Abstract
Nuclear receptors transduce hormonal signals by binding directly to DNA target sites in promoters and modulating the transcription of linked genes. Receptor-mediated transactivation appears to be potentiated in response to ligand by a number of coactivators that may provide key interactions with components of the transcription preinitiation complex and/or alter chromatin structure. Here, we use the vitamin D3 receptor ligand-binding domain (VDR LBD) as an affinity matrix to identify components of a transcriptionally active nuclear extract that interact with VDR in response to ligand. We describe the purification of a complex of at least 10 VDR interacting proteins (DRIPs) ranging from 65 to 250 kD that associate with the receptor in a strictly 1,25-dihydroxyvitamin D3-dependent manner. These proteins also appear to interact with other, but not all, nuclear receptors, such as the thyroid hormone receptor. The DRIPs are distinct from known nuclear receptor coactivators, although like these coactivators, their interaction also requires the AF-2 transactivation motif of VDR. In addition, the DRIP complex contains histone acetyltransferase activity, indicating that at least one or more of the DRIPs may function at the level of nucleosomal modification. However, we show that the DRIPs selectively enhance the transcriptional activity of VDR on a naked DNA template utilizing a cell-free, ligand-dependent transcription assay. Moreover, this activity can be specifically depleted from the extract by liganded, but not unliganded, VDR-LBD. Overexpression of DRIP100 in vivo resulted in a strong squelching of VDR transactivation, suggesting the sequestration of other limiting factors, including components of the DRIP complex. These results demonstrate the existence of a new complex of novel functional nuclear receptor coactivators.
Keywords: Vitamin D3 receptor, ligand-binding domain, nuclear receptor coactivators, VDR transactivation, cell-free transcription
Steroid, retinoid, vitamin D3, and thyroid hormones signal through ligand-dependent transcription factors that collectively comprise a superfamily of intracellular, soluble receptors (hereafter collectively called nuclear receptors) that reside in the nucleus or translocate there in response to hormonal signals. As the largest known family of eukaryotic transcriptional regulators, nuclear receptors are implicated via the target genes they modulate in the control of cell growth and differentiation, homeostasis, development, and several physiological processes (for review, see Freedman 1997 and references therein). Moreover, because they are regulated tightly by small lipophilic molecules, they are extremely attractive as pharmacologic targets.
Nuclear receptors all share a common organization in functional domains and extensive homologies in structure. A DNA-binding domain allows the receptors to bind as homodimers, or heterodimers with a common partner, retinoid X receptor (RXR), to specific DNA response elements typically composed of two hexameric half-sites organized as direct or inverted repeats. The carboxy-terminal half of the prototype nuclear receptor includes a ligand-binding domain (LBD) with a superimposed dimerization surface, and a ligand-dependent transcriptional activation function called AF-2, located at the extreme carboxyl terminus of the receptor (Danielian et al. 1992; Barettino et al. 1994; Durand et al. 1994). Crystallographic analyses have revealed that binding of a specific ligand, all-trans retinoic acid (ATRA), to retinoic acid receptor γ (RARγ) induces a conformational change in its structure that modifies the orientation of the AF-2 core motif, contained within the last of 12 α-helices that comprise the LBD (Bourguet et al. 1995; Renaud et al. 1995). This ligand-induced conformational change presumably permits protein–protein contacts between the AF-2 and the basal transcription machinery, thereby promoting initiation of transcription by RNA polymerase II and its associated factors. An intermediary class of proteins termed coactivators have been proposed to act as a bridge between these two components of transactivation (for review, see Horwitz et al. 1996; Glass et al. 1997). Several putative coactivators of nuclear receptors have been identified recently based on their ligand-dependent interactions with the AF-2 domain. They include RIP-140 (Cavailles et al. 1995), SRC-1/p160 (Onate et al. 1995; Takeshita et al. 1996) renamed NCoA-1 (Torchia et al. 1997), TIF1 (Le Douarin et al. 1995; vom Baur et al. 1996), SUG1/TRIP1 (Lee et al. 1995), CBP/p300 (Hanstein et al. 1996; Kamei et al. 1996), TIF-2/GRIP1 (Voegel et al. 1996; Hong et al. 1997), and ARA70 (Yeh and Chang 1996). Several, but not all, of these proteins can potentiate nuclear receptor transactivation in transient transfection assays. High homologies found between SRC-1/NCoA-1 and TIF2/GRIP1, and more recently p/CIP (Torchia et al. 1997), RAC3 (most likely an alternatively spliced form of p/CIP (Li et al. 1997), ATCR/AIB1 (Anzick et al. 1997; Chen et al. 1997; Li et al. 1997), and TRAM1 (Takeshita et al. 1997) suggest the existence of a family of nuclear receptor coactivators. These proteins are also homologous in the distribution of a leucine-rich sequence (LXXLL) that is required for their interaction with nuclear receptors (Heery et al. 1997; Torchia et al. 1997).
This emerging family of coactivators appears to function, at least in part, through the modification of the nucleosomal structure of DNA, as demonstrated both by the discovery of functional interactions of SRC-1 and ACTR with histone acetyltransferases (HAT) such as p/CAF (Yang et al. 1996), and more recently by the identification of an intrinsic HAT activity in CBP and SRC-1 and its related proteins (Ogryzko et al. 1996; Chen et al. 1997; Spencer et al. 1997). This suggests that activation of transcription by nuclear receptors and their cognate ligands involves the induction of promoter accessibility for the basal machinery through chromatin remodeling mediated directly by an enzymatic activity of coactivators. Interestingly, two highly related receptor corepressors, N-CoR and SMRT, interact with thyroid hormone receptor (TR) and RAR in the absence of their ligands (Chen and Evans 1995; Horlein et al. 1995; Sande and Privalsky 1996), and they also appear to act at the level of chromatin structure via interactions with histone deacetylases (Heinzel et al. 1997; Nagy et al. 1997), leading to a repression of transcription. Their interaction with TR and RAR is in turn disrupted by ligand binding.
The fact that many of the newly isolated and characterized nuclear receptor coactivators are structurally homologous and appear to act primarily by modifying histones suggests a generality in how all coactivators function to mediate the transactivation activity of all nuclear receptors. Whereas they clearly define an important new class of transcription factors, the complexity of a functionally active nuclear receptor poised at its target response element within a promoter may be even greater than we now suspect for at least three reasons. First, with one exception (AIB1), the SRC-1 type coactivator does not appear to display any kind of distinct tissue-specific expression pattern, and therefore cannot solely account for such behavior by nuclear receptors in target tissues. Second, not all of the coactivators interact or coactivate strongly with all nuclear receptors. And third, a putative complex of ligand-dependent TR-interacting proteins known as TRAPs (Fondell et al. 1996) are apparently unrelated to any member of the SRC-1 family of coactivators, underlining the existence of other mechanisms of nuclear receptor transactivation mediated by other distinct classes of proteins.
We have been studying the transcriptional regulatory properties of the vitamin D3 receptor (VDR). To more directly investigate the molecular details of VDR-mediated transactivation, we developed a cell-free transcription system responsive to 1,25(OH)2D3 signaling by utilizing crude nuclear extracts and a G-free cassette-based assay. In this system, transcriptional enhancement in vitro is dependent on purified, exogenous VDR as well as RXR, and is responsive to physiological concentrations of 1,25(OH)2D3 (Lemon et al. 1997). We have reported that 1,25(OH)2D3 enhances RXR–VDR-mediated stabilization or assembly of preinitiation complexes (PICs) to effect transcriptional enhancement from VDRE-linked promoter-containing DNA templates, in part by stabilizing the binding of TFIIA and TFIIB to a TATA box-bound TBP (Lemon et al. 1997).
In addition to direct interactions with basal factors, we presume that VDR also interacts with bridging factors that would act functionally as coactivators. To identify putative VDR coactivators, we designed an affinity column immobilizing the LBD of VDR. As presented here, this column was used to isolate a fraction from Namalwa B-cell nuclear extracts comprised of 8–10 proteins that bind selectively to the VDR LBD as a complex and only in the presence of 1,25(OH)2D3. One or more of the proteins present in this complex possesses HAT activity. Importantly, this complex enhanced 1,25(OH)2D3-dependent transcription by VDR/RXR, since VDR-mediated activation was specifically depleted from the nuclear extract by liganded, but not unliganded, VDR LBD, and the complex stimulated cell-free transactivation by VDR RXR when added back to a transcription extract. We have begun to isolate, clone, and sequence these proteins, here called DRIPs (for vitamin D receptor interacting proteins), and we present the sequence and preliminary functional analysis of one such DRIP. Thus we have demonstrated the existence of a putative complex of novel proteins that associates with the VDR LBD in vitro in a ligand-dependent manner to affect the receptor’s transcriptional activity.
Results
Several nuclear proteins bind selectively to liganded, immobilized VDR LBD
The nuclear receptor LBD encompasses the transcriptional activation domain AF-2, which interacts with auxiliary proteins that appear to communicate with components of the preinitiation complex and/or nucleosomes, and in doing so act as coactivators of RNA polymerase II transcription. The LBD, presumably through the AF-2 subdomain, possesses potent transactivation activity on its own that appears to be entirely hormone-dependent. For example, when the VDR LBD was fused to the GAL4 DNA-binding domain, it conferred strong activation to a reporter regulated by four GAL-binding sites after addition of 1,25(OH)2D3 (Forman et al. 1995; J. Ward and L.P. Freedman, unpubl.), suggesting that most if not all of VDR’s hormone-dependent transcriptional activation function resides in the LBD.
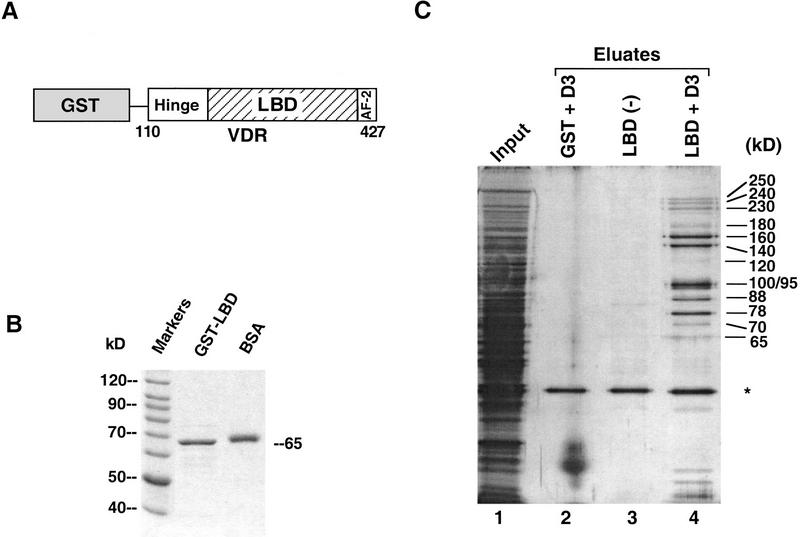
To isolate cofactors of 1,25(OH)2D3-dependent VDR transactivation that interact with this region of the receptor, we first overexpressed VDR LBD and some additional residues immediately amino-terminal to the LBD (amino acids 110–427), fused in-frame to glutathione-S-transferase (GST) (Fig. 1A). The GST moiety was used to immobilize overexpressed, purified fusion protein (Fig. 1B) to glutathione–Sepharose, over which nuclear extracts from human Namalwa B cells were passed. We chose to prepare extracts from Namalwa cells because we found previously that that such extracts support strong 1,25(OH)2D3-enhanced transactivation by exogenously added VDR RXR purified from a baculovirus expression system (Lemon et al. 1997). Namalwa extracts must therefore contain the components necessary to confer transactivation by the VDR-RXR heterodimer in response to ligand. When transcription extracts were run over a GST or unliganded GST LBD column, very few proteins were detected following extensive washing (Fig. 1C, lanes 2,3). In contrast, 10 proteins of molecular weights ranging from 65 to 250 kD selectively bound to the GST LBD column in the presence of 1,25(OH)2D3 (ligand both bound to the LBD and present in the extract) (Fig. 1C, lane 4). These ligand-dependent interacting proteins, hereafter called DRIPs, are distinct from several known nuclear receptor coactivators, such as SRC-1, TIF2, and CBP, or other components of the basal transcription complex that interact with VDR (i.e., TFIIA; Lemon et al. 1997 and TAFII135; Mengus et al. 1997), since antibodies to these proteins did not detect them among the 10 interacting DRIPs when tested by immunoblotting (C. Rachez and L.P. Freedman, unpubl.). In fact, partial microsequencing and mass spectrometric analysis performed thus far on five of the DRIPs revealed that four are encoded by novel genes, since three are not present in any eukaryotic genomic database; a fourth gene, encoding DRIP100 (100 kD) is a cloned human cDNA of unknown function (Nagase et al. 1995; see below). A fifth protein, with an apparent molecular mass of 65 kD, was determined by microsequencing to be human RXRβ (P. Tempst, H. Erdjument-Bromage, C. Rachez, and L.P. Freedman, unpubl.). Moreover, TFIIB, which has been shown to interact with VDR (Blanco et al. 1995; MacDonald et al. 1995; Nagase et al. 1995; Jurutka et al. 1997), was also detected on the GST–VDR LBD column by immunoblotting, but bound VDR independently of ligand (C. Rachez and L.P. Freedman, data not shown). The presence of bound RXR and TFIIB confirms the efficacy of our pull-down strategy, given that both proteins interact with VDR and influence its function.
Figure 1.
The VDR ligand-binding domain interacts with several nuclear proteins in a hormone-dependent manner. (A) Schematic representation of the human VDR LBD fused to GST. The carboxyl terminus of VDR (110–427), was fused to GST. The VDR LBD includes, in addition to the ligand-binding domain, a ligand-dependent transactivation motif AF-2, and a region between the LBD and the DNA-binding domain that has been called the “hinge” to which corepressors N-CoR/SMRT interact with TR and RAR. The numbers correspond to the human VDR amino acid sequence (Baker et al. 1988). (B) Overexpression and purification of GST–VDR LBD. The bacterially overexpressed GST–VDR LBD was purified on glutathione-Sepharose beads and used as an immobilized bait [see (C)]. Five microliters of bead slurry and 0.5 μg of BSA used as a size reference are shown following separation on SDS-PAGE and detected by Coomassie blue staining.(C) Ligand-dependent interactions between VDR LBD and a number of proteins from Namalwa B-cell nuclear extracts. Immobilized GST–VDR LBD was incubated with a Namalwa nuclear extract (input, lane 1) in the absence (ethanol, lane 3) or presence of 1 μm 1,25(OH)2D3 (lane 4). VDR interacting proteins (DRIPs) were eluted from the GST–VDR LBD column by incubation with N-lauroyl sarkosine (Sarkosyl). The eluates were separated by SDS-PAGE and analyzed by silver nitrate staining. Immobilized GST (lane 2) was used as a control protein in the presence of ligand. The approximate, apparent molecular masses of each interacting protein is shown at right. The asterisk denotes a nonspecific binding protein.
Specificity of DRIP interactions with other steroid and nuclear receptors
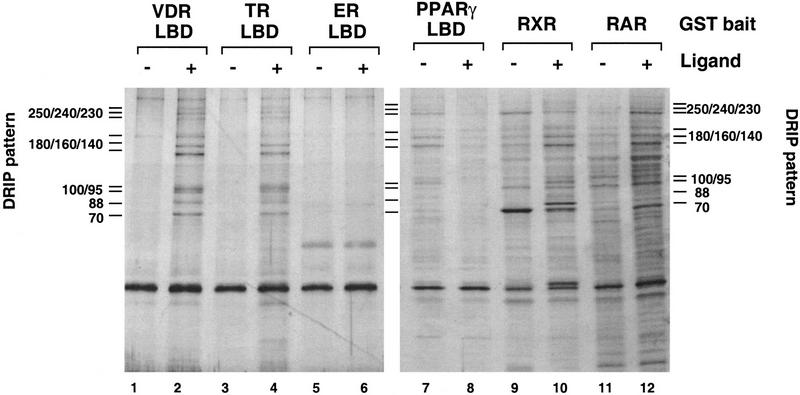
To determine the specificity of the DRIPs for VDR and other members of the nuclear receptor family, the same GST-binding assay was carried out with immobilized LBDs for thyroid hormone, peroxisomal proliferating activated receptor γ, and estrogen receptors (TR, PPARγ, and ER, respectively), as well as full-length RXR, and RAR, all fused to GST and all in the absence and presence of their cognate ligands. As is apparent from the elution profiles shown in Figure 2, a similar if not identical pattern of interacting proteins detected with VDR was seen with TR and PPARγ LBDs, but not with ER LBD, suggesting that a subclass of nuclear receptors interact with DRIPs, but other receptors do not. The patterns observed with RXR and RAR exhibited proteins in common with the DRIPs, but also contained distinct bands (lanes 9–12). This may imply that some of the DRIPs are common components to several nuclear receptors. Interestingly, the DRIP interaction with PPARγ LBD was ligand independent in this assay, and was actually inhibited by 15-deoxy-Δ12,14-PJ2 (lanes 7,8).
Figure 2.
Selectivity of ligand-dependent in vitro interactions of DRIPs with various steroid and nuclear receptors. Namalwa nuclear extracts were incubated, as described in Fig. 1, with immobilized GST fusions to VDR LBD (lanes 1,2), TR LBD (lanes 3,4) ER LBD (lanes 5,6), PPARγ LBD (lanes 7,8), full-length RXR (lanes 9,10) and RAR (lanes 11,12) in the absence (−) or presence (+) of their cognate ligands: 1,25(OH)2D3, TRIAC, estradiol, 15-deoxy-Δ12,14-prostaglandin J2, 9-cis retinoic acid, and all-trans retinoic acid, respectively. The characteristic DRIP binding pattern to VDR LBD is denoted on each side of the gel as a reference.
DRIP interactions with VDR are AF-2-dependent
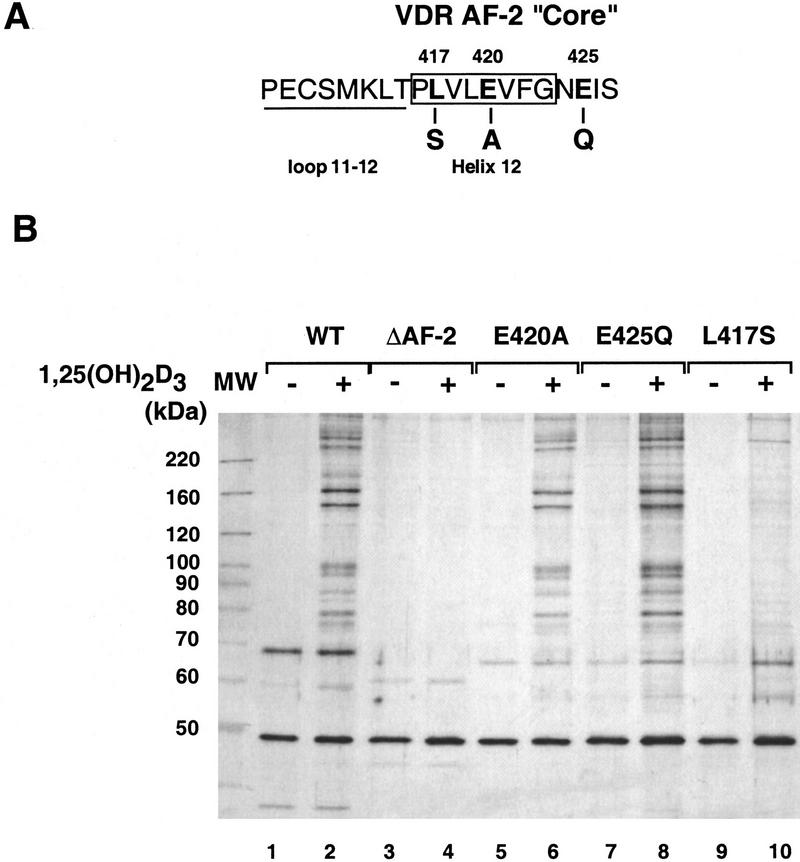
The SRC-1 family of nuclear receptor coactivators appear to interact with the extreme carboxy-terminal subdomain of receptors that coincides with the AF-2 core. We asked if the DRIPs also required this region of VDR, whose sequence is shown in Figure 3A, by immobilizing four AF-2 mutants and assaying DRIP interaction. One mutant lacks the entire AF-2 core (ΔAF-2; 403–427); two additional mutants alter either a conserved Glu at position 420 or a Leu at 417. All three mutations abolish VDR’s ability to transactivate in response to 1,25(OH)2D3 in vivo (Masuyama et al. 1997; J. Ward and L.P. Freedman, unpubl.). A fourth mutation changes a nonconserved Glu at residue 425 to Gln; this mutation has no affect on VDR’s ability to transactivate in vivo. As shown in Figure 3B, the ΔAF-2 and L417S mutants were unable to interact with the DRIPs in either the absence or presence of ligand (lanes 3,4,9,10). Taking into account the lower ligand-binding affinity of the ΔAF-2 mutant (Kd = 9.1 nm; Masuyama et al. 1997) relative to the VDR LBD (Kd = 0.9 nm), we also tested DRIP binding in the presence of 10−5 m 1,25(OH)2D3, and obtained the same result as in lane 5 (data not shown). As expected, E425Q retained its ability to interact with the DRIPs (lanes 7,8). However, E420A also bound the DRIPs in a pattern indistinguishable from the wild-type LBD (lanes 5,6). These results differentiate the DRIP–VDR interaction from the typical SRC-1/nuclear receptor interaction, in that the latter requires the conserved AF-2 E420 for the interaction, but the DRIPs clearly do not. Nevertheless, as is the case with SRC-1, DRIP association with VDR absolutely depends on another key residue, L417, and ultimately on an intact AF-2 core.
Figure 3.
DRIP interactions with VDR are AF-2-dependent. (A) Amino acid sequence of the extreme carboxyl terminus of VDR. Based on the crystal structures of three related nuclear receptor LBDs, the final loop and α-helix (helix 12), overlapping the AF-2 core, are denoted. (B) DRIP interactions with AF-2 mutants. Namalwa nuclear extracts were incubated, as described in Fig. 1, with immobilized GST fusions to the VDR LBD (WT; lanes 1,2); VDR-LBDΔAF-2 (deletion of residues 404–427, lanes 3,4); VDR LBD/E420A (lanes 5,6); VDR LBD/E425Q (lanes 7,8); VDR LBD/L417S (lanes 9,10). Each pair is shown in the absence (−) or presence (+) of 10−6 m 1,25(OH)2D3.
DRIPs interact with VDR as a large complex
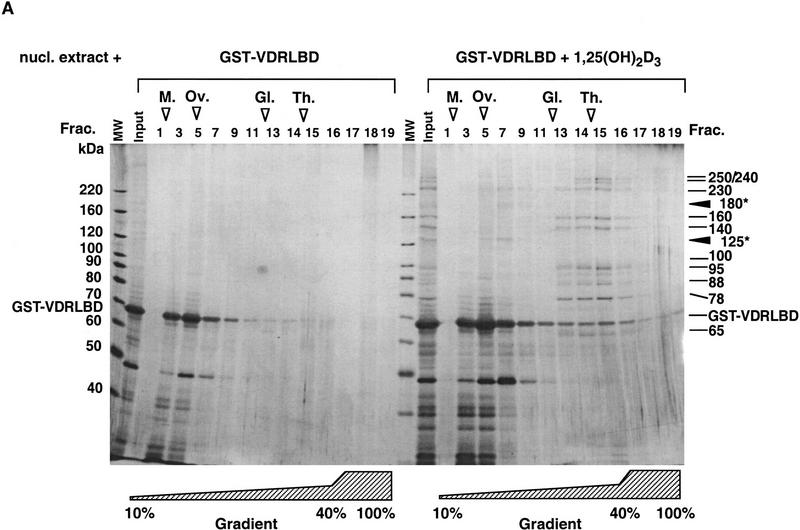
The fact that several proteins bound VDR in the presence of ligand suggested that the DRIPs interact as a single high-molecular-weight complex. We could not rule out, however, that each protein was independently making direct, individual interactions with VDR LBD (or as an array of several different small complexes). To distinguish between these possibilities, we used glycerol gradient sedimentation to estimate the size of the complex bound to VDR in the presence of 1,25(OH)2D3. When GST–VDR LBD and associated DRIPs were run through a glycerol gradient, 10 DRIPs comigrated (Fig. 4A, peak fraction 15), in an area of the gradient in which a complex >700 kD would be expected to sediment. This result indicates that the DRIPs bound together with VDR as a large multiprotein complex. Two proteins identified initially from the affinity purification, DRIP125 and DRIP180 (Fig. 1C), were not detected in the peak fractions of the gradient, but rather appeared to cosediment with GST–VDR LBD (fractions 5–7). This suggested that these two proteins might preferentially partition with GST–VDR LBD, inferring that they interact directly with VDR. Consistent with this, immunoblotting of the gradient with an anti-RXRβ antibody placed RXRβ in fraction 5 of the gradient, in which the majority of GST–VDR LBD sedimented (C. Rachez and L.P. Freedman, unpubl.).
Figure 4.
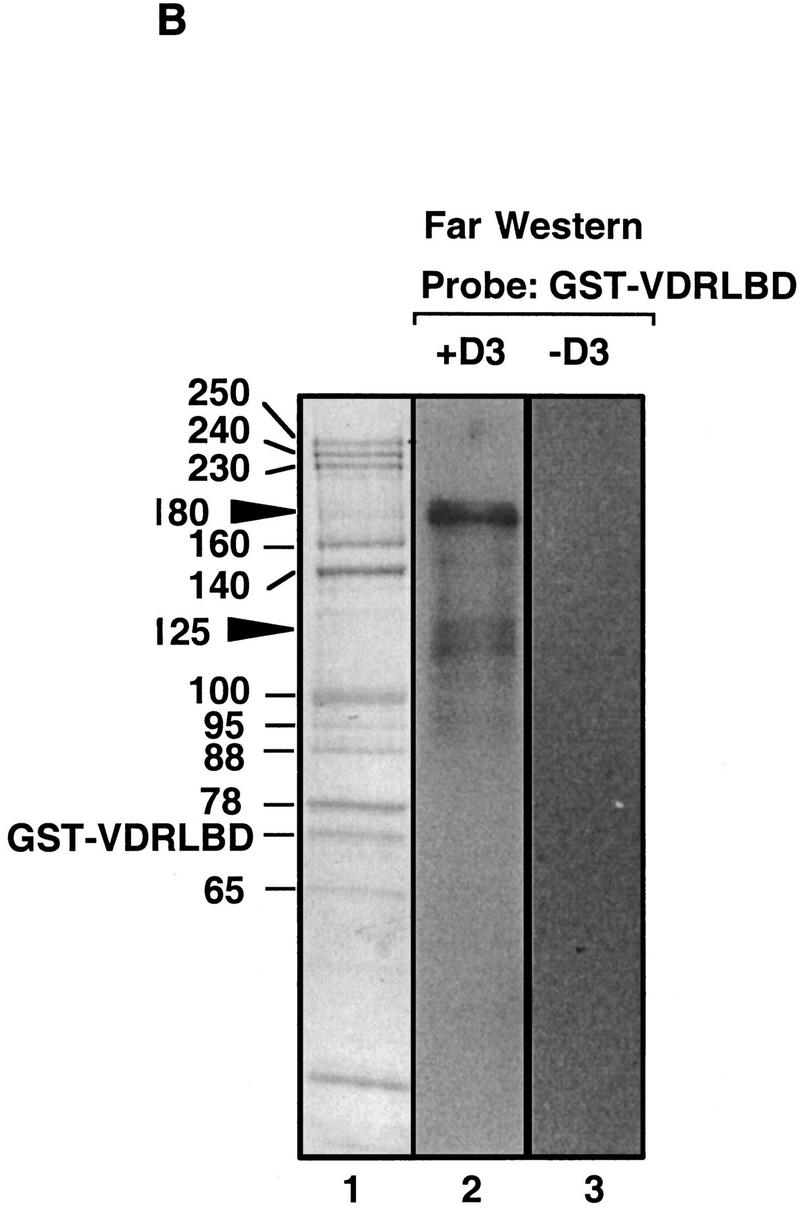
DRIPs bind to VDR LBD as a complex. (A) Glycerol gradient analysis: Nuclear extracts were passed over immobilized GST–VDR LBD in the absence or presence of 1,25(OH)2D3; receptors were then eluted from glutathione beads with reduced glutathione, and applied to 10%–40% glycerol gradients. (Left) SDS-PAGE analysis of glycerol gradient fractions of GST–VDR LBD in the absence of 1,25(OH)2D3; (right) fractions of GST–VDR LBD in the presence of 1,25(OH)2D3. Gradient fractions are from 1–17, with a 100% cushion at the bottom of the gradient (fractions 18 and 19). The position of marker proteins of known molecular masses as they sedimented through the gradient are shown above the fraction numbers: Myoglobin, 17 kD; ovalbumin, 44 kD; gamma globulin, 158 kD; thyroglobulin, 670 kD. Note that uncomplexed GST–VDR LBD, with an apparent molecular mass of 70 kD, sedimented between fractions 5–7 in both gradients. Asterisks denote two proteins of 180 and 125 kD, present in the input that cosediment with GST–VDR LBD (fractions 5–7). (B) Far Western blot. (Lane 1) Coomassie stain of the 1,25(OH)2D3-dependent DRIP complex. (Lanes 2,3) Far Western blot of the DRIP protein eluate (10 μg total protein) probed with 32P-labeled GST–VDR LBD in the presence (lane 2) or absence (lane 3) of 10−7 m 1,25(OH)2D3. Note that the exposure time of the blot probed with unliganded VDR LBD was four times longer than the same blot probed with liganded VDR LBD (4 days vs. 1 day). The solid arrowheads denote the detectable protein bands on the blot, running with apparent molecular mass of 180 and 125/130 kD.
To address which DRIP proteins might be interacting directly with VDR, we carried out a Far Western assay on the DRIP eluate, using unliganded and liganded GST–VDR LBD as probes. As shown in Figure 4B, no detectable signal was discernable among the purified DRIPs when probed with unliganded VDR LBD (lane 3), but one prominent band and two weaker bands were apparent when the same filter was probed with radiolabeled 1,25(OH)2D3-VDR-LBD (lane 2). The stronger of the two signals corresponded to a protein with an apparent molecular mass of 180 kD, and the weaker two bands to proteins just larger than 120 kD. Interestingly, the bands correspond to the same proteins that cosedimented with GST–VDR LBD, but not the rest of the complex, in the glycerol gradient (Fig. 4A, fractions 5–7 vs. 13–15), suggesting that they are able to bind VDR-LBD as individual partners of the receptor, whereas the predominant pool of DRIP proteins associated with VDR (i.e., the high-molecular-weight complex) cannot bind the receptor when dissociated in the denaturing conditions of the Far Western assay. However, given the limitations of the Far Western blot, we cannot rule out the possibility that the majority of the DRIPs simply are not properly renatured in this assay.
Functional analysis of the VDR–DRIP complex in cell-free transcription assays
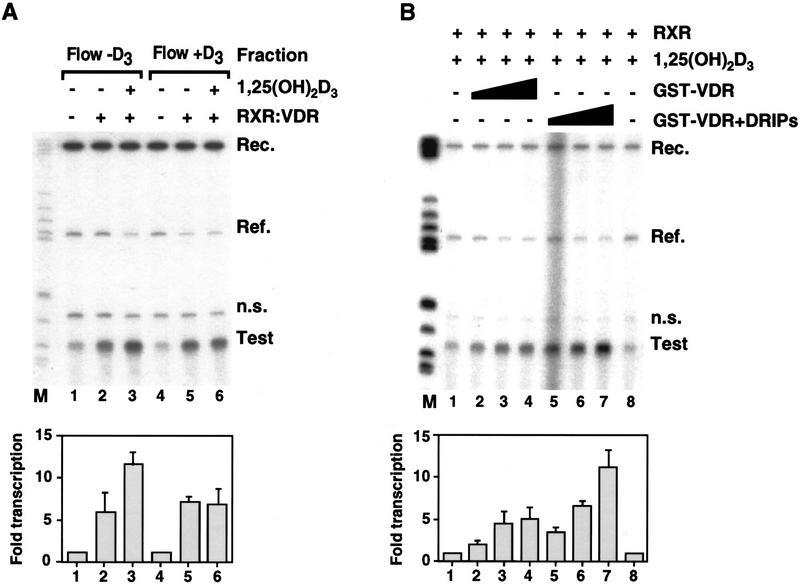
Because the DRIP complex derives from nuclear extracts, previously we were able to support transactivation by purified VDR–RXR heterodimers in vitro, we used this cell-free transcription assay to test the ability of the DRIP complex to mediate transcriptional activation by VDR. To do so, we first tested the ability of immobilized GST–VDR LBD to deplete the nuclear extract of its VDR-enhancing activity, specifically in the presence of ligand. Flow-through fractions collected from VDR LBD columns without and with 1,25(OH)2D3 (Fig. 5A, Flow − D3 and Flow + D3, respectively) were tested in the presence of exogenously added, purified VDR and RXR, in the absence or presence of 1,25(OH)2D3. Flow − D3 fractions supported a sevenfold ligand-dependent transcriptional enhancement by VDR–RXR and 1,25(OH)2D3 on a VDRE-containing G-less cassette template (Test, lanes 1–3). In contrast, the Flow + D3 fraction supported very little, if any, 1,25(OH)2D3-dependent transactivation (lane 5 vs. 6), although the constitutive activation observed typically in this system (Lemon et al. 1997) was not affected (cf. lanes 2 and 5). Thus, immobilized, liganded GST–VDR LBD appears to specifically deplete an activity that is necessary for 1,25(OH)2D3-dependent VDR–RXR transactivation.
Figure 5.
Functional analysis of the DRIP complex in a VDR/1,25(OH)2D3-responsive in vitro transcription assay. (A) Depletion experiment: Flow-through fractions run over GST–VDR LBD columns in the absence (Flow − D3; lanes 1–3) or presence (Flow + D3; lanes 4–6) of 1,25(OH)2D3 were used as transcription extracts and tested for their ability to support RXR–VDR activated transcription from a VDRE-linked E1B promoter G-less cassette template. RXR–VDR (25 ng) or 25 ng of RXR–VDR plus 10−7 m 1,25(OH)2D3 was added to each flowthrough. Transcription was allowed to proceed for 45 min, and radiolabeled transcripts 105 nucleotides in length (Test) recovered and separated on a 6% denaturing polyacrylamide gel. A reference E1B promoter-containing G-less cassette of 200 bases devoid of VDREs was included in each reaction as an internal control (Ref). (n.s.) Nonspecific transcript consistently seen in our assay (Lemon et al. 1997). 32P-End-labeled DNA recovery control (Rec.) and molecular mass markers (M) are also shown. (B) Co-eluted GST–VDR–DRIP complex potentiates VDR–RXR transactivation in vitro. Full-length GST–VDR was immobilized on glutathione—Sepharose beads, and nuclear extracts or GST-binding buffer were run over liganded columns as described for the LBD derivative. VDR–DRIP complexes were eluted with 15 mm reduced glutathione and added directly to 25 μg of transcription extracts supplemented with 20 ng of exogenously added RXR plus 10−7 m 1,25(OH)2D3 (lanes 1–8). In vitro transcription assays were carried out as described in A. (Lanes 1,8) No GST-VDR added; (lanes 2–4) 25, 50, and 100 ng of GST–VDR in the absence of DRIPs; (lanes 5–7) 25, 50, and 100 ng GST–VDR in the presence of bound DRIPs. Equivalent amounts of eluted GST–VDR with or without accompanying DRIPs was determined by SDS-PAGE (data not shown). In both A and B, quantitation data is from digitally scanned autoradiographs. Fold transcription (test/reference) were relative to basal transcription (lanes 1,8) set at a value of 1.0.
To directly test the contribution of the DRIPs when added back to the transcription extract, we formed DRIP complexes on full-length GST–VDR immobilized to glutathione beads, running nuclear extracts over the beads in the presence or absence of 1,25(OH)2D3. Because dissociation and recovery of the DRIPs from GST–VDR LBD required rather harsh buffer conditions, we recovered the DRIPs as an intact complex with full-length VDR by elution with reduced glutathione. Both GST–VDR or GST–VDR plus associated DRIPs were then tested in the cell-free transcripton assay, supplemented with added RXR and 1,25(OH)2D3 (Fig. 5B). The presence of DRIPs in the assay provided a two to threefold stimulation of VDR–RXR transcription above that observed with VDR–RXR in the absence of these proteins (lanes 4–6 vs. 1–3, respectively). The ligand-dependent association of one or all of the DRIP proteins with VDR selectively affects receptor function by enhancing its ability to transactivate from a VDRE (vitamin D response element)-linked promoter.
The DRIP complex possesses HAT activity
Recently, several proteins described that act to coactivate transcription appear to do so by acetylating histones, presumably at specific residues, resulting in a destabilization of nucleosomal structure and an accompanying increase in accessibility of DNA to other transcription factors. The SRC family of nuclear receptor coactivators possess HAT activity, as does CBP. To determine if a HAT activity is present among the DRIP proteins, we carried out a filter binding assay with the DRIP complex in the presence of 3H-labeled acetyl CoA, using either calf thymus histones as substrate or BSA as a negative control. Whereas GST–VDR LBD alone did not exhibit HAT activity, a complex of GST–VDR and the DRIPs (eluted from glutathione beads with reduced glutathione), or the DRIPs eluted from VDR with low concentrations of the detergent Sarkosyl, conferred strong HAT activity when histones, but not BSA, served as the substrate (Fig. 6). This activity was considerably greater than any endogenous HATs from CV-1 cell extracts, or from CV-1 cells transfected transiently with GRIP-1 (TIF-2) or CBP.
Figure 6.
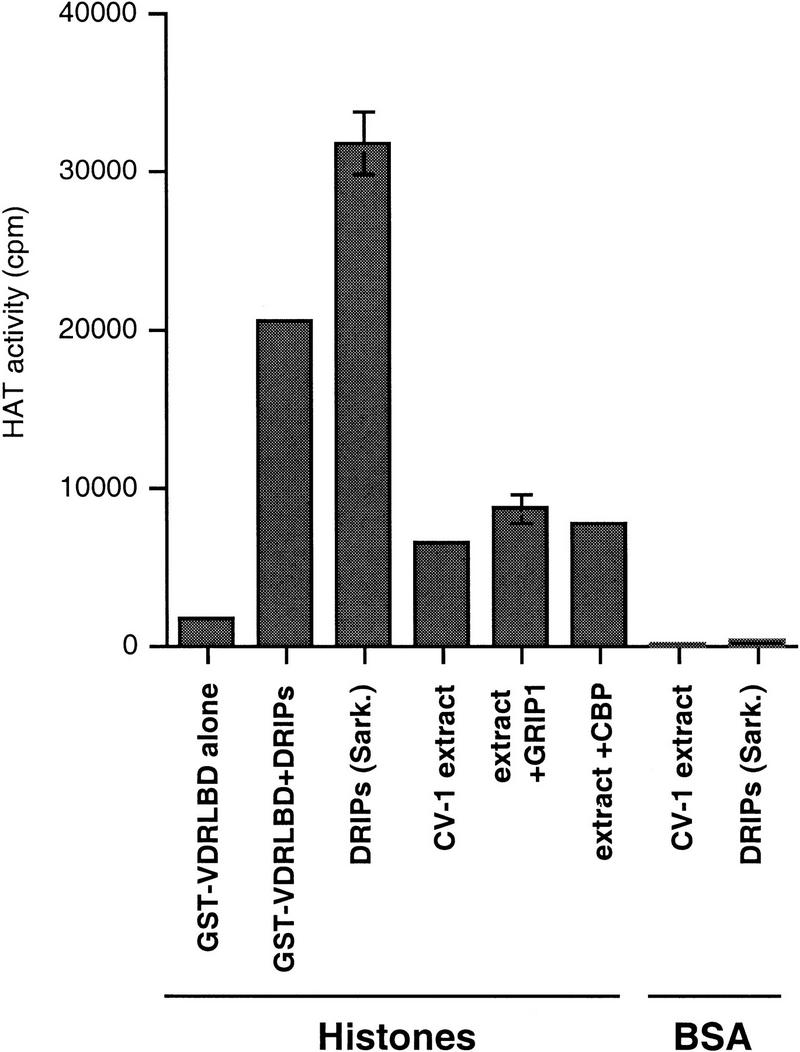
A component of the DRIP complex contains HAT activity. The indicated fractions were incubated with either free histones or BSA, together with 3H-labeled acetyl-CoA, and assayed for HAT activity in a filter-binding assay as described by (Brownell and Allis 1995). The amount of HAT activity is quantitated as cpm 3H-labeled acetate transferred from acetyl CoA to histones. GST–VDR LBD + DRIPs are the glutathione-eluted complex used in the transcription experiments described in Fig. 5B. [DRIPs (Sark.)] represent DRIPs eluted from GST–VDR LBD beads with the detergent Sarkosyl. CV-1 extract, extract + GRIP-1, and extract + CBP represent nuclear extracts from untransfected cells, or GRIP-1 or CBP-transfected CV-1 cells, respectively.
DRIP100 is encoded by a novel gene and contains LXXLL signature motifs
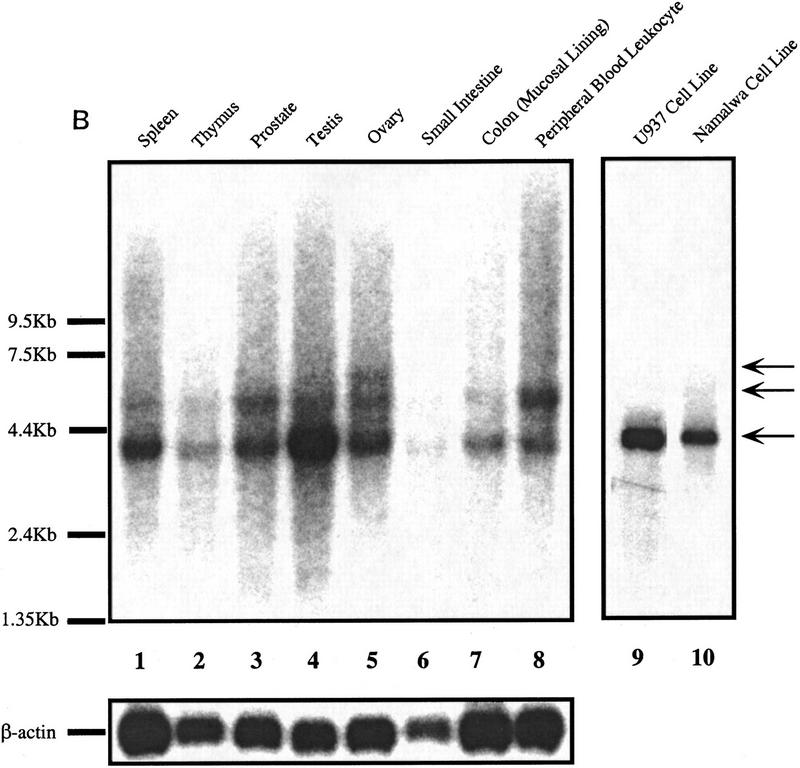
As mentioned earlier, immunoblotting of the DRIP complex with antibodies to a series of known nuclear receptor coactivators and other components of the transcription apparatus did not reveal any of these proteins to be associated with VDR. As such, we have begun a systematic approach to cloning and characterizing each of the 10 DRIPs. At present, we have protein microsequence and mass spectrometric information on five of the proteins (DRIP65 [RXRβ], 78, 100, 140, and 160), and it is clear from this data that three of the five proteins are encoded by novel genes or genes for which no function has been assigned (Z. Suldan and L.P. Freedman, unpubl.). We are in the process of cloning and sequencing full-length cDNAs encoding these proteins. DRIP100 already existed in public databases, previously cloned as KIAA0130 (GenBank accession no. D50920), which was isolated from a myeloid leukemic KG-1 cell cDNA library but has not been functionally characterized (Nagase et al. 1995). We isolated this gene from U937 cells by RT–PCR, and its translated sequence is presented in Figure 7A. The expression of DRIP100 mRNA, which appears as major and minor species of ∼4 and 5 kb, respectively, is not tissue-specific; however, it exhibited relatively low expression in the colon and thymus, whereas it was highly expressed in testes (Fig. 7B). As expected, DRIP100 mRNA was readily detected in Namalwa cells, as well as in a myelomonocytic cell line that differentiates in response to 1,25(OH)2D3, U937 (lanes 9,10).
Figure 7.
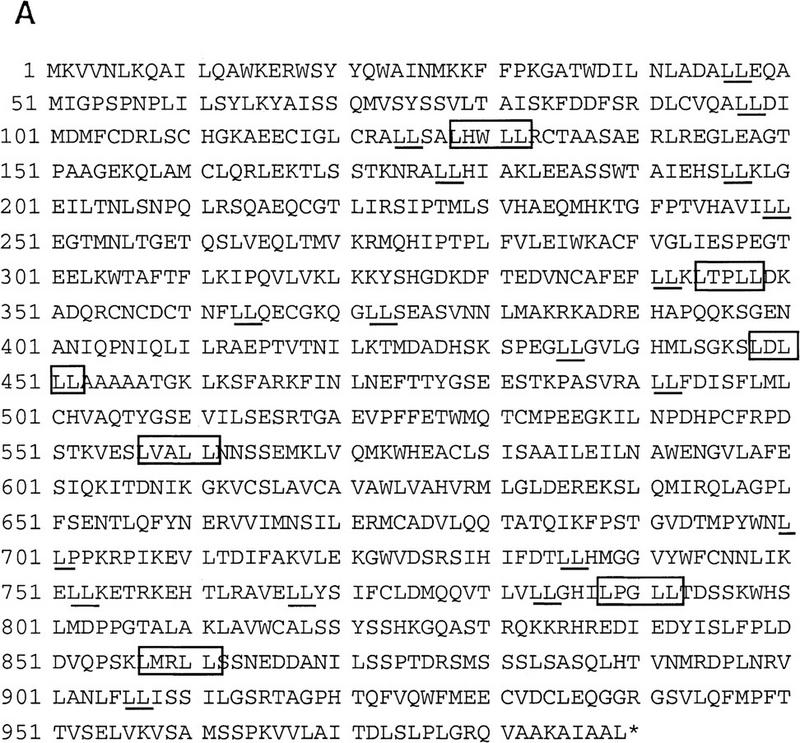
DRIP100, a novel protein that makes up part of a larger, ligand-dependent coactivator complex. (A) Primary sequence of DRIP100. Boxed residues denote a LXXLL signature motif previously identified as a short region that confers interaction with steroid/nuclear hormone receptors (Heery et al. 1997; Torchia et al. 1997). Note also several leucine doublets that are nonconsensus but nevertheless might be functional motifs (Heery et al. 1997). (B) Multiple tissue Northern blot of DRIP100. Two micrograms each of poly(A)+ RNA from the indicated human tissues (lanes 1–8) and from two cell lines (lanes 9,10) were probed with a radiolabeled 2.7-kb fragment of DRIP100 cDNA. (Arrows) One major and two minor RNA species; the predominant species is ∼4 kb. The blot was stripped and reprobed with a β-actin DNA fragment (shown directly below the DRIP100 blot).
Examination of the sequence indicated that DRIP100 is unrelated to the SRC-1 family of coactivators, but it does contain six LXXLL signature motifs previously identified as nuclear receptor interaction domains in SRC-1 and CBP family members (Heery et al. 1997; Torchia et al. 1997). In vitro transcribed and translated DRIP100, however, did not appear to interact directly with GST-VDR when the latter was used as bait in an in vitro pull-down assay. In addition, bacterially overexpressed DRIP100 was found to lack any detectable HAT activity (C. Rachez and L.P. Freedman, unpubl.).
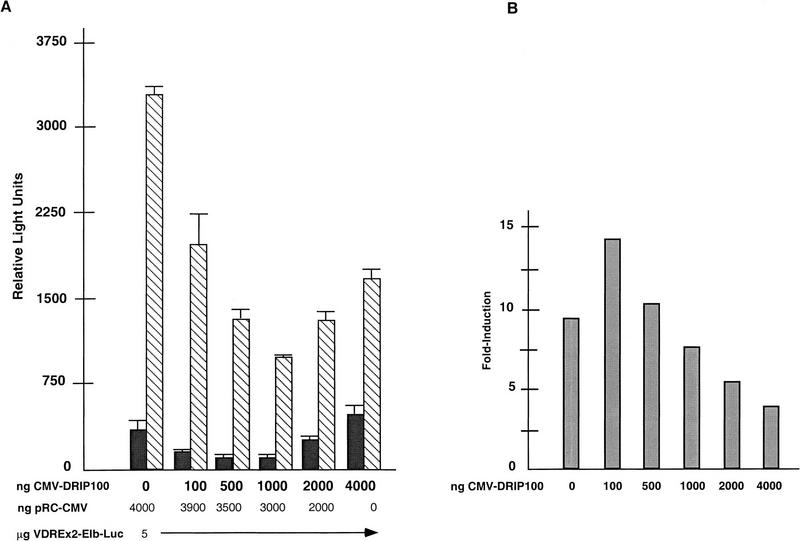
Whereas DRIP100 does not interact directly with VDR, it presumeably interacts with other DRIPs as part of the overall complex. Its overexpression in vivo might therefore lead to an imbalance and possible sequestration of limiting factors (i.e., other DRIPs) that make up the functional VDR transactivation complex, leading to transcriptional squelching (Ptashne 1988). When DRIP100 was transiently overexpressed together with VDR and a responsive reporter gene plasmid in HeLa cells, a modest coactivation, and then an increasing attenuation of 1,25(OH)2D3-dependent transactiva-tion, was detected with increasing amounts of DRIP100 transfected (Fig. 8). These effects were not seen from a reporter gene constituitively expressed from an SV40 enhancer/promoter when the same DRIP100 titration was carried out (Z. Suldan and L.P. Freedman, unpubl.). This result suggests that the overexpression of one component, DRIP100, can act to sequester limiting factors, including other components of the DRIP complex, specifically required for VDR transactivation.
Figure 8.
Effect of overexpression of DRIP100 on VDR transactivation in HeLa cells. (A) In each transfection, 5.0 μg of (VDRE)×2–E1B–LUC reporter, and 0–4.0 μg of CMV–DRIP100 DNA was used. Each transfection was carried out without (EtOH) or with 10−8 m 1,25(OH)2D3 and 2.0 μg of a normalizing reporter, CMV–βgal. Transactivation was from endogenous VDR. The amount of CMV expression plasmids was kept constant by balancing the total amount with empty CMV vector (pRc-CMV). Quantitation is from a representative experiment done in triplicate, although the same trend was observed in multiple transfections. (B) Fold induction in response to 1,25(OH)2D3 (ratios of +ligand/−ligand taken from A).
Discussion
Whereas the multiple functions and even partial structures of the various members of the steroid/nuclear receptor superfamily have been elegantly described since the first receptor cDNA for glucocorticoid receptor was isolated 11 years ago, much less is understood about how precisely these proteins act as ligand-regulated transcription factors. Specifically, how does ligand binding translate directly into transcriptional activation? It is clear that one result of the ligand-receptor interaction is an influence on dimerization, whereby homodimerizing steroid receptors are stabilized and nuclear receptors move toward partnering with RXR (Cheskis and Freedman 1997). A second effect is on the global fold of the LBD, in which helical subregions reconfigure their positions relative to the unliganded state (Bourguet et al. 1995; Renaud et al. 1995). However, the molecular details of how these changes manifest themselves to ultimately increase or enhance the number of initiation events at a RNA polymerase II promoter remain obscure. Presuming that transcriptional enhancement involves the recruitment or stabilization of key components of the preinitiation complex (Ptashne and Gann 1997), the identification and characterization of these other players are essential to sort out the mechanism of ligand-inducible transactivation by nuclear receptors.
In the work presented here, we used a biochemical approach to isolate putative coactivators of VDR. The advantage of this strategy over the widely used yeast two-hybrid assay was twofold. First, it allowed us to isolate what we believe is a large macromolecular complex of at least 10 nuclear proteins that interact with VDR in a fully ligand- and AF-2-dependent manner. Consistent with the idea that the DRIPs comprise an actual complex is that 10 of the factors cosedimented with VDR through a glycerol gradient. We envision these proteins as a macromolecular complex nucleated around one or more DRIPs whose direct interaction with VDR must be 1,25(OH)2D3-dependent. Far-Western blotting indicated that DRIP180, and perhaps two smaller proteins, may associate directly with the receptor in a ligand-dependent manner. Additional evidence of this proposed arrangement awaits the cDNA cloning of all the genes encoding the DRIPs and subsequent generation of antibodies and mutant proteins.
A second advantage of the biochemical approach was that the source of the DRIPs was a nuclear extract that we found capable of supporting VDR-RXR transactivation in vitro, suggesting that all the components necessary for 1,25(OH)2D3-responsive transcription is present in this extract. This in turn provided us with a direct, cell-free assay system to test the functionality of the DRIPs. A similar strategy has been used to fractionate crude nuclear extracts that are apparently distinct from known general transcription factors but are nevertheless required for retinoic acid-dependent transcription in vitro (Valcarcel et al. 1997). Here, the addition of the VDR–DRIP complex to the transcription extract enhanced VDR–RXR transcription from a VDRE template to a greater extent than the addition of exogenous VDR and RXR alone, implying that one or all of the DRIPs are limiting in the extract. Consistent with this, selective depletion of the DRIPs from the extract by liganded VDR LBD led to a decrease in the ability of VDR–RXR to activate transcription in response to 1,25(OH)2D3. Because the DRIPs appear to associate as a multiprotein complex, the net effect of the complex might depend on the relative stoichiometries of the DRIPs to each other and to VDR. Overexpression of one DRIP might lead to the sequestration of one or more components of the rest of the complex or other factors that are part of the Pol II transcription apparatus, any one of which could be limiting. Indeed, although low amounts of transfected DRIP100 led to a modest coactivation, overexpression of higher levels of DRIP100 in transient transfection experiments led to a strong attenuation of VDR transactivation. A similar effect on nuclear receptor transactivation was observed with TAFII135 (Mengus et al. 1997), and by overexpression of TIF-1 (Le Douarin et al. 1995) and TAFII28 (May et al. 1996). We suggest that this kind of transcriptional squelching may be occurring at the level of competition for one or several components comprising the DRIP complex.
The DRIP complex appears to be unrelated to any of the coactivators of the SRC1/NCoA family, since immunoblotting of the purified DRIPs did not identify SRC1 or TIF2 within the complex; CBP was also not detected. Moreover, partial protein sequences of 5 of the 10 DRIPs thus far obtained by microsequencing did not reveal any homology at the amino acid or nucleic acid levels with previously cloned coactivators; RXRβ, as might be predicted, did appear as part of the hormone-dependent complex. Although it has no homology with the SRC1 family, DRIP100 contains several signature motifs (LXXLL) apparently required for interaction with nuclear receptors. It did not, however, appear to bind directly to VDR in in vitro pull-down assays (J. Ward, C. Rachez, and L.P. Freedman, unpubl.) suggesting that the signature motif alone might not be the sole determinant for this interaction. In addition, the DRIP interaction, like SRC-1 and its homologs, requires VDR’s AF-2 domain. However, the structural requirements for the interaction may be somewhat different from that of SRC-1, since a conserved Glu within the AF-2 core (E420 in VDR), shown previously to be essential for ligand-dependent transactivation and association with SRC-1 (Masuyama et al. 1997), was not necessary for DRIP association with VDR, whereas a second residue, L417, also critical for VDR transactivation, was required for DRIP binding to VDR. Besides distinct contact points within the AF-2 as compared to SRC-1, we cannot rule out that DRIP binding to VDR may involve additional regions of the LBD. TR’s interaction with a putative coactivator called TRAM-1 (Takeshita et al. 1997) appears to require helix 3 within the TR LBD. Interestingly, helix 3 is not part of the AF-2 core but is folded in close proximity to the AF-2 helix in the liganded TR crystal structure (Wagner et al. 1995).
Although apparently distinct from the SRC-1 family of coactivators, the molecular weight pattern of the DRIPs resolved by gel electrophoresis is very similar if not identical to a complex of proteins first described by Fondell and Roeder as TR activating proteins, called TRAPs (Fondell et al. 1996). The TRAPs form a thyroid hormone-dependent complex with an epitope-tagged TR stably expressed in HeLa cells in vivo, and strongly enhanced TR/RXR transcription in a purified transcription system in vitro. Our complex appears to have a less potent effect on VDR-RXR transactivation, at least in our in vitro assay, but this might be because the GST-VDR/DRIP interaction in vitro is much less quantitative (∼30:1, VDR/DRIPs; see Fig. 5B) than the TR/TRAP stoichiometry in vivo (1:1). As a result, eluted GST–VDR would bring comparatively less DRIPs when added back to the cell-free assay.
Subtle but potentially important differences may distinguish our DRIP complex from the TRAPs. We identified RXRβ as a component of our complex by microsequencing. The TRAP complex did not contain any of the three RXR isoforms. We also detected strong HAT activity in the DRIP complex (Fig. 6). These differences leave open the possibility that some DRIPs are common to the TRAPs, whereas a subset are unique (and vice versa), perhaps conferring a level of specificity to vitamin D3 versus thyroid hormone signaling. As each member of the DRIP complex is cloned and overexpressed, we will begin to piece together the function or functions of what may be yet another level of complexity and perhaps specificity imposed on VDR and most likely other members of the nuclear receptor superfamily.
Materials and methods
Plasmids
The GST–VDR LBD (amino acids 110–427) was constructed by PCR amplification of full-length VDR from CMV–VDR (Lemon and Freedman 1996) using primers designed to add a BamHI site to both ends of the amplified product. The PCR product was inserted into pGEX-3X digested by BamHI to create an in-frame fusion of GST and VDR LBD. VDR-AF-2 mutants ΔAF-2(110–403), L417S, E420Q, and E425Q were obtained from P. MacDonald (St. Louis University School of Medicine, MO) as full-length constructs in pSG5. They were PCR amplified to generate the same LBD fragment described above (110–427), and were ligated in the same way into pGEX-3X. Bacterial overexpression plasmids encoding GST–TRβLBD(145–456), GST–PPARγLBD (163–475), GST–ER-LBD(312–595), and GST–RAR were kindly provided by M. Bagchi (Population Council, New York, NY), M. Lazar (University of Pennsylvania School of Medicine, Philadelphia), M. Garabedian (New York University Medical Center, NY), and C. Glass (University of California, San Diego), respectively. GST–VDR (full-length), GST–RXR, and the G-less cassette vectors used in in vitro transcription were described previously (Cheskis et al. 1995; Lemon et al. 1997). For transient expression and assay in tissue culture, the plasmids pRC–CMV (Invitrogen), pCMV–VDR, (VDRE)2E1B–LUC, pOTCO, and pCMVβ–gal were used and have been described previously (Lemon et al. 1997).
Ligands
Crystalline 1,25(OH)2D3 and estradiol were diluted in ethanol, TRIAC was diluted in 20 mm Tris-HCl (pH 9), and 9-cis retinoic acid was diluted in DMSO in the dark. They were generously provided by M. Uskokovic (Hoffman La Roche, Nutley, NJ), M. Garabedian, M. Bagchi, and R. Clerc (Hoffman La Roche, Basel, Switzerland), respectively. 15-deoxy-Δ12,14-PGJ2 was purchased from Cayman Chemicals. All trans retinoic acid was a gift of P. Pandolfi (MSKCC).
Overexpression and purification of recombinant proteins
Recombinant full-length VDR and Flag-tagged RXR were overexpressed in a baculovirus system as previously described (Lemon et al. 1997). All GST fusion proteins were overexpressed as described previously (Freedman et al. 1994). Briefly, 500 ml of bacterial cultures expressing the recombinant GST fusion proteins were grown at 37°C to an OD600 of 0.3, at which time the temperature was reduced to 20°C. Cells were induced by the addition of 0.1 mm isopropyl-β-d-thiogalactopyranoside at OD600 0.6. After 3.5 hr, bacteria were collected by centrifugation and resuspended in 5 ml of lysis buffer [PBS containing 0.5 mm PMSF, 0.5 mg/ml leupeptin, and 1 mm DTT], sonicated, and centrifuged. Soluble extracts were incubated with a glutathione-sepharose matrix (Pharmacia) for 1 hr at 4°C before washing three times in lysis buffer. The amounts of protein immobilized on beads were estimated by SDS-PAGE by comparison with a titration of bovine serum albumin (BSA, Sigma) after Coomassie blue staining.
GST LBD affinity binding assay
Immobilized GST fusion proteins (20 μg) were preincubated for 1 hr at 4°C with 1 mm ligand or carrier in GST-binding buffer [20 mm Tris-HCl (pH 7.9), 180 mm KCl, 0.2 mm EDTA, 0.05% NP-40, 0.5 mm PMSF, 1 mm DTT] containing 1 mg/ml BSA. Immobilized proteins on beads were then incubated at 4°C for 6–10 hr with 2–4 mg of Namalwa nuclear extract adjusted to 180 mm KCl, plus 10−6 m 1,25(OH)2D3 or carrier. After three washes in 1 ml of GST wash buffer (GST-binding buffer containing 0.1% NP-40), elution was performed by incubation in GST wash buffer containing 0.2% N-lauroyl sarkosine (Sarkosyl, Sigma). Samples were resolved by SDS-PAGE, and analyzed by silver nitrate staining. For functional assays of GST–VDR with or without associated DRIPs in an in vitro transcription assay (see below), the GST-binding assay was performed in the same conditions as above, except that the GST–VDR was incubated with nuclear extract or GST binding buffer containing 1 mg/ml BSA, both in the presence of 1 μm 1,25(OH)2D3. After washing, GST–VDR with or without associated DRIPs was eluted from its matrix with 15 mm reduced glutathione in GST binding buffer.
Liquid HAT assay
HAT activity in DRIP fractions and cell extracts was assayed essentially as described by Brownell and Allis (1995). Briefly, various DRIP fractions were mixed with 10 μg of calf thymus histones (Type IIA, Sigma) or BSA and 3H-labeled acetyl CoA (4.7 Ci/mmole, Amersham). The reaction was carried for 30 min at 30°C, spotted onto Whatman P-81 filters, and washed extensively with sodium carbonate buffer, (pH 9.1). Radioactivity remaining on the filter was then quantitated by liquid scintillation counting.
Glycerol density gradient
Fractions bound to immobilized GST–VDR LBD with and without 1,25(OH)2D3 were eluted with 15 mm reduced glutathione in elution buffer [50 mm Tris-HCl (pH 8.3), 150 mm KCl, 0.5 mm EDTA, 0.5 mm PMSF, 5 mm NaF, 0.08% NP-40, 0.5 mg/ml BSA, and 10% glycerol]. Eluates were layered on top of a 4.5-ml linear 10%–40% glycerol gradient in GST-binding buffer. The gradient was preformed on top of a 250-μl cushion of 100% glycerol; this was done to separate the highest molecular weight complexes from potential large aggregates. Gradients were centrifuged for 16 hr at 4°C at 40,000 rpm in an SW55 Ti rotor (Beckman). Fractions of 250 μl were then collected from the top of the tubes and analyzed by silver staining of SDS-PAGE following TCA precipitation. Protein standards run in parallel gradients, included vitamin B12 (1.3 kD), myoglobin (17 kD), ovalbumin (44 kD), γ-globulin (158 kD), and thyroglobulin (667 kD).
Far Western blot
GST–VDR LBD immobilized on the glutathione–Sepharose matrix was 32P-labeled by using 100 U/ml of the catalytic subunit of protein kinase A (Sigma) in DK buffer [50 mm Na-phosphate (pH 7.5), 10 mm MgCl2, 5 mm NaF, 4.5 mm DTT] and 8 μl of [γ-32P]ATP (3000 Ci/mmole, Amersham) for 30 min at 30°C. Beads were then washed immediately three times in DK buffer, and the labeled probe was eluted using 15 mm reduced glutathione in elution buffer [50 mm Tris-HCl (pH 8.3), 150 mm KCl, 0.5 mm EDTA, 0.5 mm PMSF, 5 mm NaF, 0.08% NP-40, 0.5 mg/ml BSA]. The probe was analyzed by SDS-PAGE and its specific activity quantitated by scintillation counting. For the Far Western blot assay, DRIP eluates were resolved by SDS-PAGE, then transferred onto nitrocellulose membrane (Transblot 0.45, BioRad) in Towbin buffer (25 mm Tris-Cl 192 mm glycine, 15% methanol). The transferred material was denatured in HB buffer [20 mm Na-phosphate (pH 7.5), 75 mm NaCl, 2.5 mm MgCl2, 0.1 mm EDTA, 1 mm DTT] containing 6 m guanidine-HCl (Fisher) and then gradually renatured by 1:1 serial dilutions in HB buffer without guanidine-HCl. The membrane was blocked in HB buffer containing 1% nonfat dry milk, and 5 mm NaF, and incubated for 6–10 hr with 20,000 cpm/ml of 32P-labeled GST–VDR LBD in the same buffer containing 10−7 m 1,25(OH)2D3. After three washes in HB buffer containing 1% milk, 5 mm NaF, and 0.05% NP-40, the membrane was dried and exposed for autoradiography with intensifying screen at −70°C.
Cell culture, nuclear extract preparation, and transient transfection
Namalwa B cells (ATCC) were cultured as previously described (Lemon et al. 1997) except that cells were grown in 5% calf serum (GIBCO), and harvested at a density of 2 × 106 cells/ml. Nuclear extracts were prepared according to the method of Dignam (Dignam et al. 1983). HeLa cells were transfected and assayed as described previously (Lemon and Freedman 1996). Briefly, cells were maintained in Dulbecco’s modified Eagle medium (high glucose) supplemented with 10% fetal bovine serum (Gemini), penicillin, and streptomycin. Calcium phosphate coprecipitation was used with 2.0 μg of pCMV–β-gal as an internal control, 5.0 μg of VDRE×2–E1B–LUC reporter, a total of 4.0 μg of CMV expression vector containing varying amounts of pCMV–DRIP100 (balanced with pRc–CMV to a total 6.0 μg in each transfection), and 4.0 μg of a pUC-derived vector containing no enhancer or promoter sequences as carrier DNA. HeLa cells were seeded 24 hr prior to transfection at 2.5 × 105 cells per plate and transfected for 12 hr. Coprecipitates were removed with Tris-buffered saline (TBS). The TBS was replaced with the above-described media containing 10% fetal bovine serum twice stripped with dextran-coated charcoal for ligand treatment. 1,25(OH)2D3 was delivered in ethanol to 0.1% at a final concentration of 10−8 m. Ligand treatment was for 24 hr and cells were harvested 39 hr post-transfection. Harvested cells were resuspended in 52 μl of 250 mm Tris (pH 7.5). Ten micrograms of whole cell extracts, prepared by freeze–thaw lysis, were assayed for luciferase activity by dilution in cell culture lysis reagent (Promega) and measurement in 100 μl of luciferase assay reagent (Promega) in a luminometer. Luciferase activity was normalized to β-Gal activity and expressed as relative luciferase units.
In vitro transcription assay
Transcription assays were performed as described previously (Lemon et al. 1997) with the following modifications: The volume of receptor incubation mix with ligand was decreased from 10 to 6 μl to allow more diluted fractions of Namalwa extracts to be tested in the assay. The different fractions of extract tested (i.e., flow-through fractions from the various GST columns) were stripped of any residual 1,25(OH)2D3 by incubation twice for 1 hr at 4°C with 10:1 volume of dextran-coated charcoal [5% charcoal (Sigma), 0.5% dextran T-70 (Sigma)], equilibrated in BC100 buffer [20 mm Tris-HCl (pH 7.9), 100 mm KCl, 20% glycerol 0.2 mm EDTA, 0.5 mm PMSF, 1 mm DTT] followed by centrifugation for 10 min at 7000g. These fractions were then dialyzed twice against BC100 buffer. The transcription assays for the depletion experiments were performed with 35 μg of the extract fractions with or without 50 ng of purified VDR/RXR and 10−7 m 1,25(OH)2D3 per assay. The assays of GST–VDR plus or minus DRIPs were carried out with 50 and 100 ng of GST–VDR eluates plus or minus DRIPs, as measured by BioRad assay and by comparison in Coomassie-stained gels (reflecting amounts of GST–VDR, not the total amounts of proteins). These proteins were added together with 25 μg of extract, 25 ng of purified RXR, and 10−7 m 1,25(OH)2D3 per assay.
Protein microsequencing
Protein samples were prepared according to the GST binding assay procedure scaled up proportionally: 1.2 mg of bait protein was incubated with 150 mg of nuclear extract. Proteins were transferred onto nitrocellulose membranes as described above. Ponceau S-stained bands were excised, in situ tryptic-digested, and fractionated by reverse-phase HPLC using a 0.8-mm Vydac C18 column (Lui et al. 1996). Selected peak fractions were analyzed by a combination of matrix-assisted laser desorption time-of-flight mass spectrometry (Reflex III; Bruker-Franzen, Bremen, Germany) and an automated Edman sequencing (477A; Applied Biosystem, Foster City, CA) (Tempst et al. 1994).
cDNA isolation of DRIP100
The cDNA encoding DRIP100 was obtained by RT–PCR using 1.2 μg of total RNA obtained from U-937 cells induced 12 hr by 1,25(OH)2D3. cDNA was synthesized using Superscript reverse transcriptase II (GIBCO-BRL); PCR was carried out using the Expand High Fidelity PCR System (Boehringer Mannheim) according to the manufacturer’s protocol. Primer sequences encompassing both ends of the cDNA were designed from the published sequence of the KIAA0130 mRNA (Nagase et al. 1995). Primers also contained restriction sites for ClaI and NdeI at the 5′ end of the cDNA, and for BamHI at its 3′ end. The 3.1-kb PCR product obtained was ligated directly into pGEM-T (Promega), and then subcloned into pCMV5 via ClaI and BamHI restriction sites. The DRIP100 cDNA clone was reverified by sequencing (Utah State University DNA sequencing facility).
Northern analysis
Human multiple tissue blots (Clontech), and 10 μg of poly(A)+RNA prepared from U937 and Namalwa B cells, were hybridized with a DRIP100 probe generated as a 2.7-kb ClaI–BamHI restriction fragment from the cDNA inserted into pGEM-T (Promega); this fragment was gel purified and random primed to a specific activity of 9.0 × 105 cpm. Following hybridizaton, the blot was dried and exposed to X-ray film.
Acknowledgments
We thank Milan Bagchi, Barry Forman, Michael Garabedian, Chris Glass, Mitch Lazar, Paul MacDonald, and Michael Stallcup for plasmids; and Pierre Chambon, Paul Lieberman, Noko Tanese, Robert Tjian, and Ming Jer-Tsai for antibodies. We are grateful to Michael Garabedian, Bryan Lemon, and Ben Luisi for discussions and constructive comments on the manuscript. This work was supported by grants from the National Institutes of Health and the Human Frontier Science Program to L.P.F., and MSKCC support grant CA-08748. L.P.F. is a Scholar of the Leukemia Society of America.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Note added in proof
Since acceptance of this paper, we have identified DRIP230 by mass spectrometry as RB18A/PBP/TRIP2 (Drane et al. 1997, Oncogene 15: 3013–3024; Zhu et al. 1997, J. Biol. Chem. 272: 25500–25506; Lee et al. 1995, Mol. Endocrinol. 9: 243–254). Furthermore, we find that a fragment of this protein interacts strongly with liganded, but not unliganded, VDR in vitro.
Footnotes
E-MAIL l-freedman@ski.mskcc.org; FAX (212) 717-3298.
References
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- Baker AR, McDonnell DP, Hughes M, Crisp TM, Mangelsdorf DJ, Haussler MR, Pike JW, Shine J, O’Malley BW. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci. 1988;85:3294–3298. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barettino D, Ruiz MV, Stunnenberg HG. Characterization of the ligand-dependent transactivation domain of thyroid hormone receptor. EMBO J. 1994;13:3039–3049. doi: 10.1002/j.1460-2075.1994.tb06603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco J, Wang I, Tsai S, Tsai M, O’Malley B, Jurutka P, Haussler M, Ozato K. Transcription factor TFIIB and the vitamin D receptor cooperatively activate ligand-dependent transcription. Proc Natl Acad Sci. 1995;92:1535–1539. doi: 10.1073/pnas.92.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguet W, Ruff M, Chambon P, Groyenmeyer H, Moras D. Crystal structure of the ligand-binding domain of the human nuclear receptor RXR-α. Nature. 1995;375:377–388. doi: 10.1038/375377a0. [DOI] [PubMed] [Google Scholar]
- Brownell J, Allis C. An activity gel assay detects a single, catalytically active histone acetyltransferase subunit in Tetrahymena macronuclei. Proc Natl Acad Sci. 1995;92:6364–6368. doi: 10.1073/pnas.92.14.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavailles V, Dauvois S, L’Horset F, Lopez G, Hoare S, Kushner PJ, Parker MG. Nuclear factor RIP140 modulates transcriptional activates by the estrogen receptor. EMBO J. 1995;14:3741–3751. doi: 10.1002/j.1460-2075.1995.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HW, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- Cheskis B, Freedman LP. Modulation of steroid/nuclear receptor dimerization and DNA binding by ligands. In: Freedman LP, editor. Molecular biology of steroid and nuclear hormone receptors. Boston, MA: Birkhauser; 1997. pp. 133–158. [Google Scholar]
- Cheskis B, Lemon BD, Uskokovic M, Lomedico PT, Freedman LP. Vitamin D3-retinoid-X receptor dimerization is differentially affected by analogues of 1,25 Dihydroxyvitamin D3. Mol Endocribol. 1995;9:1814–1824. doi: 10.1210/mend.9.12.8614417. [DOI] [PubMed] [Google Scholar]
- Danielian PS, White R, Lees JA, Parker MG. Identification of a conseved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992;11:1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mamalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand B, Saunders M, Gaundon C, Roy B, Losson R, Chambon P. Activation function (AF-2) of retinoic acid receptor and 9-cis retinoic acid receptor: Presence of a conserved autonomous constitutive activating domain and influence of the nature of the response element on AF-2 activity. EMBO J. 1994;13:5370–5382. doi: 10.1002/j.1460-2075.1994.tb06872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondell JD, Ge H, Roeder RG. Ligand induction of a transcriptionally active thyroid hormone receptor co-activator complex. Proc Natl Acad Sci. 1996;93:8329–8333. doi: 10.1073/pnas.93.16.8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman BM, Umesono K, Chen J, Evans RM. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Freedman LP, editor. Molecular biology of steroid and nuclear hormone receptors. Boston, MA: Birkhauser; 1997. [Google Scholar]
- Freedman LP, Arce V, Perez Fernandez R. DNA sequences that act as high affinity targets for the vitamin D3 receptor in the absence of the retinoid-X receptor. Mol Endocrinol. 1994;8:265–273. doi: 10.1210/mend.8.3.8015545. [DOI] [PubMed] [Google Scholar]
- Glass CK, Rose DW, Rosenfeld MG. Nuclear receptor coactivators. Curr Opin Cell Biol. 1997;9:222–232. doi: 10.1016/s0955-0674(97)80066-x. [DOI] [PubMed] [Google Scholar]
- Hanstein B, Eckner R, DiRenzo J, Halachmi S, Liu H, Searcy B, Kurokawa R, Brown M. p300 is a component of an estrogen receptor coactivator complex. Proc Natl Acad Sci. 1996;93:11540–11545. doi: 10.1073/pnas.93.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- Heinzel T, Lavinsky RM, Mullen TM, Soderstrom M, Laherty CD, Torchia J, Yang WM, Brard G, Ngo SD, Davie JR, Seto E, Eisenman RN, Rose DW, Glass CK, Rosenfeld MG. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature. 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- Hong H, Kohli K, Garabedian MJ, Stallcup MR. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol Cell Biol. 1997;17:2735–2744. doi: 10.1128/mcb.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlein AJ, Naar AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass CK, Rosenfeld MG. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–403. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- Horwitz KB, Jackson TA, Bain DL, Richer JK, Takimoto GS, Tung L. Nuclear receptor coactivators and corepressors. Mol Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- Jurutka P, Hsieh J-C, Remus L, Whitfield G, Haussler C, Blanco J, Ozato K, Haussler M. Mutations in the 1,25-dihydroxyvitamin D3 receptor identifying C-terminal amino acids required for transcriptional activation that are functionally dissociated from homrone binding, heterodimeric DNA binding, and interaction with TFIIB. J Biol Chem. 1997;272:14592–14599. doi: 10.1074/jbc.272.23.14592. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S-C, Heyman RA, Rose DW, Glass CK, Rosenfeld MG. A CBP-integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- Le Douarin B, Zechel C, Garnier J-M, Lutz Y, Tora L, Pierrat B, Heery D, Gronenmyer H, Chambon P, Losson R. The N-terminal part of TIF1, a putative mediator of the ligand dependent activation function (AF-2) of nuclear receptors, is fused to B-Raf in the oncogenic protein T18. EMBO J. 1995;14:2020–2033. doi: 10.1002/j.1460-2075.1995.tb07194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Ryan F, Swaffield JC, Johnston SA, Moore DD. Interaction of thyroid-hormone receptor with a conserved transcriptional mediator. Nature. 1995;374:91–94. doi: 10.1038/374091a0. [DOI] [PubMed] [Google Scholar]
- Lemon BD, Freedman LP. Selective effects of ligands on vitamin D3 receptor- and retinoid X receptor-mediated gene activation in vivo. Mol Cell Biol. 1996;16:1006–1016. doi: 10.1128/mcb.16.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon BD, Fondell JD, Freedman LP. Retinoid X receptor: Vitamin D-3 receptor heterodimers promote stable preinitiation complex formation and direct 1,25-dihydroxyvitamin D-3-dependent cell-free transcription. Mol Cell Biol. 1997;17:1923–1937. doi: 10.1128/mcb.17.4.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Gomes PJ, Chen JD. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci. 1997;94:8479–8484. doi: 10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui M, Tempst P, Erdjument-Bromage H. Methodical analysis of protein-nitrocellulose interactions to design a refined digestion protocol. Anal Biochem. 1996;241:156–166. doi: 10.1006/abio.1996.0393. [DOI] [PubMed] [Google Scholar]
- MacDonald P, Sherman D, Dowd D, Jefcoat SJ, DeLisle R. The vitamin D receptor interacts with general transcription factor IIB. J Biol Chem. 1995;270:4748–4752. doi: 10.1074/jbc.270.9.4748. [DOI] [PubMed] [Google Scholar]
- Masuyama H, Brownfield CM, St. Arnaud R, MacDonald PN. Evidence for ligand-dependent intramolecular folding of the AF-2 domain in vitamin D receptor-activated transcription and coactivator interaction. Mol Endocrinol. 1997;11:1507–1517. doi: 10.1210/mend.11.10.9990. [DOI] [PubMed] [Google Scholar]
- May M, Mengus G, Lavigne AC, Chambon P, Davidson I. Human TAF(II28) promotes transcriptional stimulation by activation function 2 of the retinoid X receptors. EMBO J. 1996;15:3093–3104. [PMC free article] [PubMed] [Google Scholar]
- Mengus G, May M, Carre L, Chambon P, Davidson I. Human TAF(II)135 potentiates transcriptional activation by the AF-2s of the retinoic acid, vitamin D3, and thyroid hormone receptors in mammalian cells. Genes & Dev. 1997;11:1381–1395. doi: 10.1101/gad.11.11.1381. [DOI] [PubMed] [Google Scholar]
- Nagase T, Seki N, Tanaka A, Ishikawa K, Nomura N. Prediction of the coding sequences of unidentified human genes. IV. The coding sequences of 40 new genes (KIAA0121-KIAA0160) deduced by analysis of cDNA clones from human cell line KG-1. DNA Res. 1995;2:167–174. doi: 10.1093/dnares/2.4.167. [DOI] [PubMed] [Google Scholar]
- Nagy L, Kao HY, Chakravarti D, Lin RJ, Hassig CA, Ayer DE, Schreiber SL, Evans RM. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- Ogryzko V, Schlitz R, Russanova V, Howard B, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- Onate S, Tsai S, Tsai M-J, O’Malley B. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988;335:683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M, Gann A. Transcriptional activation by recruitment. Nature. 1997;386:569–577. doi: 10.1038/386569a0. [DOI] [PubMed] [Google Scholar]
- Renaud J-P, Rochel N, Ruff M, Vivat V, Chambon P, Groyenmeyer H, Moras D. Crystal structure of the RAR-g ligand-binding domain bound to all-trans retinoic acid. Nature. 1995;378:681–689. doi: 10.1038/378681a0. [DOI] [PubMed] [Google Scholar]
- Sande S, Privalsky M. Identification of TRACs (T3 receptor-associating cofactors), a family of cofactors that associate with, and modulate the activity of, nuclear hormone receptors. Mol Endocrinol. 1996;10:813–825. doi: 10.1210/mend.10.7.8813722. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou JX, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, Omalley BW. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- Takeshita A, Yen PM, Misiti S, Cardona GR, Liu Y, Chin WW. Molecular cloning and properties of a full-length putative thyroid hormone receptor coactivator. Endocrinology. 1996;137:3594–3597. doi: 10.1210/endo.137.8.8754792. [DOI] [PubMed] [Google Scholar]
- Takeshita A, Cardona GR, Koibuchi N, Suen CS, Chin WW. TRAM-1, A novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J Biol Chem. 1997;272:27629–27634. doi: 10.1074/jbc.272.44.27629. [DOI] [PubMed] [Google Scholar]
- Tempst P, Geromanos S, Elicone C, Erdjument-Bromage H. Improvements in microsequencer performances for low picomole sequence analysis. Methods: Companion Methods Enzymol. 1994;6:248–261. [Google Scholar]
- Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK, Rosenfeld MG. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- Valcarcel R, Meyer M, Meisterernst M, Stunnenberg H. Requirement of cofactor for RXR/RAR-mediated transcriptional activation in vitro. Biochim Biophys Acta. 1997;1350:229–234. doi: 10.1016/s0167-4781(96)00234-5. [DOI] [PubMed] [Google Scholar]
- Voegel JJ, Heine MJ, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- vom Baur E, Zechel C, Heery D, Heine M, Garnier J, Vivat V, Le Douarin B, Gronemeyer H, Chambon P, Losson R. Differential ligand-dependent interactions between the AF-2 activating domain of nuclear receptors and the putative transcriptional intermediary factors mSUG1 and TIF1. EMBO J. 1996;15:110–124. [PMC free article] [PubMed] [Google Scholar]
- Wagner RL, Apriletti JW, McGrath ME, West BL, Baxter JD, Fletterick RJ. A structural role for hormone in the thyroid hormone receptor. Nature. 1995;378:690–697. doi: 10.1038/378690a0. [DOI] [PubMed] [Google Scholar]
- Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- Yeh S, Chang C. Cloning and characterization of a specific coactivator, ARA70, for the androgen receptor in human prostate cells. Proc Natl Acad Sci. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]