Abstract
Although human mast cells express G protein coupled receptors for the anaphylatoxin C3a, previous studies indicated that C3a causes mast cell degranulation, at least in part, via a C3a receptor-independent mechanism similar to that proposed for polycationic molecules such as compound 48/80. The purpose of the present study was to delineate the receptor specificity of C3a-induced degranulation in human mast cells. We found that C3a, a C3a receptor “superagonist” (E7) and compound 48/80 induced Ca2+ mobilization and degranulation in a differentiated human mast cell line, LAD2. However, C3a and E7 caused Ca2+ mobilization in an immature mast cell line, HMC-1 but compound 48/80 did not. We have previously shown that LAD2 cells express MrgX1 and MrgX2 but HMC-1 cells do not. To delineate the receptor specificity for C3a and compound 48/80 further, we generated stable transfectants expressing MrgX1 and MrgX2 in a rodent mast cell line, RBL-2H3 cells. We found that compound 48/80 caused degranulation in RBL-2H3 cells expressing MrgX1 and MrgX2 but C3a did not. By contrast, E7 activated RBL-2H3 cells expressing MrgX2 but not MrgX1. These findings demonstrate that in contrast to previous reports, C3a and compound 48/80 do not use a shared mechanism for mast cell degranulation. It shows that while compound 48/80 utilizes MrgX1 and MrgX2 for mast cell degranulation C3a does not. It further reveals the novel finding that the previously characterized synthetic peptide, C3a receptor “superagonist” E7 activates human mast cells via two mechanisms; one involving the C3a receptor and the other MrgX2.
Keywords: Mast cell, degranulation, C3a, Compound 40/80, MrgX1, MrgX2
1. Introduction
The anaphylatoxin C3a is one of the most potent mast cell chemoattractants known (Hartmann et al., 1997; Nilsson et al., 1996). It also induces degranulation in purified human skin mast cells, peripheral blood CD34+ cell-derived mast cells and a newly developed human mast cell line, LAD2 cells (Fukuoka et al., 2008; Lappalainen et al., 2007; Oskeritzian et al., 2005; Venkatesha et al., 2005; Woolhiser et al., 2004). By contrast, C3a does not induce degranulation in murine peritoneal mast cells, bone marrow-derived mast cells or rat basophilic leukemia, RBL-2H3 cells (Erdei et al., 2004; Soruri et al., 2008). C3a, however, causes substantial degranulation in rat peritoneal mast cells via a pathway that appears to be independent of cell surface C3a receptors (Fukuoka and Hugli, 1990; Mousli et al., 1992). These findings raise the interesting possibility that C3a-induced degranulation in human mast cells may involve C3a receptor-dependent and independent pathways (el-Lati et al., 1994).
Studies with synthetic C3a peptides indicated that a 20 amino acid carboxyl terminal fragment of C3a (C3aP: 58-77; Asn-Tyr-Ile-Thr-Glu-Leu-Arg-Arg-Gln-His-Ala-Arg-Ala-Ser-His-Leu-Gly-Leu-Ala-Arg) expresses biological potency equal to natural C3a (Lu et al., 1984). However, incorporation of two tryptophanyl residues at the N-terminus of a 15-residue C3a analogue (E7; Trp-Trp-Gly-Lys-Lys-Tyr-Arg-Ala-Ser-Lys-Leu-Gly-Leu-Ala-Arg), results in ~1500% increase in guinea pig platelet aggregation activity when compared to the C3aP (Ember et al., 1991). The effect of E7 was shown to be specific for the C3a receptor, as it cross-desensitized the ability of C3a but not C5a to induce guinea pig ileum contraction. Furthermore, compared to C3aP, much lower concentrations of E7 were required to induce vascular permeability in guinea pig skin, a response which presumably depends on mast cells (Ember et al., 1991).
Recently, a large family of G protein coupled receptors (Mas related genes; Mrgs, also known as sensory neuron-specific receptors, SNSR) has been identified in rodents (Dong et al., 2001; Lembo et al., 2002). These receptors are selectively expressed in small-diameter sensory neurons of dorsal root ganglia and are thought to be involved in the sensation and modulation of pain. Interestingly, a subgroup of these receptors (MrgX1 - MrgX4), are expressed in human but not murine neurons (Burstein et al., 2006; Dong et al., 2001). Furthermore, MrgX1 and MrgX2 are expressed in human cord blood-derived mast cells and compound 48/80 activates transfected cells expressing MrgX2 but not MrgX1 (Tatemoto et al., 2006). Previous studies indicated that C3a could activate both human skin mast cells and rat peritoneal mast cells via a pathway similar to that mediated via compound 48/80 (el-Lati et al., 1994; Mousli et al., 1992; 1994). This raises the intriguing possibility that C3a-induced mast cell degranulation could involve both C3a receptor and MrgX2.
The purpose of this study was to determine the receptor specificity of C3a-induced degranulation in human mast cells. Here, we demonstrate that although compound 48/80 induces mast cell degranulation via MrgX1 and MrgX2, C3a does not utilize these receptors for degranulation. We further show that C3a receptor “superagonist” E7 acts as a dual agonist for C3a receptor and MrgX2.
2. Materials and Methods
2.1 Materials
All cell culture reagents were purchased from Invitrogen (Gaithersburg, MD). Monoclonal anti-DNP specific IgE and anti-human IgE were purchased from Sigma Life Sciences, Inc (St. Louis, MO). Human IgE was purchased from EMD Biosciences (San Diego, CA). Amaxa cell transfection kits and reagents were purchased from Lonza (Gaithersburg, MD). Plasmids encoding hemagglutinin (HA)-tagged human MrgX1, and MrgX2 in pReceiver–M06 vector were obtained from Genecopeia (Rockville, MD). All recombinant human cytokines were purchased from Peprotech (Rocky Hill, NJ). Cortistatin-14 (CST) and Bovine Adrenal Medulla Docosapeptide (BAM-22P) were obtained from American Peptide (Vista, California). Human C3a was from Complement Technology (Tyler, Texas). Compound 48/80 was from MP Biomedicals (Solon, OH) and Pertussis toxin was obtained from List Biological Laboratories (Campbell, CA).
2.2 Synthesis and purification of C3a receptor peptides
Linear peptides were synthesized by using standard solid-phase methodology and were assembled with a Fmoc protection on an automatic peptide synthesizer (430A, Applied Biosystems) on a preloaded Fmoc-L-Arginine(Pmc)-HMP resin (Applied Biosystems) (Qu et al., 2011). Peptide deprotection and cleavage was performed using TFA/phenol/water/ thioanisole/ 1,2ethanedithiol, (82.5/5/5/5/2.5) for 3 h at room temperature. All synthetic peptides were purified on a C-18 reverse-phase HPLC column to a ~95% purity and the identity of the peptides was monitored by laser desorbtion mass spectrometry (MALDI-TOF).
2.3 Differentiation of human mast cells from CD34+ progenitors and culture of mast cell lines
Human CD34+ progenitors were cultured in StemPro-34 medium supplemented with L-glutamine (2 mM), penicillin (100 IU/ml), streptomycin (100 µg/ml), SCF (100 ng/ml), IL-6 (100 ng/ml) and IL-3 (30 ng/ml) (first week only). Hemidepletions were performed weekly with media containing SCF (100 ng/ml) and IL-6 (100 ng/ml). Cells were used for experiments after 7–10 weeks in culture (Radinger et al., 2011; Venkatesha et al., 2005). LAD2 cells were maintained in StemPro-34 medium containing nutrient supplements (Invitrogen) supplemented with L-glutamine (2 mM), penicillin (100 IU/ml), streptomycin (100 µg/ml) and 100 ng/ml SCF (Kirshenbaum et al., 2003; Radinger et al., 2011). Cell culture medium was hemi-depleted weekly with fresh culture medium (Kirshenbaum et al., 2003). Human mast cell line, HMC-1 cells were cultured in Iscove’s modified Eagle’s medium supplemented with 10% FCS, L-glutamine (2 mM), penicillin (100 IU/ml) and streptomycin (100 µg/ml). RBL-2H3 cells were maintained as monolayer cultures in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, L-glutamine (2 mM), penicillin (100 IU/ml) and streptomycin (100 µg/ml) (Ali et al., 1994).
2.4 Stable transfection of RBL-2H3 cells
RBL-2H3 cells were detached with versene, washed twice with DMEM and 1 × 106 cells were transfected with plasmids encoding HA-tagged MrgX1 or MrgX2, using the Amaxa nucleofector device and Amaxa kit V according to the manufacturer’s protocol. Following nucleofection, cells were cultured in the presence of G418 (1mg/ml) and cells expressing equivalent receptors were sorted using an anti-HA specific antibody/FITC-conjugated anti-mouse-IgG and used for studies on Ca2+ mobilization and degranulation (Subramanian, 2011).
2.5 Calcium mobilization
Ca2+ mobilization was determined as described previously (Ali et al., 2000; Ali et al., 1993). Briefly, cells (human mast cells; 0.2 × 106 and RBL-2H3 cells; 1.0 × 106) were loaded with 1 µM indo-1 AM in the presence of 1 µM pluronic F-127 for 30 min at room temperature. Cells were washed and resuspended in 1.5 ml of HEPES-buffered saline. Ca2+ mobilization was measured in a Hitachi F-2500 spectrophotometer with an excitation wavelength of 355 nm and an emission wavelength of 410 nm (Ali et al., 2000).
2.6 Degranulation assay
LAD2 mast cells, CD34+ cell-derived mast cells (5 × 103) and RBL-2H3 cells (5 × 104) were seeded into 96-well plates overnight in the presence of human IgE (1µg/ml) or mouse IgE (1µg/ml), respectively. The following day, cells were washed and incubated in a total volume of 50 µl of buffer containing 0.1% BSA and exposed to anti-human IgE (human mast cells), DNP-BSA (RBL-2H3) or different concentrations of peptides. For total β-hexosaminidase release, control cells were lysed in 50 µl of 0.1% Triton X-100. Aliquots (15 µl) of supernatants or cell lysates were incubated with 15 µl of 1 mM p-nitrophenyl-N-acetyl-β-D-glucosamine for 1.5 h at 37�C. Reaction was stopped by adding 250 µl of a 0.1 M Na2CO3/0.1 M NaHCO3 buffer and absorbance was measured at 405 nm (Ali et al., 1994).
3. RESULTS
3.1 Activation of human mast cells by C3a, a C3a receptor superagonist and compound 48/80
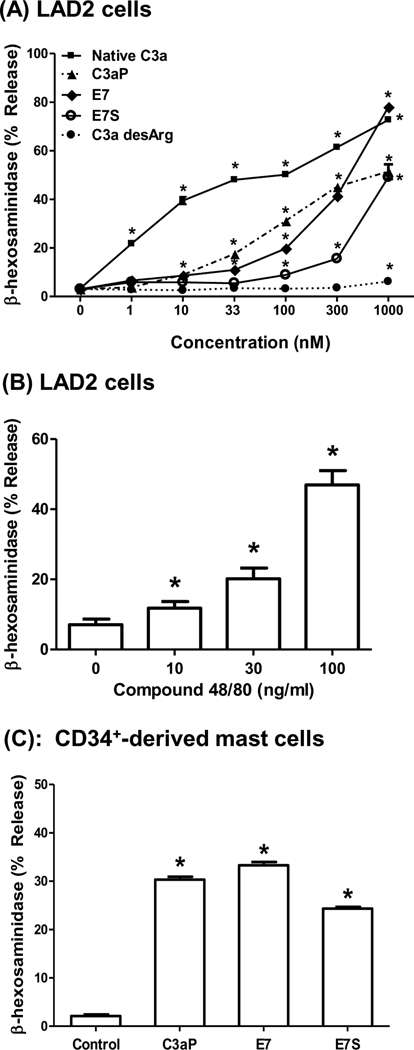
Previous studies showed that recombinant C3a induces degranulation in human skin mast cells, CD34+-derived primary mast cells and a relatively new mast cell line, LAD2 cells (Fukuoka et al., 2008; Lappalainen et al., 2007; Venkatesha et al., 2005; Woolhiser et al., 2004). For the present study, we initially utilized LAD2 cells and determined the effects of purified human C3a, C3a desArg, a peptide corresponding to the 20 carboxyl terminal amino acid sequence of C3a (C3aP), a synthetic C3a receptor superagonist E7 (see Table 1 for peptide sequences) and compound 48/80 on β-hexosaminidase release as a measure of degranulation. As shown in Fig. 1A, C3a induced a dose-dependent degranulation of LAD2 mast cells with a maximal release of ~75% with an EC50 value of 3 nM. C3a desArg, however, did not induce degranulation at any of the concentrations tested. The peptide E7, which was previously shown to be ~ 12 –15 fold more potent than C3a receptor agonist peptide (C3aP) in guinea pigs (Ember et al., 1991), induced a dose-dependent increase in degranulation in LAD2 mast cells. However, its potency for mast cell degranulation was lower than that of C3a. To determine whether the effect of E7 on mast cell degranulation is specific for the C3a receptor, we used a scrambled E7 peptide (E7S) of the same amino acid composition (see Table 1). Surprisingly, E7S also induced mast cell degranulation in a dose-dependent manner. Similar to previous findings in primary human mast cells (Oskeritzian et al., 2005), compound 48/80 also induced degranulation in LAD2 cells (Fig. 1B). To determine the relevance of studies using human LAD2 cells for C3aP, E7, and E7S we tested their effects on degranulation of primary human CD34+-derived mast cells. We found that, as with LAD2 cells, these peptides induced degranulation in primary mast cells (Fig. 1C).
Table I.
Amino acid sequences of the peptides used.
| Peptides | Amino acid sequence |
|---|---|
| C3a peptide (C3aP;C3a 58-77) | Asn-Tyr-Ile-Thr-Glu-Leu-Arg-Arg-Gln-His-Ala-Arg-Ala-Ser-His-Leu-Gly-Leu-Ala-Arg |
| C3a receptor superagonist (E7) | Trp-Trp-Gly-Lys-Lys-Tyr-Arg-Ala-Ser-Lys-Leu-Gly-Leu-Ala-Arg |
| E7 scrambled peptide (E7S) | Leu-Arg-Ala-Gly-Ser-Arg-Tyr-Lys-Lys-Trp-Ala-Leu-Lys-Trp-Gly |
| Cortistatin | Pro-[Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Ser-Ser-Cys]-Lys |
| BAM-22P | Tyr-Gly-Gly-Phe-Met-Arg-Arg-Val-Gly-Arg-Pro-Glu-Trp-Trp-Met-Asp-Tyr-Gln-Lys-Arg-Tyr-Gly |
Figure 1. Effects of C3a receptor agonists and compound 48/80 on degranulation in human mast cells.
(A) LAD2 mast cells were stimulated with different concentrations of C3a, C3 desArg, C3aP, C3a receptor superagonist E7 and scrambled E7 (E7S) for 30 min and percent degranulation (β-hexosaminidase release) was determined. (B) LAD2 cells were stimulated with different concentrations of compound 48/80 and degranulation was determined. (C) CD34+-derived human mast cells were exposed to buffer (control), C3a (100 nM), E7 or E7S (1 µM) for 30 min and percent degranulation was determined. Data are mean ± S.E.M of 3 – 4 experiments. Statistical significance was tested using two way (A) or one way (B and C) ANOVA with Bonferroni’s post test. * indicates P<0.05
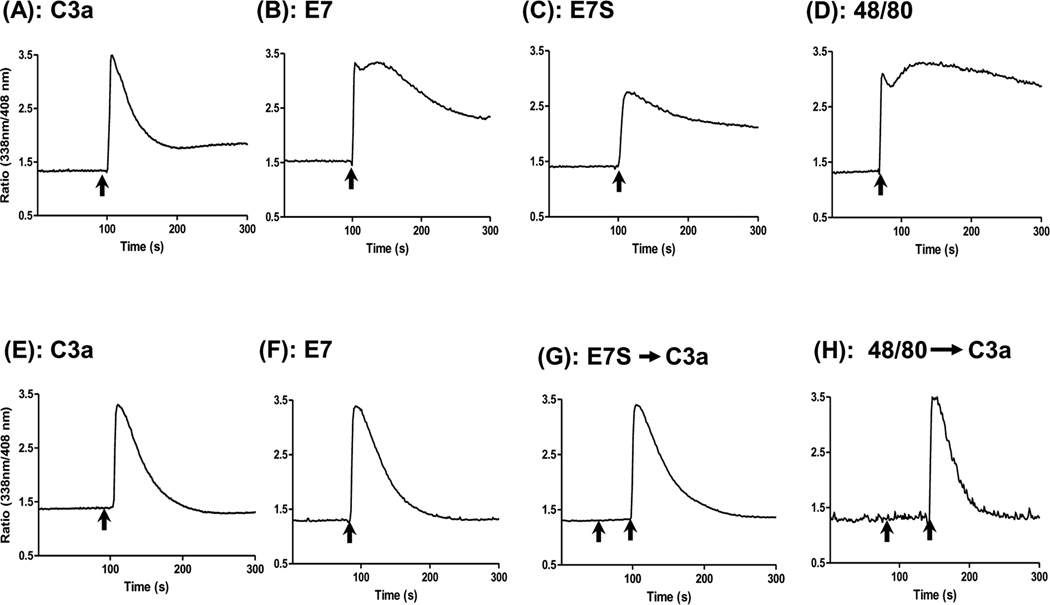
We next sought to determine the signaling pathways via which C3a, its receptor peptides and compound 48/80 activate human mast cells, using intracellular Ca2+ mobilization assay. As shown in Fig. 2A and 2B, C3a and E7 induced rapid Ca2+ responses in LAD2 cells which peaked within seconds after stimulation. However, there was a prominent and sustained response in E7-stimulated cells (Fig. 2B) that was markedly reduced in cells stimulated with C3a (Fig 2A). Interestingly, E7S and compound 48/80 also induced robust and sustained Ca2+ responses in LAD2 mast cells (Fig. 2C and D). HMC-1 cell is a relatively immature mast cell line that expresses C3a receptors and responds to C3a for a transient Ca2+ mobilization (Ahamed et al., 2004; Ali et al., 2000). We therefore tested the effects of C3a, E7, E7S and compound 48/80 on Ca2+ mobilization in HMC-1 cells. Unlike the situation in LAD2 mast cells, C3a and E7 induced Ca2+ mobilization of similar magnitude and duration in HMC-1 cells (Fig 2E, F). However, E7S and compound 48/80 did not initiate Ca2+ responses in this cell line while they were fully responsive to C3a (Fig. 2G, H). These findings suggest that the C3a receptor superagonist E7 activates LAD2 cells via two mechanisms; one involving the C3a receptor and the other independent of the C3a receptor. Furthermore, the C3a receptor-independent effect of E7 does not require an ordered sequence of its amino acids (see Table 1) and may share a common mechanism with compound 48/80.
Figure 2. Ca2+ mobilization by C3a, E7, E7S and compound 48/80 in LAD2 mast cells and HMC-1 cells.
(A– D) LAD2 mast cells (0.2 × 106/ml) and (E– H) HMC-1 cells (1 × 106/ml) were incubated with Indo-1AM and, as indicated by arrows, were stimulated with C3a (10 nM; A and E), E7 (1 µM, B and F), E7S (1 µM; C and G), compound 48/80 (1 µg/ml, D and H) and intracellular Ca2+ mobilization was determined. For panels G and H first arrow (from the left) indicates E7S or compound 48/80 but the second arrow indicates C3a (10 nM). Data shown are representative of 3 similar experiments.
3.2 Distinct roles of MrgX1 and MrgX2 on C3a, E7 and compound 48/80-induced mast cell degranulation
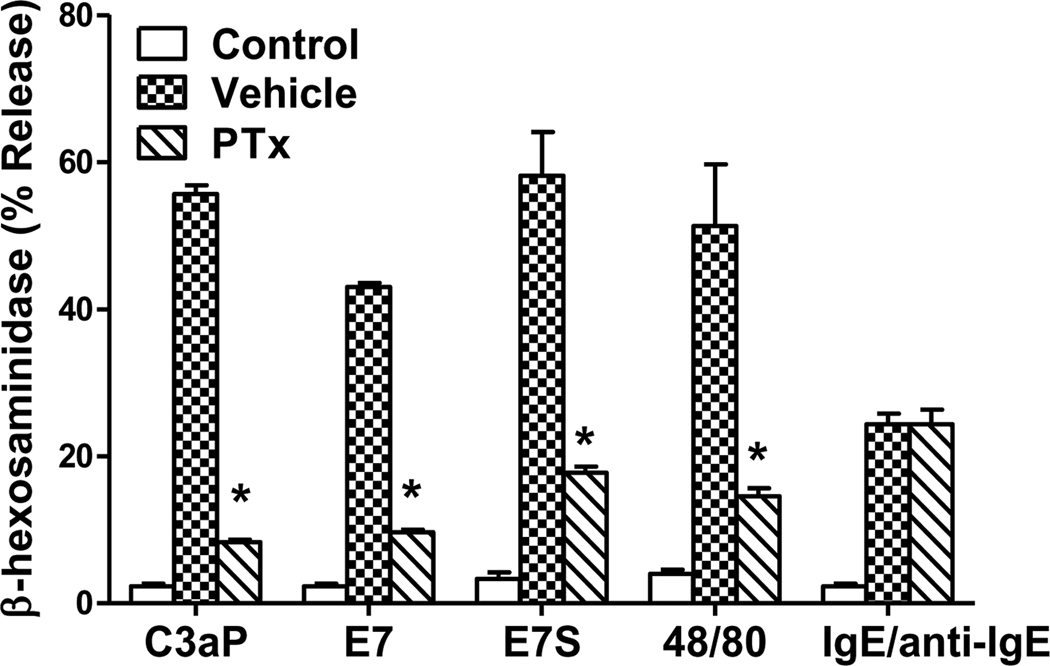
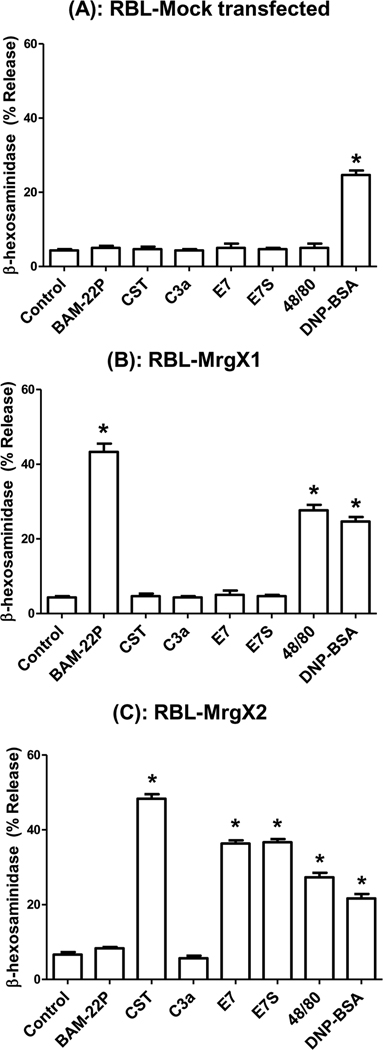
C3a and compound 48/80 induce mast cell degranulation via signaling pathways that require the activation of Gi family of G Proteins (Ahamed et al., 2001; Mousli et al., 1994). To determine the role of G proteins on degranulation in LAD2 mast cells, Pertussis toxin (PTx)-treated or untreated cells were stimulated by C3a, E7, E7S, compound 48/80 and for control anti-IgE in IgE-primed cells. As shown in Fig. 3, PTx treatment inhibited degranulation in response to C3a, E7, E7S and compound 48/80 but not IgE/anti-IgE. Human cord blood-derived mast cells express G protein coupled receptor MrgX1 at low levels and MrgX2 at higher levels (Tatemoto et al., 2006). In transfected HEK293 cells compound 48/80 utilizes MrgX2 but not MrgX1 for Ca2+ mobilization (Tatemoto et al., 2006). We have recently shown that LAD2 and CD34+-derived mast cells express MrgX1 and MrgX2 (Subramanian, 2011). Ligands for MrgX1 and MrgX2 have been identified as neuropeptides Bovine Adrenal Medulla Docosapeptide (BAM-22P) and cortistatin (CST), respectively (Lembo et al., 2002; Robas et al., 2003). To determine the roles MrgX1 and MrgX2 on mast cell degranulation by peptides used in this study, we generated stable transfectants in a rodent mast cell line, RBL-2H3 cells, separately expressing equivalent numbers of HA-tagged MrgX1 and MrgX2 (Subramanian, 2011). We found that none of the peptides or compound 48/80 induced degranulation in mock transfected RBL-2H3 cells (Fig. 4A). However, cells expressing MrgX1 responded only to BAM-22P and compound 48/80 for degranulation (Fig. 4B). By contrast, MrgX2-expressing cells responded to CST, E7, E7S and compound 48/80 (Fig. 4C). Interestingly, C3a (1 µM) did not activate RBL-2H3 cells expressing MrgX1 or MrgX2 (Fig. 4B and C). As expected mock transfected RBL-2H3 cells and cells expressing MrgX1 or MrgX2 responded to antigen for degranulation.
Figure 3. Effects of Pertussis toxin (PTx) on degranulation induced by C3a, E7, E7S, compound 48/80 and antigen in LAD2 mast cells.
Mast cells were cultured with human IgE (1 µg/ml) in the presence or absence of PTx for 16 h. Cells were then washed and stimulated with C3a (100 nM), E7 (1 µM), E7S (1 µM), compound 48/80 (100 ng/ml) or anti-human IgE (1 µg/ml) for 30 min. Degranulation is expressed as percent of total β-hexosaminidase present in granules. Data are mean ± S.E.M of 3 experiments. Statistical significance was tested using two way ANOVA with Bonferroni’s post test. * indicates P<0.05 comparing vehicle to PTx-treated cells.
Figure 4. Roles of MrgX1 and MrgX2 on C3a,E7 and compound 48/80-induced mast cell degranulation.
(A) Mock transfected RBL-2H3 cells (B) cells stably expressing MrgX1 or (C) MrgX2 were incubated with anti-DNP specific IgE (1 µg/ml, 16 hours) and stimulated with C3a (100 nM) or the indicated peptides (1 µM), compound 48/80 (100 ng/ml) or antigen (DNP-BSA, 30 ng/ml) for 30 min and β-hexosaminidase release was measured. Data shown are representative of 3 experiments. Statistical significance was tested using one way ANOVA with Bonferroni’s post test. * indicates P<0.05.
4. DISCUSSION
In the present study, we utilized C3a and its receptor agonist E7, compound 48/80, CD34+-derived primary mast cells, two human mast cell lines as well as RBL-2H3 cells stably expressing human MrgX1 and MrgX2 to determine the receptor specificity of C3a-induced degranulation in human mast cells. Using these systems, we demonstrate that although compound 48/80 activates human mast cells via MrgX1 and MrgX2, C3a does not utilize these receptors for degranulation. We further show that C3a receptor superagonist E7 acts as a dual agonist for the C3a receptor and MrgX2 for mast cell degranulation.
MrgX1 and MrgX2 are expressed in cord blood-derived human mast cells (Tatemoto et al., 2006). Furthermore, compound 48/80 promotes Ca2+ mobilization in HEK293 cells expressing MrgX2 (Tatemoto et al., 2006). We found that compound 48/80 induced degranulation in human mast cells and in RBL-2H3 cells expressing MrgX1 and MrgX2. Since previous studies indicated that C3a activates degranulation in rat peritoneal mast cells via a mechanism similar to compound 48/80 (Fukuoka and Hugli, 1990; Mousli et al., 1992; 1994), it raised the interesting possibility that C3a could also activate mast cell degranulation via MrgX1 and MrgX2. We showed that C3a at concentrations up to 1 µM did not induce degranulation in cells expressing either human MrgX1 or MrgX2. These findings demonstrate that, in contrast to previous suggestions, (Fukuoka and Hugli, 1990; Mousli et al., 1992; 1994), C3a and compound 48/80 do not utilize a shared mechanism for degranulation in human mast cells.
Unlike most GPCRs, Mrg receptors display substantial species specific differences. Interestingly, human Mrg receptors share only 45 – 65% amino acid sequence identity with the mouse and rat receptors. In addition, while there are only four Mrg genes known in humans, 32 distinct Mrg sequences are present in mice. By contrast, the rat genome possesses one each of the MrgA, MrgC, and MrgD genes and ten MrgB genes (Zylka et al., 2003). An important feature of Mrg receptors that distinguishes them from the C3a receptor is that they are low affinity receptors and require ligand in the micromolar range to induce full biological responses (Fukuoka et al., 2008; Robas et al., 2003; Tatemoto et al., 2006). It is noteworthy that rat and mouse peritoneal mast cells do not express the C3a receptor (Mousli et al., 1992; 1994; Soruri et al., 2008) but C3a causes degranulation in rat peritoneal mast cells with an EC50 value of ~1 µM (Mousli et al., 1992), which is in contrast to the EC50 value of ~3 nM in human mast cells (Fig. 1). This raises the interesting possibility that while C3a induces degranulation in human mast cells via the C3a receptor it promotes the same response in rat peritoneal mast cells via one of the Mrg receptors. Rat peritoneal mast cells express a number of Mrg receptors including MrgB1, MrgB2, MrgB3 MrgB6, MrgB8 and MrgB9 (Tatemoto et al., 2006). Compound 48/80 caused reporter gene activation in HEK293 cells expressing rat MrgB3 and given that compound 48/80 and C3a activate rat peritoneal mast cells via a similar mechanism, this suggests both secretagogues may induce degranulation in rat peritoneal mast cells via MrgB3.
An important shared property of the peptides that activate mast cells via MrgX receptors is that they possess amino acids with hydrophobic and positively charged side chains (Table 1). The neuropeptides cortistatin-14 and cortistatin-17 (CST-14 and CST-17) were identified as the first ligands for MrgX2 (Robas et al., 2003). Structural activity studies demonstrated that for CST-14 and CST-17 both the N-terminal proline and the C-terminal lysine are required for MrgX2 activation. Previous studies demonstrated that incorporation of two tryptophanyl residues at the N-terminus of a 15-residue C3a analogue (E7; Trp-Trp-Gly-Lys-Lys-Tyr-Arg-Ala-Ser-Lys-Leu-Gly-Leu-Ala-Arg), results in ~1500% increase in guinea pig platelet aggregation activity when compared to the C3aP (See Table 1). Our studies with C3aP, E7 and E7S clearly demonstrate that for human mast cells, tryptophanyl-residues has no positive impact on the ability of synthetic peptide to activate C3a receptor, but it renders the peptide an agonist for MrgX2. We have recently shown that a potent C5a receptor antagonist PMX-53, activates human mast cells via MrgX2 and its ability to do so requires the presence of tryptophan and a positively charged amino acid residue (Subramanian, 2011). These findings demonstrate that peptides possessing hydrophobic and positively charged side chains including the neuropeptide cortistatin, PMX-53, E7 and E7S act as MrgX2 agonists. The demonstration that compound 48/80 activates both MrgX1 and MrgX2 whereas E7 and E7S (present study) and PMX-53 (Subramanian, 2011) activate MrgX2 suggests distinct structural requirements for the activation of MrgX1 and MrgX2 receptors in mast cells..
In summary, we found that in contrast to previous reports, C3a and compound 48/80 do not share a common mechanism for inducing mast cell degranulation. Previous studies demonstrated that E7 is a C3a receptor superagonist for guinea pig platelet aggregation, smooth muscle contraction and increased vascular permeability, presumably via mast cells activation. The study described herein demonstrates that the C3a receptor superagonist activates human mast cells via two mechanisms; one involving the C3a receptor and the other MrgX2. Given that MrgX2 is expressed only in humans but not in rodents, great care should be exercised when extrapolating data using amphiphilic receptor agonist peptides with aromatic side chains in animal models to humans.
Acknowledgements
We are grateful to Dr. Joseph Butterfield (Mayo Clinic, Rochester, MN) for supplying us with HMC-1 cells. We also thank Drs. Arnold Kirshenbaum and Dean Metcalfe (NIAID/NIH) for providing LAD2 mast cells and the FACS core facilities of the Schools of Medicine and Dental Medicine, University of Pennsylvania for acquisition, analysis and cell sorting. This work was supported by National Institutes of Health Grants HL085774, AI080852 to HA and AI068730 JDL]
REFERENCES
- Ahamed J, Haribabu B, Ali H. Cutting edge: Differential regulation of chemoattractant receptor-induced degranulation and chemokine production by receptor phosphorylation. J Immunol. 2001;167:3559–3563. doi: 10.4049/jimmunol.167.7.3559. [DOI] [PubMed] [Google Scholar]
- Ahamed J, Venkatesha RT, Thangam EB, Ali H. C3a enhances nerve growth factor-induced NFAT activation and chemokine production in a human mast cell line, HMC-1. J Immunol. 2004;172:6961–6968. doi: 10.4049/jimmunol.172.11.6961. [DOI] [PubMed] [Google Scholar]
- Ali H, Ahamed J, Hernandez-Munain C, Baron JL, Krangel MS, Patel DD. Chemokine production by G protein-coupled receptor activation in a human mast cell line: roles of extracellular signal-regulated kinase and NFAT. J Immunol. 2000;165:7215–7223. doi: 10.4049/jimmunol.165.12.7215. [DOI] [PubMed] [Google Scholar]
- Ali H, Richardson RM, Tomhave ED, Didsbury JR, Snyderman R. Differences in phosphorylation of formylpeptide and C5a chemoattractant receptors correlate with differences in desensitization. J Biol Chem. 1993;268:24247–24254. [PubMed] [Google Scholar]
- Ali H, Richardson RM, Tomhave ED, DuBose RA, Haribabu B, Snyderman R. Regulation of stably transfected platelet activating factor receptor in RBL-2H3 cells. Role of multiple G proteins and receptor phosphorylation. J Biol Chem. 1994;269:24557–24563. [PubMed] [Google Scholar]
- Burstein ES, Ott TR, Feddock M, Ma JN, Fuhs S, Wong S, Schiffer HH, Brann MR, Nash NR. Characterization of the Mas-related gene family: structural and functional conservation of human and rhesus MrgX receptors. Br J Pharmacol. 2006;147:73–82. doi: 10.1038/sj.bjp.0706448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- el-Lati SG, Dahinden CA, Church MK. Complement peptides C3a- and C5a-induced mediator release from dissociated human skin mast cells. J Invest Dermatol. 1994;102:803–806. doi: 10.1111/1523-1747.ep12378589. [DOI] [PubMed] [Google Scholar]
- Ember JA, Johansen NL, Hugli TE. Designing synthetic superagonists of C3a anaphylatoxin. Biochemistry. 1991;30:3603–3612. doi: 10.1021/bi00229a003. [DOI] [PubMed] [Google Scholar]
- Erdei A, Andrasfalvy M, Peterfy H, Toth G, Pecht I. Regulation of mast cell activation by complement-derived peptides. Immunol Lett. 2004;92:39–42. doi: 10.1016/j.imlet.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Fukuoka Y, Hugli TE. Anaphylatoxin binding and degradation by rat peritoneal mast cells. Mechanisms of degranulation and control. J Immunol. 1990;145:1851–1858. [PubMed] [Google Scholar]
- Fukuoka Y, Xia HZ, Sanchez-Munoz LB, Dellinger AL, Escribano L, Schwartz LB. Generation of anaphylatoxins by human beta-tryptase from C3, C4, and C5. J Immunol. 2008;180:6307–6316. doi: 10.4049/jimmunol.180.9.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K, Henz BM, Kruger-Krasagakes S, Kohl J, Burger R, Guhl S, Haase I, Lippert U, Zuberbier T. C3a and C5a stimulate chemotaxis of human mast cells. Blood. 1997;89:2863–2870. [PubMed] [Google Scholar]
- Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Lindstedt KA, Kovanen PT. A protocol for generating high numbers of mature and functional human mast cells from peripheral blood. Clin Exp Allergy. 2007;37:1404–1414. doi: 10.1111/j.1365-2222.2007.02778.x. [DOI] [PubMed] [Google Scholar]
- Lembo PM, Grazzini E, Groblewski T, O'Donnell D, Roy MO, Zhang J, Hoffert C, Cao J, Schmidt R, Pelletier M, Labarre M, Gosselin M, Fortin Y, Banville D, Shen SH, Strom P, Payza K, Dray A, Walker P, Ahmad S. Proenkephalin A gene products activate a new family of sensory neuron--specific GPCRs. Nat Neurosci. 2002;5:201–209. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- Lu ZX, Fok KF, Erickson BW, Hugli TE. Conformational analysis of COOH-terminal segments of human C3a. Evidence of ordered conformation in an active 21-residue peptide. J Biol Chem. 1984;259:7367–7370. [PubMed] [Google Scholar]
- Mousli M, Hugli TE, Landry Y, Bronner C. A mechanism of action for anaphylatoxin C3a stimulation of mast cells. J Immunol. 1992;148:2456–2461. [PubMed] [Google Scholar]
- Mousli M, Hugli TE, Landry Y, Bronner C. Peptidergic pathway in human skin and rat peritoneal mast cell activation. Immunopharmacology. 1994;27:1–11. doi: 10.1016/0162-3109(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Nilsson G, Johnell M, Hammer CH, Tiffany HL, Nilsson K, Metcalfe DD, Siegbahn A, Murphy PM. C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin-sensitive signal transduction pathway. J Immunol. 1996;157:1693–1698. [PubMed] [Google Scholar]
- Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, Schwartz LB. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol. 2005;115:1162–1168. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H, Magotti P, Ricklin D, Wu EL, Kourtzelis I, Wu YQ, Kaznessis YN, Lambris JD. Novel analogues of the therapeutic complement inhibitor compstatin with significantly improved affinity and potency. Mol Immunol. 2011;48:481–489. doi: 10.1016/j.molimm.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radinger M, Jensen BM, Kuehn HS, Kirshenbaum A, Gilfillan AM. Generation, isolation, and maintenance of human mast cells and mast cell lines derived from peripheral blood or cord blood. Curr Protoc Immunol Chapter. 2011;7(Unit 7):37. doi: 10.1002/0471142735.im0737s90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robas N, Mead E, Fidock M. MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J Biol Chem. 2003;278:44400–44404. doi: 10.1074/jbc.M302456200. [DOI] [PubMed] [Google Scholar]
- Soruri A, Grigat J, Kiafard Z, Zwirner J. Mast cell activation is characterized by upregulation of a functional anaphylatoxin C5a receptor. BMC Immunol. 2008;9:29. doi: 10.1186/1471-2172-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian H, Kashem SW, Collington S, Qu K, Lambris JD, Ali H. PMX-53 as a dual CD88 antagonist and an agonist for Mas-related gene 2 (MrgX2) in Human mast cells. Mol Pharmacol. 2011 doi: 10.1124/mol.111.071472. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, Ogasawara H, Edamura K, Takagi H, Iwamura H, Noguchi M, Naito T. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 2006;349:1322–1328. doi: 10.1016/j.bbrc.2006.08.177. [DOI] [PubMed] [Google Scholar]
- Venkatesha RT, Berla Thangam E, Zaidi AK, Ali H. Distinct regulation of C3a-induced MCP-1/CCL2 and RANTES/CCL5 production in human mast cells by extracellular signal regulated kinase and PI3 kinase. Mol Immunol. 2005;42:581–587. doi: 10.1016/j.molimm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Woolhiser MR, Brockow K, Metcalfe DD. Activation of human mast cells by aggregated IgG through FcgammaRI: additive effects of C3a. Clin Immunol. 2004;110:172–180. doi: 10.1016/j.clim.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci U S A. 2003;100:10043–10048. doi: 10.1073/pnas.1732949100. [DOI] [PMC free article] [PubMed] [Google Scholar]