Abstract
Objective
Olfactory identification deficits (OIDs) are seen in schizophrenia, but it is unclear whether they are state- or trait-related.
Methods
We examined the prevalence of OIDs, as assessed by the University of Pennsylvania Smell Identification Test (UPSIT), and their correlations with prodromal symptoms in young relatives at risk for schizophrenia or schizoaffective disorder (HR-S).
Results
UPSIT scores were lower in HR- S than in healthy controls, but were non-significant after covarying the effects of age, gender and IQ. OID deficits in HR-S were correlated, after covarying out the effects of age and IQ, with prodromal disorganisation.
Conclusion
The potential value of OID deficits as markers of psychopathological vulnerability in young relatives at risk for schizophrenia deserves further investigation.
Keywords: cognition, olfactory identification, prodrome, schizophrenia
Introduction
Olfactory identification deficits (OID) have been observed in patients with schizophrenia (1–4). OIDs are present in neuroleptic naíve psychosis patients, are stable (2–4) and are associated with negative symptoms, diminished emotional responsiveness (5), deficiencies in verbal memory (6) and prolonged duration of the illness (3).
It remains unclear whether OIDs found in patients with schizophrenia are associated with the illness itself or susceptibility to the disorder. Studies examining adult first or second degree family members at high risk for schizophrenia (HR-S) (7) and monozygotic unaffected twin brothers of patients with schizophrenia (8) suggest that OIDs may be associated with the familial susceptibility to the illness, and may represent useful intermediate phenotypes. However, relatively few studies focus on adolescent non-psychotic relatives of patients with schizophrenia, who represent a high risk population for the disorder.
Previous literature has shown that olfactory deficits are associated with cognitive deficits and negative symptoms in schizophrenia patients (2,6). Brewer et al. (9) reported that impairment in olfactory identification ability in ultra-high-risk subjects (defined clinically by the presence of prodromal symptoms) predicted later emergence of a schizophrenia spectrum disorder. However, few previous studies have examined the relationship between OID abnormalities and severity of prodromal symptoms in the genetic high risk populations.
From an ongoing study of young first degree relatives at risk for schizophrenia, we studied OIDs in HR-S subjects using the University of Pennsylvania Smell Identification Test (UPSIT). In this preliminary report, we examined the prevalence of OIDs in HR-S subjects, and the relation between UPSIT scores and prodromal symptoms of schizophrenia in these subjects.
Methods
Subjects
A group of 50 adolescent and young adult high-risk first degree relatives (HR-S; 39 offspring and 11 siblings) were recruited in this study from outpatient clinics at the Western Psychiatric Institute and Clinic (WPIC), Pittsburgh. An age-matched healthy control (HC) group of 42 subjects were recruited from the same communities as where the HR subjects were drawn using flyers posted in community settings and by word of mouth. HR-S subjects were required to have at least one first degree family member with a DSM-IV diagnosis of a schizophrenia (n = 41) or schizoaffective disorder (n = 9; subtype not documented). Affected family members were ascertained by Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) verified by clinical consensus diagnosis. Clinical evaluation of the HR subjects was conducted by using the structured clinical interview for DSM IV Axis I disorders (SCID-I), supplemented by the Behavioral Disorders sections of the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS) (10).
HR-S subjects with a DSM-IV diagnosis of a schizophrenia spectrum disorder, mental retardation, significant head injury, significant history of or current medical or neurological illness were excluded from the study. All subjects were presented with a description of the study and provided written informed consent in accordance with guidelines provided by the Institutional Review Board (IRB) of the University of Pittsburgh. Subjects less than 18 years of age were required to have a parent or guardian give informed written consent for the study.
Assessments
Subjects were assessed for aptitude in odor identification using UPSIT (11), a standardised 40-item forced choice test of olfactory identification. The olfactory stimuli were embedded in 'scratch and sniff' microcapsules that were fixed and positioned on strips at the bottom of each page. There were four response alternatives for each item, located above the odorant strip. The subjects were asked to scratch the microencapsulated strip on the bottom on the page, smell the area and then choose the response choice which best described the odor.
IQ was measured using either the Ammon's Quick IQ test (12) or the Peabody Picture Vocabulary Test (13), or the average of these two, when both test data were available in the same subjects. The Structured Interview for Prodromal Syndromes (SIPS) and the Scale of Prodromal Symptoms (SOPS) scale was used to assess prodromal symptoms (14); these data were available in the 41 HR-S subjects only. The SOPS scale has four sub-scales: positive, negative, disorganisation and general symptoms.
Analysis of Covariance (ANCOVA) using gender and age as covariates (given the fact that these two variables may be modifying variables for olfactory function) was used to examine overall group differences between the HR-S subjects and controls. We also conducted an analysis of covariance to examine the effect of IQ on these differences. The relations between UPSIT scores and the SOPS measures were analysed using partial correlations with IQ, and age as covariates. Statistica software (version 8) was used in data analyses.
Results
The study included HR-S subjects (n = 50; 26 males; age 16.36 + 3.50 years), and HCs (n = 42; 15 males; age 17.17 + 3.14 years). The groups did not differ in age (t = 1.11; DF = 90; p = 0.27) or gender (Mann Whitney U = 879; p = 0.18) but the HR-S subjects, compared to controls, had significantly lower IQ (104.27 ± 15.2 vs. 114.24 ± 10.7; t = 3.56; p < 0.001).
ANCOVA with age and gender as covariates revealed that compared to HC subjects (33.91 + 2.6), reductions of UPSIT scores were seen in HR-S subjects (31.79; + 5.15) (Wald = 3.76 DF = 1; p = 0.05). No significant gender effects or gender by group interactions were seen. When IQ was additionally used as a covariate, the difference between HR-S and HC subjects was no longer significant (Wald = .65; p = 0.42). When the analysis was repeated including only HR-S subjects with parents diagnosed with schizophrenia, the results remained the same.
Since it is possible that siblings may differ from offspring with regard to the degree of risk for schizophrenia (15), we examined the OIDs in the offspring only versus HC subjects, again with age, gender and IQ as covariates. The UPSIT scores did not significantly differ with this comparison.
We carried out correlational analyses in the HR-S subjects (n = 43) between UPSIT scores and prodromal measures after partialing out the effects of age and IQ. UPSIT scores were inversely correlated with SOPS disorganised symptoms (Partial r = −0.47; p = 0.002) (Fig. 1) but not with the SOPS positive (Partial r = −0.14; p = 0.38), negative (Partial r = −0.19; p = 0.25); or general (Partial r = −0.21; p = 0.18) symptoms. The relation between SOPS disorganised symptoms and UPSIT scores was significant when the analysis was confined to the offspring only (n = 35; partial r = −0.43; p = 0.013).
Fig. 1.
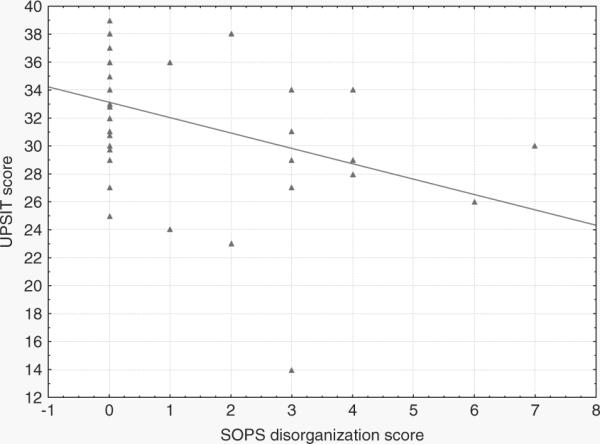
Partial correlation (age and IQ partialled out) between UPSIT scores and SOPS disorganisation symptoms in HR-S subjects (n = 43).
Discussion
We observed a modest reduction in UPSIT scores in HR-S subjects that were nonsignificant after covarying out the effects of age, gender and IQ. This result is perhaps related to the fact that OIDs have been shown to be related to cognitive abilities in some (6, 16, 17). though not all previous studies of schizophrenia patients (18). This observation underlines the importance of addressing the potential confounding effect of cognitive deficits while investigating OID deficits. An alternative view is that cognitive impairment is not a confound, but essentially a co-morbid condition. It is also to be kept in mind that olfactory identification abnormalities, being examined in this study, are more likely to be associated with broader cognitive deficits, in comparison with olfactory discrimination tasks that directly target olfactory perceptual processes.
Previous research has shown that OIDs are associated with the severity of symptoms in Schizophrenia (4) as well as in prodromal patients (9). Therefore, we hypothesised that olfactory deficits will be greater in HR individuals with prodromal psychopathology, especially as they emerge during late adolescence, proximal to the typical age of onset of schizophrenia. As predicted, olfactory deficits were correlated with prodromal disorganisation symptoms, though not with other prodromal symptoms. This finding suggests that olfactory deficits may be a marker of risk for the emergence of prodromal symptoms, and perhaps may have predictive value for later emergence of psychotic symptoms.
The strengths of our study include the investigation of a unique, well characterised group of HR-S subjects. A larger pool of data as well as follow-up will shed more light on the predictive value of olfactory deficits in schizophrenia for the emergence of serious psychopathology during early adulthood.
Acknowledgements
This study was supported by NIMH grants MH 64023 and 01180 and an Established Investigator Award from NARSAD (MSK) and a NARSAD young Investigator award (VAD).
References
- 1.Hurwitz T, Kopala L, Clark C, Jones B. Olfactory deficits in schizophrenia. Biol Psychiatry. 1988;23(2):123–128. doi: 10.1016/0006-3223(88)90081-9. [DOI] [PubMed] [Google Scholar]
- 2.Brewer WJ, Pantelis C, Anderson V, Velakoulis D, Singh B, Copolov DL, McGorry PD. Stability of olfactory identification deficits in neuroleptic-naive patients with firstepisode psychosis. Am J Psychiatry. 2001;158(1):107–115. doi: 10.1176/appi.ajp.158.1.107. [DOI] [PubMed] [Google Scholar]
- 3.Moberg PJ, Doty RL, Turetsky BI, Arnold SE, Mahr RN, Gur RC, Bilker W, Gur RE. Olfactory identification deficits in schizophrenia: Correlation with duration of illness. Am J Psychiatry. 1997;154(7):1016–1018. doi: 10.1176/ajp.154.7.1016. [DOI] [PubMed] [Google Scholar]
- 4.Moberg PJ, Arnold SE, Doty RL, Gur RE, Balderston CC, Roalf DR, Gur RC, Kohler CG, Kanes SJ, Siegel SJ, Turetsky BI. Olfactory functioning in schizophrenia: Relationship to clinical, neuropsychological, and volumetric mri measures. J Clin Exp Neuropsychol. 2006;28(8):1444–1461. doi: 10.1080/13803390500434409. [DOI] [PubMed] [Google Scholar]
- 5.Schneider F, Habel U, Reske M, Toni I, Falkai P, Shah NJ. Neural substrates of olfactory processing in schizophrenia patients and their healthy relatives. Psychiatry Res. 2007;155(2):103–112. doi: 10.1016/j.pscychresns.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Compton MT, McKenzie Mack L, Esterberg ML, Bercu Z, Kryda AD, Quintero L, Weiss PS, Walker EF. Associations between olfactory identification and verbal memory in patients with schizophrenia, first-degree relatives, and non-psychiatric controls. Schizophr Res. 2006;86(1–3):154–166. doi: 10.1016/j.schres.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Kopala LC, Good KP, Morrison K, Bassett AS, Alda M, Honer WG. Impaired olfactory identification in relatives of patients with familial schizophrenia. Am J Psychiatry. 2001;158(8):1286–1290. doi: 10.1176/appi.ajp.158.8.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ugur T, Weisbrod M, Franzek E, Pfuller U, Sauer H. Olfactory impairment in monozygotic twins discordant for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2005;255(2):94–98. doi: 10.1007/s00406-004-0536-8. [DOI] [PubMed] [Google Scholar]
- 9.Brewer WJ, Wood SJ, McGorry PD, Francey SM, Phillips LJ, Yung AR, Anderson V, Copolov DL, Singh B, Velakoulis D, Pantelis C. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry. 2003;160(10):1790–1794. doi: 10.1176/appi.ajp.160.10.1790. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (k-sads-pl): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 11.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of pennsylvania smell identification test: A rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94(2.1):176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Otto W, McMenemy R. An appraisal of ammon's quick test in a remedial reading program. J Educ Meas. 1965;2(2):193–198. [Google Scholar]
- 13.Dunn LM, Dunn LM, Robertson GJ, Eisenberg JL. Peabody picture vocabulary test – revised. American Guidance Service; Circle Pines, MN: 1981. [Google Scholar]
- 14.Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW. Prospective diagnosis of the initial prodrome for schizophrenia based on the structured interview for prodromal syndromes: Preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159(5):863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- 15.Varma SL, Zain AM, Singh S. Psychiatric morbidity in the first-degree relatives of schizophrenic patients. Am J Med Genet. 1997;74(1):7–11. doi: 10.1002/(sici)1096-8628(19970221)74:1<7::aid-ajmg2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 16.Stedman TJ, Clair AL. Neuropsychological, neurological and symptom correlates of impaired olfactory identification in schizophrenia. Schizophr Res. 1998;32(1):23–30. doi: 10.1016/s0920-9964(98)00021-8. [DOI] [PubMed] [Google Scholar]
- 17.Saoud M, Hueber T, Mandran H, Dalery J, d'Amato T. Olfactory identification deficiency and wcst performance in men with schizophrenia. Psychiatry Res. 1998;81(2):251–257. doi: 10.1016/s0165-1781(98)00104-8. [DOI] [PubMed] [Google Scholar]
- 18.Seidman LJ, Talbot NL, Kalinowski AG, McCarley RW, Faraone SV, Kremen WS, Pepple JR, Tsuang MT. Neuropsychological probes of frontolimbic system dysfunction in schizophrenia. Olfactory identification and wisconsin card sorting performance. Schizophr Res. 1991;6(1):55–65. doi: 10.1016/0920-9964(91)90021-i. [DOI] [PubMed] [Google Scholar]


