjuvant chemotherapy: a median follow-up of 63.4 months. BMC Cancer. 2009;9:375. doi: 10.1186/1471-2407-9-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine and science in sports and exercise. 2010;42:1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]