Abstract
Size-related traits are common targets of natural selection, yet there is a relative paucity of data on selection among mammals, particularly from studies measuring lifetime reproductive success (LRS). We present the first phenotypic selection analysis using LRS on size-related traits in a large terrestrial carnivore, the spotted hyena, which displays a rare pattern of female-biased sexual size dimorphism (SSD). Using path analysis, we investigate the operation of selection to address hypotheses proposed to explain SSD in spotted hyenas. Ideal size measures are elusive, and allometric variation often obfuscates interpretation of size proxies. We adopt a novel approach integrating two common methods of assessing size, and demonstrate lifetime selection on size-related traits that scale hypoallometrically with overall body size. Our data support selection on hypoallometric traits in hyenas, but not on traits exhibiting isometric or hyperallometric scaling relationships, or on commonly used measures of overall body size. Our results represent the first estimate of lifetime selection on a large carnivore, and suggest a possible route for maintenance of female-biased SSD in spotted hyenas. Finally, our results highlight the importance of choosing appropriate measures when estimating animal body size, and suggest caution in interpreting selection on size-related traits as selection on size itself.
Keywords: selection, sexual size dimorphism, lifetime reproductive success, allometry, body size, Crocuta crocuta
1. Introduction
Both body size and morphological scaling relationships are critical factors in ecology and evolution, as they are central to energetics, niche partitioning, life-history strategies, reproduction (e.g. [1–4]) and ultimately fitness [5,6]. However, selection on size can be complex. Consistent positive sexual selection is common among male mammals for access to females, or for control of resources required by females, and is hypothesized to result in the observed pattern where males are generally larger than female conspecifics [5,6]. In many classes of animals, females are often larger than males, probably because fecundity increases with increasing body size [3]. By contrast, female mammals are predicted to be smaller than males owing to a trade-off between somatic growth and reproduction [5]. Deviations from these common patterns probably result either from relaxation of widespread selection, the imposition of novel selection or both.
Selection analyses are most reliable when performed on lifetime fitness data [7–10]. Lifetime reproductive success (LRS) is often considered a gold standard for measuring fitness [7,9], performing well even against rate-sensitive measures of lifetime fitness such as lambda [11]. However, estimates of lifetime selection remain rare owing to the difficulty of conducting long-term evolutionary studies, particularly those involving large carnivorous mammals (see [12] and references therein). We use LRS data from a long-term field study to evaluate selection on size-related traits among female spotted hyenas (Crocuta crocuta); spotted hyenas exhibit female-biased sexual size dimorphism (SSD), a condition that is arguably unique among terrestrial carnivores [13]. We apply the results of our selection analysis to test a number of the most commonly invoked hypotheses explaining reversed SSD in spotted hyenas.
One of the first hypotheses forwarded to explain female-biased SSD in spotted hyenas, the ‘infant defence’ hypothesis, suggests that larger mothers might be better at preventing infanticide by conspecifics [13,14]. Another possibility is suggested by the observation that increased size decreases the relative metabolic cost of nursing in a variety of species [2], permitting larger mothers to invest more heavily in offspring (e.g. [15]). The metabolic costs of lactation are extremely high among spotted hyenas [16], and this ‘inexpensive lactation’ hypothesis provides an explanation for why female size might be under positive selection. The third hypothesis we test is the ‘hunting success’ hypothesis, suggesting that hunting success increases with size. Finally, the ‘feeding competition’ hypothesis suggests that larger females fare better during intense competition to obtain food at kills dominated by individuals of high social rank. All hypotheses predict a positive relationship between female size and fitness. Thus, we would fail to support any of these hypotheses without evidence for positive selection on morphological size traits in female spotted hyenas. However, the infant defence and inexpensive lactation hypotheses both further predict that one fitness component in particular, cub survival, should increase with maternal size, whereas the feeding competition and hunting success hypotheses make no specific prediction regarding which fitness components might be affected by maternal size.
Despite its clear importance in ecology and evolution, there is no well-accepted method for measuring animal body size, nor is there a consensus on what body size truly represents; this may result in widely divergent interpretations of covariation of fitness with size. Body mass can be problematic as a size measure, because it may fluctuate temporally because of feeding, reproduction and other factors [17]. Mammalian carnivores such as spotted hyenas represent extreme examples, as they consume large amounts of tissue in a single meal (e.g. [14]). A common approach to estimating body size is use of taxon-specific univariate proxies, such as snout-vent length in reptiles or body mass in birds and mammals. However, this approach assumes both a strong correlation and an isometric relationship, or 1 : 1 log ratio, between the trait and overall size [17]. We define overall size here as a measure of all morphological traits where all traits increase isometrically. Any allometric variation, or deviation from isometry, represents a change in shape rather than size. A common alternative to univariate size measures is the use of the first axis (PC1) from a principal components analysis (PCA) on the covariance matrix of a set of log-transformed morphological measures (e.g. [18,19]). However, this approach also assumes an isometric relationship between overall size and each trait, indicated by the loading of the trait with PC1. Failure to meet this assumption suggests that traits do not contribute equally to the size measure, and thus that PC1 represents both size and shape, obscuring interpretation. Interestingly, however, this assumption is probably seldom met, as allometric variation is more the rule than the exception [20].
The current approach in selection analysis is to use all available size-related morphological traits in a selection gradient analysis to assess direct and indirect components of selection [10]. However, there are potential conceptual and practical drawbacks to this approach. Conceptually, if all of the size-related traits in fact reflect an underlying, but unmeasured, body size factor, then the contribution of the size factor to fitness will be spread among the traits. The high degree of multicollinearity in the model will inflate standard errors for the partial regression coefficients. This can lead to an inability to statistically detect selection, even when it is operating. In practice, unrealistically large sample sizes may be required to obtain reasonable estimates with many traits and few a priori expectations concerning those under selection.
Here, we use a novel approach for estimating body size that represents a compromise between using PC1 as the sole size proxy and a selection gradient analysis that includes all traits. Our approach integrates multivariate allometric techniques [21,22], identifies unequal contribution of traits to PC1 and, if necessary, allows groups of traits to be chosen for inclusion in multivariate measures of size based on allometric relationships. We then test for selection among female hyenas on three composite size traits grouped by their multivariate allometric coefficients, using LRS as a measure of fitness. We demonstrate an explicit link between fitness and a composite size trait that scales hypoallometrically in adult female spotted hyenas, and use path analysis to identify fitness components influenced by size. Interestingly, we do not observe a significant relationship between fitness and either mass or PC1 from a PCA performed on all traits. We discuss these results within the context of measuring size as a target of selection, and with respect to the evolution of the rare form of SSD reversal observed in spotted hyenas.
2. Material and methods
(a). Study organisms
Spotted hyenas are characterized by small litter sizes, slow life histories and unusual genital monomorphism [14,23–25]. Spotted hyenas live in social groups called clans, consisting of up to 90 individuals including multiple females born in the clan and their young, as well as several adult immigrant males. Each clan is structured by a strict linear dominance hierarchy [26–28], and an individual's position in this hierarchy has profound effects on both survival and reproduction by mediating differential access to food at kills [14,16,24]. Female spotted hyenas are physiologically competent to breed after 24 months of age, but first parturition usually occurs in the third or fourth year of life; the timing of first parturition varies greatly with rank [16,23]. Female hyenas are philopatric, whereas nearly all males emigrate and join neighbouring clans after puberty [29,30]. Spotted hyenas live up to 19 years in the wild [31].
(b). Study site, population and field methods
We used data from a total of 170 immobilizations: 68 immobilizations of 46 females in the Talek clan, 22 immobilizations of 14 females in the Mara River clan and 80 immobilizations of 80 females in other Mara clans; the latter 80 females were only included in the allometric and correlation analyses, as we had no reproductive data for them. For individual hyenas immobilized more than once as adults, we used their mean values. Males could often not be monitored or immobilized after dispersal from their birth clan, so their lifetime fitness could not be accurately assessed here. Therefore, our analyses were performed only on adult females. From each immobilized hyena, we obtained the four cranial and nine post-cranial linear morphological measurements shown in figure 1. We only included measurements taken after 36 months of age or after first parturition, whichever came first; 36 months represent a conservative estimate of the age at which reproductive and morphological maturity is achieved among females [23,24]. Females were included if they met these criteria even if they died without giving birth. All morphological data were natural log transformed prior to analysis.
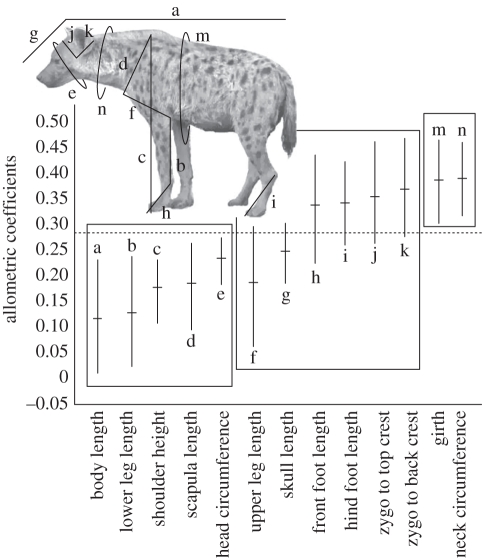
Figure 1.
Morphological measurements taken from 140 adult female spotted hyenas, displayed with allometric intervals and labelled by corresponding letters; each letter refers to only one trait. The isometric hypothesis for overall body size is designated by the horizontal dotted line. Values on the y-axis represent allometric coefficients, or the loadings on PC1, for each trait.
(c). Fitness measures
As our measure of fitness we used LRS, defined as the total number of offspring produced that survived to weaning. As fitness components, we included cub survival to weaning, average annual reproductive success (ARS) and reproductive lifespan. Measuring fitness of mothers and their offspring accurately can be difficult, and it may not be clear whether to assign a particular component of fitness to the mother or to her offspring. Assigning the fitness component of cub survival to weaning as a maternal fitness component is common in mammalian studies. This practice has been criticized because selection theory emphasizes that the fitness of individuals in one generation (e.g. offspring) should not be assigned to individuals in another generation (e.g. parents), as this can result in incorrect predictions regarding evolutionary dynamics [10]. However, when the effect of the parental phenotype on a component of offspring fitness greatly overshadows the effect of the offspring phenotype and there is no genetic correlation between the parental trait of interest and the component of offspring fitness, then it may be beneficial to assign this component of fitness to the parent [32]. Explicit consideration of a genetic correlation between the parental trait and the component of offspring fitness of interest has specifically been suggested, based on researchers' knowledge of the species' biology and the traits in question [32]. In the case of spotted hyenas, there is no reason to suspect a direct genetic correlation between maternal size and offspring survival. In fact, offspring survival to weaning is dominated by the mother's ability to provide milk and protect her cubs [16,33], and is strongly influenced by maternal social rank [24], which is learned and is not under genetic control [34,35].
(d). Data analysis
(i). Allometric methods
For log-transformed morphological data, the multivariate allometric coefficient for each trait is indicated by the trait's loading in the first eigenvector of the variance–covariance matrix (PC1), called the allometric vector. In order for PC1 to represent an isometric size measure, the allometric coefficients should equal 1/(p1/2), called the isometric hypothesis, where p is the number of traits included in the PCA [36]. To compare coefficients among traits, we used a bootstrap approach to estimate 99% confidence intervals (allometric CIs) on the loadings, resampling with replacement 10 000 times [21,37]. Because the allometric coefficients are estimated from loadings, they are dependent on the covariance matrix of included traits, and thus on the traits included in the analysis. Thus, the specific allometric coefficient of a trait is dependent upon the relative scaling relationship of the trait with other traits included in the PCA. If a trait's allometric CI overlapped the expected value for isometry, representing the null hypothesis, the trait was considered isometric to overall body size [36,37]. If the CI fell below the isometric value, the trait was considered to be hypoallometric to body size, or scale at less than a 1 : 1 log ratio with overall body size, whereas a CI wholly above the isometric value indicated a hyperallometric trait scaling at greater than a 1 : 1 log ratio with body size. If all included traits did not scale isometrically, PC1 would not be a good measure of overall body size because each trait would not contribute equally, and thus increases in PC1 would result in disproportionate changes in the trait in question. Disproportionate increases in some traits suggest that the size measure is conflated with shape. Because PC1 with all traits included failed to represent a good isometric measure of body size here, we split the measured traits into three groups: traits that scaled hypoallometrically, isometrically and hyperallometrically with body size, respectively. We then performed a separate PCA on each group and used each resulting PC1 as a new composite measure of size. This of course altered the resulting covariance matrix, and thus the relative scaling relationships of the traits. We therefore estimated the allometric coefficients of each group, comparing them with the predicted isometric value for each group. Any traits that demonstrated mild departures from isometry were left in their respective groups, as perfect isometry is unlikely. As an additional precaution, however, we also temporarily moved traits that exhibited mild departures from isometry to new groups, and repeated all further analyses to determine whether their placement influenced our results. Currently, we do not know whether the measured traits are functionally, developmentally or evolutionarily integrated, but merely present them as appropriate and practical proxies for size. Although we formed trait groups based on their allometric CIs, we also wanted to determine whether these or similar groups would also be generated if we used other grouping methods. If our trait groups were robust to the use of alternative methods, this would support the idea that trait groups were natural groups produced by similar evolutionary and developmental processes, and not merely artefacts of any particular covariance matrix. Therefore, we used the ‘pvclust’ package in R [38] to perform a hierarchical cluster analysis, with bootstrap support values for nodes, to further investigate relationships among the univariate morphological traits of interest. We used 10 000 bootstrap replicates with uncentred correlations subtracted from one as a measure of distance between two traits. We used a variety of agglomeration methods including Ward's, single, average, median and complete. Using a bootstrap resampling method allowed us to estimate confidences in the various topologies of each dendrogram. The combination of dimensional reduction and the preservation of some allometric information makes this technique very useful when a large number of size traits are measured, especially when there are no clear a priori hypotheses regarding the importance of specific traits.
(ii). Selection gradients and path analysis
We used Conner's [39] approach to understanding natural selection operating on one or more traits using selection gradient analysis [10], multiplicative fitness components [8] and path analysis [40]. LRS was converted to relative fitness by dividing it by mean absolute fitness for selection gradient analysis [8,10]. LRS and all fitness components were standardized for path analysis [39]. We calculated standardized selection gradients to estimate the strength of selection by regressing relative LRS on the standardized traits of interest [10,39]. Although we had full morphological trait data for 140 females, we had reproductive data on 50 females, seven from the Mara River clan and 43 from the Talek clan, and full LRS data for a reduced set of 31 individuals, all from the Talek clan. For the selection gradient analysis, we only used the 31 individuals from the Talek clan with full LRS. The females used in each analysis represent all females for which we had all necessary data. Although bias could possibly be introduced into the path analysis by inclusion of data from two clans, the ecological conditions experienced by both clans are very similar [41], and the results of the selection gradient analysis match the path analysis closely, so we believe it unlikely. The three composite size measures were included in a multiple regression along with the standardized social rank of each animal. Social rank was included to remove any correlated effects of rank, as rank affects most aspects of life history, behaviour and ecology in the spotted hyena [16,23,24,26,28,42]. However, social rank among adult female spotted hyenas is not correlated with mass or other univariate measures [23]. We also assessed the relationship between rank and morphology in our current dataset, described in the section on path analysis. We did not include mass in the current analysis because any effect of mass would conflate the effects of size and condition, either of which might influence fitness [43,44]. We did, however, perform a selection gradient analysis with only social rank and mass to assess the value of this measure in spotted hyenas, as well as a similar model with PC1 from a PCA of all 13 traits replacing mass.
We performed path analysis using ordinary least-squares (OLS) regression to determine which fitness components are influenced by size-related traits and social rank, and to elucidate the importance of different fitness components in determining total fitness [39], as well as the potential influence of social rank on size in the current dataset. Offspring achieve ranks directly subordinate to their mothers in this species with no evidence that rank acquisition is influenced by adult size [35]. We expected any correlation between rank and size observed to be due to effects of rank on size rather than vice versa. We thus fit the relationship between rank and size as a causal path. The multiplicative fitness components we included were reproductive lifespan, average number of offspring born each year, or ARS, and proportion of cubs born that survived to weaning. We confirmed this approach by using structural equation models (SEMs), which allow simultaneous estimation of all paths using maximum likelihood, using bootstrapping to construct CIs. However, we only used SEM in a confirmatory role to OLS path analysis owing to complications engendered by the estimation of all paths simultaneously (see electronic supplementary material). Because sample size limited the number of variables that could reliably be included in the path analysis, only traits found to be under significant (p ≤ 0.05) lifetime selection in the selection gradient analysis were included in the path analysis. We confirmed that this was appropriate using corrected Akaike's information criterion (AICc) to compare the fit of the model containing only the significant traits with other possible models.
To maximize sample size in our path analysis, we used all 50 females for which we had at least 3 years of fitness measures after reproductive maturity. Nineteen of 50 females included in the path analysis had left- or right-censored data; left-censored individuals started breeding before our study began, and right-censored individuals were alive at the end of the study. To test whether the effect of size differed between censored and uncensored individuals, we performed model selection on ANCOVAs using likelihood ratio tests and AICc (detailed in the electronic supplementary material). No method indicated a difference regarding effects of hypoallometric size on reproductive longevity between individuals with full lifetime data and either left- or right-censored individuals (electronic supplementary material, tables S2 and S3), so we subsequently pooled the data for these three groups. All statistical analyses were performed in R v. 2.9.2 [45].
3. Results
(a). Allometric analyses
As has been observed in studies with other organisms, the loadings on PC1 from the PCA from morphological traits were unequal among female hyenas (e.g. [21,22,46]), with allometric CIs only overlapping the expected value for isometry for six of 13 traits (figure 1). Using the observed multivariate allometric patterns, we grouped hyperallometric, isometric and hypoallometric traits separately, performed a PCA on each group and used the new PC1s as multivariate proxies for size. We refer to each resulting multivariate size measure by its original allometric relationship to isometric size (e.g. ‘the hypoallometric size trait’). The bootstrapped correlation analysis indicated that groups identified by allometric relationships were also generally robust to other clustering methods (figure 2). The meaning of multivariate traits calculated from such groupings is easier to interpret than that of a PC1 calculated from all measured traits because they can be interpreted more easily as a set of proxies for overall size, not conflated with allometric effects. For the purposes of this paper, we explicitly limit ourselves to using these measures as size proxies, and we make no inferences about possible genetic, functional or evolutionary explanations for the observed groupings. After reanalysing the allometric CIs of the new multivariate traits, skull length and upper leg length appear to be slightly hypoallometric to the new predicted value for the multivariate isometric trait. Thus, both traits appear to straddle the isometric and the hypoallometric groups. However, moving these traits into the hypoallometric group and repeating all analyses does not change any of the remaining results (see electronic supplementary material).
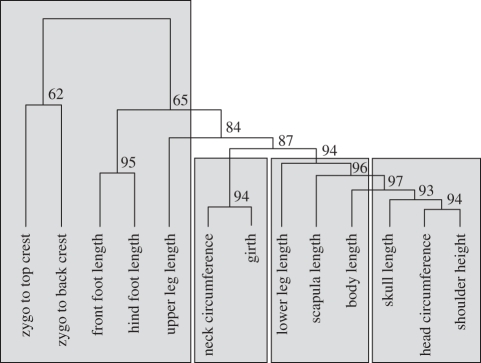
Figure 2.
Cluster diagram from hierarchical cluster analysis on univariate measures. Cluster analyses were performed using five different agglomeration methods, and distance measures calculated from uncentred correlations. Shaded regions designate groups of traits that are returned by four out of five agglomeration methods. The exact topology shown was returned by two out of five agglomeration methods.
(b). Correlation analyses
The groups chosen using the allometric CIs were robust to alternative grouping methods. Specifically, hierarchical cluster analyses using a variety of agglomeration methods, including Ward's, average, single, complete and Mcquitty's, returned topologies similar to the groupings chosen using the allometric CIs (figures 1 and 2). Although complete congruence between the correlation and allometric analyses was not universal across agglomeration methods, especially at higher dimension topologies, all agglomeration methods generally corresponded with the allometric CIs at lower levels. Specifically, small clusters of traits commonly found using one method were usually seen using others, and were also recovered by partitioning traits using allometric coefficients. Furthermore, two out of five agglomeration methods recovered nearly the same partitions identified by allometric coefficients (e.g. figure 2). The general congruence observed here suggests that the allometric CIs broadly reflect the action of the evolutionary and developmental processes that generate bivariate correlations between morphological traits. Therefore, it seems reasonable to consider the groups we identified using allometric CIs appropriate, both in terms of multivariate statistical methods and interpretation of our results, thus reinforcing the utility of our approach for partitioning morphological traits.
(c). Selection analysis
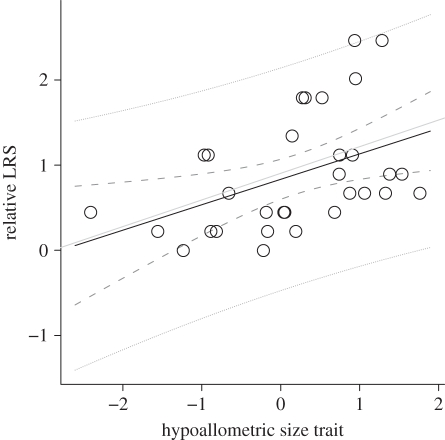
We found no selection on isometric or hyperallometric size measures (table 1). The model did, however, reveal significant positive selection on the hypoallometric trait (figure 3 and table 1), as well as a negative effect of rank on LRS (table 1). By convention, the highest ranked animal is assigned a rank of one, so a negative effect of rank on LRS indicates that higher ranked individuals have higher LRS. The observed selection on the hypoallometric trait is fairly strong (β = 0.313), as Kingsolver et al. [12] demonstrated that absolute magnitudes of selection estimates (|β|) roughly follow an exponential distribution, with a mean of 0.22 and a median of 0.16. In addition, the magnitude of selection on the hypoallometric trait is more than twice that of selection on the other traits. In the two separate selection gradient models, one with mass and rank, and the other with rank and PC1 from all size-related traits, neither commonly used size proxy contributed significantly to fitness (mass: β = 0.223, s.e. = 0.111, t = 2.000, p = 0.055; PC1: β = 0.200, s.e. = 0.120, t = 0.168, p = 0.105). Additional descriptive statistics and information on the opportunity for selection (I) appear in the electronic supplementary material and table S1.
Table 1.
Parameters from multiple regression selection analysis performed using data from 31 adult female spotted hyenas. β values are standardized selection gradients. Significant effects at α ≤ 0.05 are indicated by asterisks.
| β | s.e. | t | p-value | |
|---|---|---|---|---|
| rank | −0.276 | 0.108 | −2.545 | 0.0172* |
| hypoallometric size | 0.313 | 0.112 | 2.788 | 0.0098* |
| isometric size | −0.105 | 0.145 | −0.729 | 0.4726 |
| hyperallometric size | 0.139 | 0.143 | 0.977 | 0.3378 |
Figure 3.
Relationship between relative LRS and the standardized hypoallometric size trait observed among 31 female spotted hyenas. The black line indicates the selection differential, whereas the grey line indicates the selection gradient. The dashed lines indicate the 95% CIs while the dotted lines are the 95% predictive intervals.
(d). Fitness components
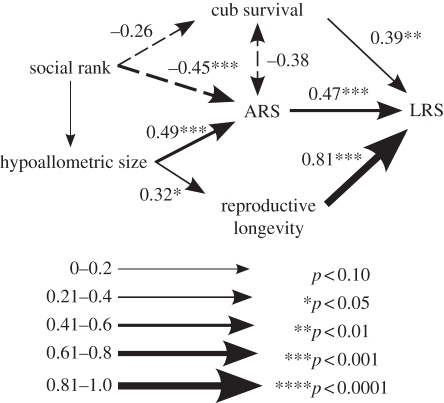
To understand how the hypoallometric trait contributes to LRS, we used a path analytic approach, which demonstrated that the hypoallometric size trait contributes to LRS through its impact on both reproductive lifespan and ARS (figure 4). Also, rank had a positive effect on ARS, a marginally significant positive effect on cub survival and no effect on reproductive lifespan (figure 4). Rank also had a non-significant positive effect on the hypoallometric trait, suggesting that low-ranking females may be larger as adults than high-ranking females (figure 4). All fitness components had strong effects on LRS, though the effect of reproductive lifespan was much stronger than ARS or cub survival (figure 4). Finally, the negative correlation between ARS and cub survival (figure 4) suggests a possible reproductive trade-off. The negative correlation between ARS and cub survival to weaning in figure 3 was taken from the SEM because the correlated errors did not meet the assumptions of a recursive model, but other path coefficients were unaffected. Using OLS path analysis, this negative correlation was present, but not significant at α ≤ 0.05. There were no other discrepancies between the traditional path analysis and the SEM (figure 4 and electronic supplementary material, table S4).
Figure 4.
Results of path analysis showing relationships between social rank, the hypoallometric size trait and fitness components among 50 adult female spotted hyenas. All paths are shown with relationships significant at p ≤ 0.20. Path coefficients are given above paths that are significant at p ≤ 0.10. Arrow width corresponds to the magnitude of the path coefficient, with positive coefficients indicated by solid lines and negative coefficients indicated by dashed lines. The highest social rank an individual can achieve is ‘1’, so negative path coefficients indicate that rank is positively related to the response variable. Path coefficients are almost identical to MLE estimates; 95% bootstrap CIs are given in electronic supplementary material, table S5, and agree in all cases with significances of path coefficients. The correlation shown between ARS and cub survival is the MLE estimate, for reasons discussed in §3.
4. Discussion
The intensive sampling and individual identification necessary to obtain long-term fitness data are difficult in free-living populations, particularly for animals that are cryptic, nocturnal, dangerous or long-lived. We present, to our knowledge, the first phenotypic selection analysis on a large carnivore using LRS as a measure of fitness. We also showed that LRS among female spotted hyenas is affected by a multivariate morphological trait that scales hypoallometrically with body size (figure 3 and table 1). However, we failed to find evidence for selection on either an isometric size trait, which is often thought of as ‘idealized size’, or a hyperallometric size trait (table 1). Although the results of the selection gradient analysis support all four adaptive hypotheses considered here, the path analysis results (figure 4) fail to support either the infant defence hypothesis or the inexpensive lactation hypothesis, which predict an effect of size-related morphological traits on cub survival. Instead, the results of our selection and path analysis (figures 3 and 4) are consistent with both the feeding competition hypothesis and the hunting success hypothesis.
The feeding competition hypothesis suggests that large body size in females might be favoured by selection if females compete more intensively than males for food [13,47]. Under this hypothesis, food is more critical to the reproductive success of females than that of males, and this appears to be the case among spotted hyenas [48]. Reproductive opportunities among female spotted hyenas are strongly limited by food; feeding competition among females is very intense, and priority of access to food has profoundly important effects on most measures of female reproductive success [16,23,48]. Although rank is the most important determinant of access to food, size may influence food access through a greater ability to steal or process food more quickly, increasing intake rates without affecting priority of access. In addition, size may be beneficial in situations where intrasexual rank plays a lesser role, such as during interspecific interactions at kills. The hunting success hypothesis, which is also supported by our data, suggests that hunting success increases with size. In contrast to other extant hyenas, spotted hyenas are proficient predators, and even solitary hunters can bring down prey up to four times their own body mass [49]. If selection is acting on hunting ability, then females with larger values of the hypoallometric trait should exhibit higher success rates during hunts. Furthermore, if the hunting success hypothesis is correct, we should see the same relationship between hunting success and hypoallometric size traits in males and females, but a greater effect of hunting success on fitness in females. Although we view these hypotheses as the most likely candidates, size data from adult males is necessary to provide conclusive evidence in support of any particular explanation for SSD in this species. Other hypotheses proposed to explain SSD in spotted hyenas, such as a pleiotropic effect of female masculinization or selection for smaller males, cannot be directly addressed with our current data.
The importance of ARS as a route through which body size influences LRS among female spotted hyenas will allow us to compare selection on size-related traits in males and females directly, shedding further light on the maintenance of female-biased SSD, which represents a derived trait in this species [50]. Yet, one remaining question concerns the source of variation in ARS. Namely, does size influence ARS via inter-litter interval, litter size or both? In a multiple regression, increases in the hypoallometric trait resulted in larger litters (β = 0.431, s.e. = 0.135, t = 3.192, p = 0.003), but rank had no effect (β = −0.229, s.e. = 0.135, t = −1.696, p = 0.097). In a separate multiple regression, females with larger values of the hypoallometric trait had more litters per year (β = 0.377, s.e. = 0.132, t = 2.859, p = 0.006), as did higher ranking females (β = −0.396, s.e. = 0.132, t = −3.007, p = 0.004). In a third multiple regression, females with more litters per year had increased ARS (β = 0.715, s.e. = 0.053, t = 13.623, p < 0.001), as did females with a greater average litter size (β = 0.388, s.e. = 0.053, t = 7.384, p < 0.001). All variables in these analyses were mean centred and standardized. Sample size was 49 because we did not have enough information on the exact frequency of one female's litters.
There is little explicit support for any current hypothesis explaining female-biased SSD in spotted hyenas, but it is commonly assumed in the literature that larger body size in female hyenas evolved as part of an integrated suite of ‘sex-role reversed’ traits, including enhanced aggressiveness, social dominance and male-like genitalia in females. However, our data suggest instead that SSD in the spotted hyena may result from direct positive selection on size-related morphological traits in females. Positive selection may also play a role in the generation of patterns of static allometry among females. It is not currently known whether the hypoallometric trait affects fitness among male spotted hyenas as it does among females. Direct evidence regarding how female-biased SSD is maintained will come from comparing effects of size on ARS between the two sexes. Selection on females that is absent or negative in males, combined with positive heritability, would provide strong support for positive selection on size-related morphological traits as a mechanism maintaining female-biased SSD in spotted hyenas. Another result relevant to future analyses comparing males and females is that our path analysis identified a negative correlation between ARS and cub survival (figure 4), possibly representing a life-history trade-off in which females that reproduce at younger ages experience lower cub survival simply because of inexperience at first parturition. However, a more likely possibility is that litter loss before weaning brings females rapidly back into oestrus [23], allowing them to produce more litters, albeit unsuccessful ones, per unit time than females whose cubs survive to weaning.
In addition to comparing selection on size in males and females in future work, it may be informative to incorporate our results concerning the specific morphological traits under selection in attempts to address the remaining hypotheses explaining female-biased SSD in spotted hyenas. Specifically, we note that we failed to find evidence for selection on mass or PC1 from a PCA on all morphological traits. We interpret this to mean that selection is probably acting on the subset of morphological traits contained within the hypoallometric measure rather than overall size itself. Notably, body length, mass and PC1 are all commonly used as proxies for overall size, yet in our study they vary greatly in their relationships to fitness. PC1 calculated from all traits is generally not as condition dependent as mass, and is widely considered a standard comprehensive measure of size. However, the effect of PC1 on fitness here is even less clear than the effect of mass. Thus, our results suggest that, despite the importance of size in biology (e.g. [1,6,51]), overall size is not always the trait of interest. Our data thus underscore the value of determining whether size itself, or specific size-related morphological traits, are under selection. In situations where size appears vital in mediating an ecological process, it may be that the proxy used for size was the relevant trait, not overall size.
Although we currently have little understanding in hyenas of how post-cranial morphological traits contribute to running speed, feeding performance or hunting ability, identifying morphological traits that are targets of selection is an important first step. Interestingly, in post hoc analyses, we found that all hypoallometric traits except scapula length significantly influenced LRS when each univariate trait was included in a multiple regression alone with social rank. However, no traits that scaled isometrically or hyperallometrically exhibited evidence for even indirect selection (electronic supplementary material, table S5). The robustness of this pattern supports the notion that our allometric grouping technique has identified a set of functionally integrated traits, and suggests that selection is acting either on the hypoallometric trait as an integrated unit or on individual hypoallometric traits.
Research on the evolutionary forces shaping allometric patterns in animals has mainly focused on sexually selected male traits, including ornaments, weaponry and genitalia (e.g. [52,53]). Although these traits are of great interest, when investigating the generation of allometric scaling relationships, such strict focus may limit our thinking, and a broader base of empirical work would most probably benefit the entire field. We do not yet know whether the hypoallometric size trait documented here among female spotted hyenas is shaped by natural or sexual selection; in keeping with Darwin's [54] original definition of sexual selection, the latter possibility would most probably not have been considered at all even a decade ago. However, Clutton-Brock [47,55] has recently argued that competition for reproductive opportunities among female animals can generate strong selection favouring competitive ability, and that in extreme cases, selection may reverse the usual direction of sex differences in behaviour and morphology. If the definition of sexual selection is broadened to encompass the consequences of reproductive competition and mate choice in both sexes [47], then the spotted hyena probably represents one such extreme case. If the hypoallometric size trait affects fitness in female but not male spotted hyenas, this would suggest it is indeed a sexually selected trait, and that selection on the hypoallometric size trait drives the rare pattern of female-biased SSD in spotted hyenas.
Acknowledgements
The work presented here was described in Animal Research Protocol no. 07/08-099-00, approved most recently on 4 June 2010 by the Institutional Animal Care and Use Committee at Michigan State University.
We thank the Kenyan Ministry of Education, Science and Technology for permission to conduct this research, and the Kenya Wildlife Service, Narok County Council and the Senior Warden of the Masai Mara National Reserve for assistance. We are deeply grateful to all those who contributed to the long-term data collection. In addition, we wish to thank Alex Shingleton, Jeff Conner, Russell Bonduriansky and three anonymous reviewers for helpful comments on an earlier version of this manuscript. This work was supported by NSF grants IBN0343381, IOB0618022 and IOS0819437 to K.E.H., NSF grants IOS0919855, IOS0919855 and MCB0922344 to I.D., Cooperative Agreement no. DBI-0939454 and graduate research fellowships from NSF and Michigan State University to E.M.S.
References
- 1.Stearns S. C. 1992. The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Peters R. H. 1983. The ecological implications of body size. Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Schmidt-Nielsen K. 1984. Scaling: why is animal size so important? Cambridge, UK: Cambridge University Press [Google Scholar]
- 4.Calder W. A. 1984. Size, function, and life history. Cambridge, MA: Harvard University Press [Google Scholar]
- 5.Blanckenhorn W. U. 2000. The evolution of body size: what keeps organisms small? Q. Rev. Biol. 75, 385–407 10.1086/393620 (doi:10.1086/393620) [DOI] [PubMed] [Google Scholar]
- 6.Bonner J. T. 2006. Why size matters. Princeton, NJ: Princeton University Press [Google Scholar]
- 7.Clutton-Brock T. H. 1991. Lifetime data and the measurement of selection. Evolution 45, (doi:10.2307/2409682) [DOI] [PubMed] [Google Scholar]
- 8.Arnold S. J., Wade M. J. 1984. On the measurement of natural and sexual selection: applications. Evolution 38, 720–734 10.2307/2408384 (doi:10.2307/2408384) [DOI] [PubMed] [Google Scholar]
- 9.Endler J. A. 1986. Natural selection in the wild. Princeton, NJ: Princeton University Press [Google Scholar]
- 10.Lande R., Arnold S. J. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226 10.2307/2408842 (doi:10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 11.Brommer J. E., Gustafsson L., Pietiäninen H., Merilä J. 2004. Single generation estimates of individual fitness as proxies for long-term genetic contribution. Am. Nat. 163, 505–517 10.1086/382547 (doi:10.1086/382547) [DOI] [PubMed] [Google Scholar]
- 12.Kingsolver J. G., Hoekstra H. E., Hoekstra J. M., Berrigan D., Vignieri S. N., Hill C. E., Hoang A., Gibert P., Beerli P. 2001. The strength of phenotypic selection in natural populations. Am. Nat. 157, 245–261 10.1086/319193 (doi:10.1086/319193) [DOI] [PubMed] [Google Scholar]
- 13.Ralls K. 1976. Mammals in which females are larger than males. Q. Rev. Biol. 51, 245–276 10.1086/409310 (doi:10.1086/409310) [DOI] [PubMed] [Google Scholar]
- 14.Kruuk H. 1972. The spotted hyena: a study of predation and social behavior. Chicago, IL: University of Chicago Press [Google Scholar]
- 15.Crocker D. E., Williams J. D., Costa D. P., Boeuf B. J. L. 2001. Maternal traits and reproductive effort in northern elephant seals. Ecology 82, 3541–3555 10.1890/0012-9658(2001)082[3541:MTAREI]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[3541:MTAREI]2.0.CO;2) [DOI] [Google Scholar]
- 16.Hofer H., East M. L. 2003. Behavioral processes and costs of co-existence in female spotted hyenas: a life history perspective. Evol. Ecol. 17, 315–331 10.1023/A:1027352517231 (doi:10.1023/A:1027352517231) [DOI] [Google Scholar]
- 17.Fairbairn D. J. 2007. Introduction: the enigma of sexual size dimorphism. In Sex, size & gender roles (eds Fairbairn D. J., Blanckenhorn W. U., Szekely T.), New York, NY: Oxford University Press, Inc [Google Scholar]
- 18.Massemin S., Korpimäki E., Wiehn J. 2000. Reversed sexual size dimorphism in raptors: evaluation of the hypotheses in kestrels breeding in a temporally changing environment. Oecologia 124, 26–32 10.1007/s004420050021 (doi:10.1007/s004420050021) [DOI] [PubMed] [Google Scholar]
- 19.Schulte-Hostedde A., Millar J. S., Gibbs H. L. 2002. Female-biased sexual size dimorphism in the yellow-pine chipmunk (Tamias amoenus): sex-specific patterns of annual reproductive success and survival. Evolution 56, 2519–2529 [DOI] [PubMed] [Google Scholar]
- 20.Lindenfors P., Gittleman J. L., Jones K. E. 2007. Sexual size dimorphism in mammals. In Sex, size and gender roles (eds Fairbairn D. J., Blanckenhorn W. U., Szekely T.). New York, NY: Oxford University Press, Inc [Google Scholar]
- 21.Klingenberg C. P. 1996. Multivariate allometry. In Advances in morphometrics (ed. Marcus L. F.), pp. 23–48 New York, NY: Plenum Press [Google Scholar]
- 22.Tzeng T. D., Yeh S. Y. 2002. Multivariate allometric comparisons for kuruma shrimp (Penaeus japonicus) off Taiwan. Fish. Res. 59, 279–288 10.1016/S0165-7836(01)00403-9 (doi:10.1016/S0165-7836(01)00403-9) [DOI] [Google Scholar]
- 23.Holekamp K. E., Smale L., Szykman M. 1996. Rank and reproduction in the female spotted hyaena. J. Reprod. Fertil. 108, 229–237 10.1530/jrf.0.1080229 (doi:10.1530/jrf.0.1080229) [DOI] [PubMed] [Google Scholar]
- 24.Watts H., Tanner J. B., Lundrigan B. L., Holekamp K. E. 2009. Post-weaning maternal effects and the evolution of female dominance in the spotted hyena. Proc. R. Soc. B 276, 2291–2298 10.1098/rspb.2009.0268 (doi:10.1098/rspb.2009.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton W. J., Tilson R. L., Frank L. G. 1986. Sexual monomorphism in spotted hyenas, Crocuta crocuta. Ethology 71, 63–73 [Google Scholar]
- 26.Holekamp K. E., Smale L. 1990. Provisioning and food sharing by lactating spotted hyenas, Crocuta crocuta (Mammalia: Hyaenidae). Ethology 86, 191–202 10.1111/j.1439-0310.1990.tb00429.x (doi:10.1111/j.1439-0310.1990.tb00429.x) [DOI] [Google Scholar]
- 27.Frank L. G. 1986. Social organization of the spotted hyaena, Crocuta crocuta. II. Dominance and reproduction. Anim. Behav. 34, 1510–1527 10.1016/S0003-3472(86)80221-4 (doi:10.1016/S0003-3472(86)80221-4) [DOI] [Google Scholar]
- 28.Smale L., Frank L. G., Holekamp K. E. 1993. Ontogeny of dominance in free-living spotted hyaenas: juvenile rank relations with adult females and immigrant males. Anim. Behav. 46, 467–477 10.1006/anbe.1993.1215 (doi:10.1006/anbe.1993.1215) [DOI] [Google Scholar]
- 29.Smale L., Nunes S., Holekamp K. E. 1997. Sexually dimorphic dispersal in mammals: patterns, causes, and consequences. Adv. Stud. Behav. 26, 181–250 10.1016/S0065-3454(08)60380-0 (doi:10.1016/S0065-3454(08)60380-0) [DOI] [Google Scholar]
- 30.Van Horn R. C., McElhinny T. L., Holekamp K. E. 2003. Age estimation and dispersal in the spotted hyena (Crocuta crocuta). J. Mammal. 84, 1019–1030 10.1644/BBa-023 (doi:10.1644/BBa-023) [DOI] [Google Scholar]
- 31.Drea C. M., Frank L. G. 2003. The social complexity of spotted hyenas. In Animal social complexity (eds de Waal F. B. M., Tyack P. L.). Cambridge, MA: Harvard University Press [Google Scholar]
- 32.Wolf J. B., Wade M. J. 2001. On the assignment of fitness to parent and offspring: whose fitness is it and when does it matter? J. Evol. Biol. 14, 347–356 10.1046/j.1420-9101.2001.00277.x (doi:10.1046/j.1420-9101.2001.00277.x) [DOI] [Google Scholar]
- 33.Watts H. 2007. Social and ecological influences on survival and reproduction in the spotted hyena, Crocuta crocuta. East Lansing, MI: Michigan State University [Google Scholar]
- 34.East M. L., Höner O. P., Wachter B., Wilhelm K., Burke T., Hofer H. 2009. Maternal effects on offspring social status in spotted hyenas. Behav. Ecol. 20, 478–483 10.1093/beheco/arp020 (doi:10.1093/beheco/arp020) [DOI] [Google Scholar]
- 35.Engh A. L., Esch K., Smale L., Holekamp K. E. 2000. Mechanisms of maternal rank ‘inheritance’ in the spotted hyaena, Crocuta crocuta. Anim. Behav. 60, 323–332 10.1006/anbe.2000.1502 (doi:10.1006/anbe.2000.1502) [DOI] [PubMed] [Google Scholar]
- 36.Jolicoeur P. 1963. The multivariate generalisation of the allometry equation. Biometrics 19, 497–499 10.2307/2527939 (doi:10.2307/2527939) [DOI] [Google Scholar]
- 37.Tzeng T. D., Yeh S. Y. 1999. Permutation tests for difference between two multivariate allometric patterns. Zool. Stud. 38, 10–18 [Google Scholar]
- 38.Suzuki R., Shimodaira H. 2009. pvclust: hierarchical clustering with p-values via multiscale bootstrap resampling, R package, version 1.2-1. See http://www.is.titech.ac.jp/~shimo/prog/pvclust/
- 39.Conner J. K. 1996. Understanding natural selection: an approach integrating selection gradients, multiplicative fitness components, and path analysis. Ethol. Ecol. Evol. 8, 387–398 10.1080/08927014.1996.9522911 (doi:10.1080/08927014.1996.9522911) [DOI] [Google Scholar]
- 40.Li C. C. 1975. Path analysis: a primer. Pacific Grove, CA: The Boxwood Press [Google Scholar]
- 41.Kolowski J. M., Holekamp K. E. 2009. Ecological and anthropogenic influences on space use by spotted hyaenas. J. Zool. 277, 23–36 10.1111/j.1469-7998.2008.00505.x (doi:10.1111/j.1469-7998.2008.00505.x) [DOI] [Google Scholar]
- 42.Holekamp K. E., Smale L. 1993. Ontogeny of dominance in free-living spotted hyaenas: juvenile rank relations with other immature individuals. Anim. Behav. 46, 451–466 10.1006/anbe.1993.1214 (doi:10.1006/anbe.1993.1214) [DOI] [Google Scholar]
- 43.Melis C., Herfindal I., Kauhala K., Andersen R., Hogda K. A. 2010. Predicting animal performance through climatic and plant phenology variables: the case of an omnivore hibernating species in Finland. Mamm. Biol. 75, 151–159 10.1016/j.mambio.2008.12.001 (doi:10.1016/j.mambio.2008.12.001) [DOI] [Google Scholar]
- 44.Fairbairn D. J., Blanckenhorn W. U., Szekely T. 2007. Sex, size and gender roles: evolutionary studies of sexual size dimorphism. Oxford, UK: Oxford University Press [Google Scholar]
- 45.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 46.Tzeng T. D. 2004. Morphological variation between populations of spotted mackerel (Scomber australasicus) off Taiwan. Fish. Res. 68, 45–55 10.1016/j.fishres.2004.02.011 (doi:10.1016/j.fishres.2004.02.011) [DOI] [Google Scholar]
- 47.Clutton-Brock T. 2009. Sexual selection in females. Anim. Behav. 77, 3–11 10.1016/j.anbehav.2008.08.026 (doi:10.1016/j.anbehav.2008.08.026) [DOI] [Google Scholar]
- 48.Holekamp K. E., Smale L. 2000. Feisty females and meek males: reproductive strategies in the spotted hyena. In Reproduction in context (eds Wallen K., Schneider J.), pp. 257–285 Cambridge, MA: MIT Press [Google Scholar]
- 49.Holekamp K. E., Smale L., Berg R., Cooper S. M. 1997. Hunting rates and hunting success in the spotted hyena (Crocuta crocuta). J. Zool. 242, 1–15 10.1111/j.1469-7998.1997.tb02925.x (doi:10.1111/j.1469-7998.1997.tb02925.x) [DOI] [Google Scholar]
- 50.Holekamp K. E., Kolowski J. M. 2009. Hyaenidae. In Handbook of mammals of the world, vol. 1 (eds Wilson D., Mittermeier R., Fonseca G.). Madrid, Spain: Lynx Edicions [Google Scholar]
- 51.Roff D. A. 1986. Predicting body size with life history models. BioScience 36, 316–323 10.2307/1310236 (doi:10.2307/1310236) [DOI] [Google Scholar]
- 52.Eberhard W. G. 2009. Static allometry and animal genitalia. Evolution 63, 48–66 10.1111/j.1558-5646.2008.00528.x (doi:10.1111/j.1558-5646.2008.00528.x) [DOI] [PubMed] [Google Scholar]
- 53.Emlen D. J. 2008. The evolution of animal weaponry. Annu. Rev. Ecol. Evol. Syst. 39, 387–413 10.1146/annurev.ecolsys.39.110707.173502 (doi:10.1146/annurev.ecolsys.39.110707.173502) [DOI] [Google Scholar]
- 54.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray [Google Scholar]
- 55.Clutton-Brock T. 2007. Sexual selection in males and females. Science 318, 1882–1885 10.1126/science.1133311 (doi:10.1126/science.1133311) [DOI] [PubMed] [Google Scholar]