Abstract
Sexual selection theory predicts that phenotypic traits used to choose a mate should reflect honestly the quality of the sender and thus, are often costly. Physiological costs arise if a signal depends on limited nutritional resources. Hence, the nutritional condition of an organism should determine both its quality as a potential mate and its ability to advertise this quality to the choosing sex. In insects, the quality of the offspring's nutrition is often determined by the ovipositing female. A causal connection, however, between the oviposition decisions of the mother and the mating chances of her offspring has never been shown. Here, we demonstrate that females of the parasitic wasp Nasonia vitripennis prefer those hosts for oviposition that have been experimentally enriched in linoleic acid (LA). We show by 13C-labelling that LA from the host diet is a precursor of the male sex pheromone. Consequently, males from LA-rich hosts produce and release higher amounts of the pheromone and attract more virgin females than males from LA-poor hosts. Finally, males from LA-rich hosts possess three times as many spermatozoa as those from LA-poor hosts. Hence, females making the right oviposition decisions may increase both the fertility and the sexual attractiveness of their sons.
Keywords: handicap hypothesis, mother knows best principle, parasitic wasp, phenotype-linked fertility hypothesis, preference-performance hypothesis, sex pheromone
1. Introduction
It is well established by sexual selection theory that signals enabling mate finding and recognition can also be used by the choosing sex (typically the female) to assess the quality of the signaller (typically the male) [1]. Benefits for females are highest if the signal reflects honestly the condition of the advertising male. Therefore, reliable signals should be insusceptible to cheating [2]. This can be achieved, for instance, if the signal is costly as predicted by the handicap hypothesis [3]. The costs of a sexually selected signal should covary with signal attractiveness thus enabling males of superior quality to bear the expenses of the signal at lower fitness losses [4–6].
Direct physiological costs accrue, for instance, if the signal intensity depends on the availability of limited nutritional resources like certain secondary metabolites being accumulated as signals themselves [7–10] or functioning as signal precursors [11–16]. But also macronutrients may influence the intensity of sexual signals. Studies addressing this aspect focused mainly on protein [4]. By contrast, the impact of dietary fats on sexual signalling has hardly ever been studied, although chemical signals are often derived from the fatty acid metabolism [17–19] and some polyunsaturated fatty acids (PUFAs) are essential nutrients for most animals [20,21] and thus, like essential amino acids, may be a limiting dietary resource. Furthermore, many of the studies addressing the impact of macronutrients on sexual signals are merely correlative and the underlying mechanisms remain poorly understood [4,22–24].
In insects, it is often the ability of the mother to find suitable oviposition sites that controls the availability and quality of food for the offspring because immature insect stages are often restricted in their mobility. Hence, females are predicted by the preference-performance hypothesis [25] to choose oviposition sites according to the nutritional and environmental requirements of their offspring. This phenomenon, often referred to as the ‘mother knows best’ principle [26], has been demonstrated in both herbivores [27] and carnivores [28] and is particularly true for parasitic wasps in which host organisms chosen by the mother are the only source of nutrients for the offspring [29–31].
A model organism for the study of parasitic wasp biology is the jewel wasp Nasonia vitripennis Walker (Hymenoptera: Pteromalidae) [30]. Females of this species parasitize puparia of numerous cyclorraphous fly species [32]. Nasonia vitripennis occurs in two ecotypes, one found in the nests of hole-breeding birds, the other living on and near decaying carcasses [33]. Males of N. vitripennis attract females by releasing a substrate-borne sex pheromone consisting of a mixture of (4R,5R)- and (4R,5S)-5-hydroxy-4-decanolides (HDLs) [34]. The response is shown by virgin females only [34–36] and is synergized by the trace component 4-methylquinazoline [37]. The HDLs are biosynthesized in the rectal vesicle of males [38] and released via the anal orifice by dabbing movements of the abdominal tip [39,40]. Females have been shown to orient along HDL concentration gradients thereby avoiding the danger of mating with sperm-depleted males that release less of the attractive chemicals than males of sufficient fertility [41]. Vernolic acid (= erythro-12,13-epoxy-octadec-9Z-enoic acid) is a precursor of HDL [38], suggesting that pheromone biosynthesis starts from linoleic acid (LA), which is a possible epoxidation substrate of cytochrome P450 enzymes [42]. LA is an essential nutrient for most animals and is, like other PUFAs, important for a number of metabolic processes in animals including sperm production [21]. In the present study, we investigated the putative function of LA as a pheromone precursor in N. vitripennis males. Furthermore, we tested the hypothesis that the availability of dietary fat rich in LA has an impact on the fertility of N. vitripennis males and increases their sexual attractiveness by enabling them to produce and release higher amounts of the abdominal sex pheromone. Finally, we tested the prediction of the preference-performance hypothesis that females are able to detect and prefer those hosts for oviposition that contain higher amounts of unsaturated fatty acids including LA.
2. Material and methods
(a). Insects
The N. vitripennis used in this study originated from an inbred strain that was collected from a bird's nest in northern Germany and reared on freeze-killed puparia of the green bottle fly Lucilia caesar as described elsewhere [43].
(b). Manipulation of host quality
To obtain hosts with high (LA+) and low (LA−) contents in LA, newly emerged L. caesar flies of both sexes were provided for 8 days with honey, water and ground meat, until females were ready to oviposit. Subsequently, females were separated into two groups and allowed to oviposit on two oviposition substrates differing in the fatty acid composition. A larval diet relatively rich in LA was prepared by adding 10 per cent of safflower oil (Goldhand, Düsseldorf, Germany) to lean ground beef fillet and homogenizing it using a scoop. A larval diet poor in LA was prepared by the same method using coconut oil instead (Palmin, Peter Köln KGaA, Elmshorn, Germany). The addition of only 10 per cent fat to lean meat ensured that the diets were comparable to natural dietary resources with respect to the total fat content. The fatty acid composition of the two fats was determined as described below (table 1). Emerging fly larvae fed on the diets for about a week and then pupated. Pupae of either type were collected daily, frozen 2 days after pupation and stored at −20°C until used for parasitoid rearing or chemical analysis (see below).
Table 1.
Relative fatty acid composition of lipids extracted from LA+ and LA− hosts and the fats used to produce them. Compounds are listed in the elution order of the FAME on the DB-5 stationary phase used for chemical analysis.
| name | trivial name | LA+ host | safflower oil | LA− host | coconut oil |
|---|---|---|---|---|---|
| hexanoic acid | caproic acid | — | — | — | 0.63 |
| octanoic acid | caprylic acid | — | — | 1.16 | 8.50 |
| decenoic acida | — | — | 0.11 | — | |
| decanoic acid | capric acid | — | — | 1.23 | 6.25 |
| dodecenoic acida | — | — | 0.67 | — | |
| dodecanoic acid | lauric acid | — | — | 17.51 | 39.12 |
| (9Z)-tetradec-9-enoic acid | myristoleic acid | — | — | 4.79 | — |
| tetradecanoic acid | myristic acid | 1.12 | 0.08 | 11.47 | 19.36 |
| hexadecenoic acida | 7.59 | 0.05 | 0.83 | — | |
| (9Z)-hexadec-9-enoic acid | palmitoleic acid | 6.18 | 0.11 | 16.28 | — |
| hexadecanoic acid | palmitic acid | 16.08 | 6.74 | 17.38 | 11.80 |
| (9Z,12Z)-octadeca-9,12-dienoic acid | linoleic acid | 12.44 | 18.24 | 3.83 | 0.70 |
| (9Z)-octadec-9-enoic acid | oleic acid | 50.86 | 69.04 | 21.07 | 3.67 |
| octadecenoic acida | 1.01 | 1.53 | — | — | |
| octadecanoic acid | stearic acid | 2.90 | 3.32 | 2.89 | 9.97 |
| octadecadienoic acida | 1.62 | — | — | — | |
| (5Z,8Z,11Z,14Z)-5,8,11,14-eicosatetraenoic acid | arachidonic acid | 0.19 | — | 0.77 | — |
| eicosenoic acid1 | — | 0.34 | — | — | |
| eicosanoic acid | arachidic acid | — | 0.54 | — | — |
| total | 100 | 100 | 100 | 100 |
aPosition and configuration of the double bond(s) not determined.
(c). Fatty acid analysis of LA+ and LA− hosts
The fatty acid composition of the two host types and the fats used to produce them was determined by coupled gas chromatography–mass spectrometry (GC–MS) of the fatty acid methyl esters (FAMEs). Groups of three 2-day-old LA+ and LA− host puparia were extracted for 30 min with 500 µl of hexane. Prior to the extraction, puparia were homogenized using a scoop. The solvent was removed under a gentle stream of nitrogen and 1 mg of extracted raw lipids was re-suspended in 100 µl of methanol and subsequently mixed with 10 µl of acetyl chloride (10%, dissolved in methanol). Samples were kept for 1 h at 60°C for transesterification. Then 200 µl of sodium hydrogen carbonate (5%, dissolved in H2O) was added and the FAMEs were extracted for 30 s with 200 µl of pentane. FAMEs were analysed on a Shimadzu QP2010 Plus GC–MS system equipped with a BPX5 capillary column (30 m × 0.32 mm inner diameter, 0.25 µm film thickness, SGE Analytical Science Europe, Milton Keynes, UK). Samples were injected at 300°C with a split ratio of 1 : 5 using an AOC 20i auto sampler. Helium was used as carrier gas at a constant flow rate of 2 ml min−1. The initial oven temperature of 50°C (held for 4 min) was increased at 3°C min−1 to 280°C (held for 15 min). The MS was operated in the electron impact mode at 70 eV. Identification of FAMEs was done by analysing reference fatty acids (Aldrich, Deisenhofen, Germany) that were derivatized under the same conditions. Relative FAME composition (as %) was determined by relating peak areas of individual compounds to the total peak area of all FAMEs in the sample.
(d). Production of parasitoid males on LA+ and LA− hosts
Groups of two virgin N. vitripennis females were allowed to oviposit for 48 h into LA+ and LA− hosts, respectively. Virgin females were used because they produce all-male broods for the subsequent experiments (haplodiploidy). Parasitoid pupae of both treatments were excised from the hosts 1–2 days before eclosion and kept individually in microcentrifuge tubes until being used for behavioural bioassays and chemical analyses, respectively (see below). Males of either treatment used for comparing experiments were generally of equal size (head width 700 µm) and age (2 days).
(e). 13C-labelling experiments
To test the hypothesis that LA from the host diet is directly incorporated into the male sex attractant, we reared L. caesar hosts on a diet enriched in [13C18]-LA (13C-LA). For this purpose, we added 100 mg of 13C-LA (Campro Scientific GmbH, Berlin, Germany) to 2 g of lean ground beef fillet as described above. Groups of 10 5-day-old LA+ host larvae (see above) were transferred to the 13C-LA diet and allowed to feed for another day until pupation. Subsequently, they were offered to virgin wasp females for parasitization. Abdomens of 2-day-old N. vitripennis males reared on these 13C-LA-labelled hosts were extracted with 25 µl of dichloromethane and used for GC–MS analysis using the equipment and conditions described below. Incorporation of 13C-LA into the two HDL stereoisomers was concluded from the appearance of diagnostic ions in the mass spectra as described elsewhere [38].
(f). Quantification of pheromone deposits released by males from LA+ and LA− hosts
Pheromone deposits were quantified by GC–MS using thermal desorption (TD) sampling. For this purpose, empty 89 × 5 mm ID TD glass tubes (Supelco, Bellefonte, PA, USA) were filled at one end with 50 mg of Tenax TA (Supelco). The adsorbent layer of 25 mm was fixed using fine-mesh metal screens (Supelco). One µl of an internal standard solution containing 100 ng µl−1 methyl undecanoate (Sigma-Aldrich, Deisenhofen, Germany) in methanol was applied to the adsorbent and the tube was purged for 5 min (adsorbent upwind) using a nitrogen flow of 100 ml min−1 to remove the solvent. Subsequently, a 2-day-old male (LA+ males, n = 25; LA− males, n = 24) was transferred together with a virgin female to the empty side of the tube and mating within the tube was observed under a stereo microscope. Then, the female was removed and the male was allowed to mark the inner side of the tube for 10 min. After removal of the male, TD tubes were purged for 3 min with nitrogen at a flow rate of 60 ml min−1 (adsorbent upwind) and capped for TD volatile sampling. By this means, both the volatilized proportion of the marking and the substrate-borne residues were subjected to quantitative analysis with losses kept to a minimum. TD sampling was done using an automated Shimadzu TD 20 thermal desorber (Shimadzu GmbH, Duisburg, Germany) coupled to a Shimadzu QP2010 Plus GC–MS system. Volatiles were desorbed from the TD tubes for 8 min at 250°C with helium at a flow rate of 60 ml min−1 and cryofocused at −20°C on an internal Tenax trap. Subsequently, volatiles were injected into the GC–MS by heating the internal trap to 280°C for 5 min at a split ratio of 1 : 20. The GC was equipped as described above but the temperature programme started at 80°C, increased at 5°C min−1 to 200°C and then at 15°C to 280°C (held for 15 min). Quantification of total HDL deposited by individual males was done by the internal standard method. For this purpose, a calibration curve was created by analysing defined amounts (5–200 ng) of synthetic (4R,5R)-HDL in methanol and an internal standard using the method described above. The reference compound was synthesized by Sharpless asymmetric dihydroxylation of ethyl (4E)-decenoate as described by Garbe & Tressl [44]. Pheromone amounts deposited by males from LA+ and LA− hosts were compared by a Mann–Whitney U-test.
(g). Quantification of pheromone titres of males from LA+ and LA− hosts
Two-day-old males from LA+ (n = 23) and LA− hosts (n = 20) of comparable size were killed by freezing and their abdomens were cut off and extracted for 30 min with 25 µl dichloromethane containing 10 ng µl−1 methyl undecanoate as an internal standard. Extracts were subjected to GC–MS analysis using the instrumentation and conditions described for the TD sampling. However, an AOC 20i auto injector operated in splitless mode was used for sampling of the extracts. Quantification of total HDL in individual males was done by the internal standard method. For this purpose a calibration curve was created by analysis of defined amounts (5–200 ng µl−1) of synthetic (4R,5R)-HDL dissolved in dichloromethane containing the internal standard at 10 ng µl−1. Pheromone titres of males from LA+ and LA− hosts were compared by a Mann–Whitney U-test.
(h). Sperm count of males from LA+ and LA− hosts
Virgin males of equal size and age (2 days) from LA+ and LA− hosts (n = 10 per host type) were killed by freezing. Testes and seminal vesicles were dissected in 10 µl of Ringer solution. One haphazardly chosen testis and seminal vesicle per male were opened and dispersed for 2 min using a fine needle. The solution was diluted with another 10 µl of Ringer solution and dispersed again. The spot was air-dried and fixed in 70 per cent ethanol. The coverslip was put on a square grid (side length 2.5 mm) and sperm numbers of three haphazardly chosen squares were counted blind under a microscope. The total sperm count for each male was estimated by extrapolation.
(i). Female response to pheromone deposits released by males from LA+ and LA− hosts
The response of virgin females to pheromone markings deposited by the two groups of males was examined using a static four-cavity olfactometer [40]. This was made of acrylic glass and consisted of a round walking arena (9 cm diameter, 8 mm thick) equipped with four symmetrically arranged spherical cavities (1 cm diameter, 4 mm deep), a low rim (2 mm high), and a glass plate to cover the arena. Males of both groups were allowed to mate simultaneously with a virgin female in opposing cavities of the olfactometer. Subsequently, females were removed and males were allowed to deposit the sex pheromone in their cavity for 10 min. The other two cavities remained empty and functioned as controls. After removal of the males, virgin females were released individually into the centre of the olfactometer and the time they spent in the four cavities was recorded for 5 min using Observer XT observational software (Noldus, Wageningen, The Netherlands). Additionally, it was noted which cavity was entered first by the females (first choice). Parasitoids were used only once and pheromone deposits were renewed after every replicate (n = 30). Residence times of females spent in the two marked cavities of the olfactometer were analysed by a Wilcoxon matched-pairs test. First choice of females for the LA+ and LA− cavity, respectively, was compared by a two-tailed binominal test.
(j). Oviposition preference of parasitoid females
To test the hypothesis that female parasitoids prefer LA+ hosts over LA− hosts for oviposition, single virgin females (n = 40) were placed into Petri dishes containing a LA+ and a LA− host of equal size for oviposition. To prevent inadvertent rolling of the hosts, these were glued at a distance of 20 mm to the bottom of the dishes using a drop of non-toxic glue (Pentel ER153, Tokyo, Japan). Females were allowed to oviposit for 24 h. After 10 days, male parasitoid pupae were excised from the hosts of either type and counted. In a second experiment, we used mated females (n = 30) to study whether females lay a higher proportion of male eggs in LA+ than in LA– hosts. In general, the experiment was performed as described above. But in this case the test lasted 3 days and the females were exposed to new hosts each day. After the third day, parasitized LA+ and LA− hosts were separated and emerging offspring was sexed and counted. Parasitoids from LA+ and LA− hosts were compared by a Wilcoxon matched-pairs test.
3. Results
(a). Linoleic acid is a precursor of the male sex pheromone
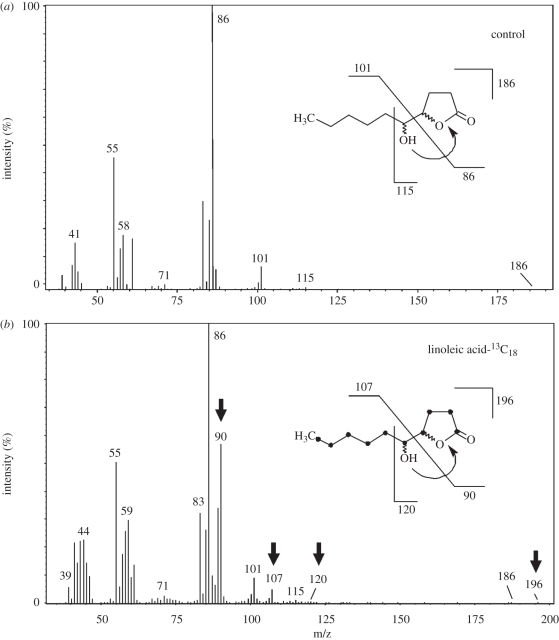
Our 13C-labelling experiments revealed that LA from the host diet is directly incorporated into the male sex pheromone of the parasitoid. Incorporation of 13C was indicated by mass shifts of the four diagnostic ions m/z 86, 101, 115 and 186 in the mass spectra of (4R,5R)- and (4R,5S)-HDL ([38], figure 1). The incorporation rate was 36 per cent each.
Figure 1.
Mass spectra of HDL from N. vitripennis males reared on (a) a normal host (control) and (b) a host fed a diet enriched in [13C18]-linoleic acid. Arrows indicate diagnostic ions resulting from the incorporation of the labelled precursor. 13C-atoms are indicated by black dots.
(b). Fatty acid composition of the host depends on its dietary fats
The fatty acid composition of LA+ and LA− hosts differed significantly and was clearly influenced by the fats used for preparing the host diets (table 1). LA− hosts contained mainly saturated and monounsaturated fatty acids with chain lengths between 8 and 18 carbon units whereas LA was a minor component amounting less than 4 per cent of the total fatty acids. A threefold higher amount of LA was found in the fatty acid profile of LA+ hosts that was dominated by oleic acid, the major component of the safflower oil used to prepare their diet. Despite the differences found in the fatty acid composition of LA+ and LA− hosts in our study, both were within the range reported earlier for potential hosts of N. vitripennis [45].
(c). Parasitoid males from LA+ hosts are more fertile
Although the diets used to prepare the two host types were comparable in terms of energetic value, N. vitripennis males from LA+ hosts had three times as many spermatozoa in their seminal vesicle as those from LA− hosts (t-test: t = 6.466, d.f. = 18, p < 0.001, figure 2).
Figure 2.
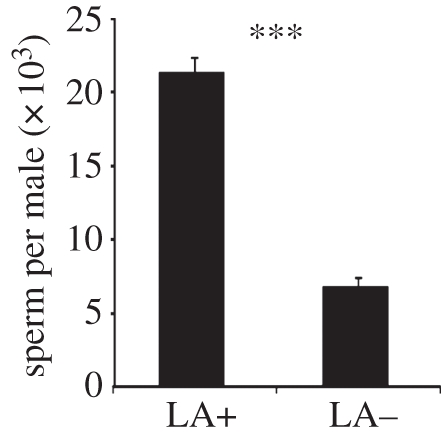
Mean sperm number (±s.e.m.) counted in the seminal vesicles of individual N. vitripennis males from LA+ and LA− hosts. Asterisks indicate significant differences at p < 0.001 (t-test).
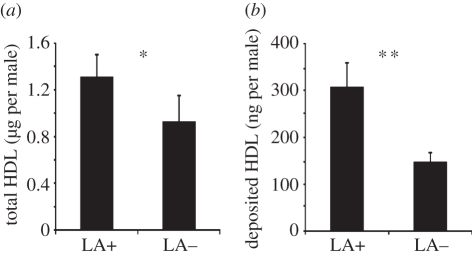
(d). Parasitoid males from LA+ hosts produce and release more of the sex pheromone
Males from LA+ hosts had significantly higher total HDL titres (Mann–Whitney U-test: U = 141, Z = −2.167, d.f. = 41, p = 0.030) (figure 3a) and deposited almost twice as much of the pheromone as those from LA− hosts (Mann-Whitney-U-test: U = 179, Z = −2.903, d.f. = 47, p = 0.004, figure 3b).
Figure 3.
Total HDL amounts (mean ± s.e.m.) (a) extracted from the abdomens and (b) deposited within an observation time of 10 min by 2-day-old N. vitripennis males from LA+ and LA− hosts, respectively. Asterisk indicates significant differences at p < 0.01 (**) and p < 0.05 (*) (Mann–Whitney U-test).
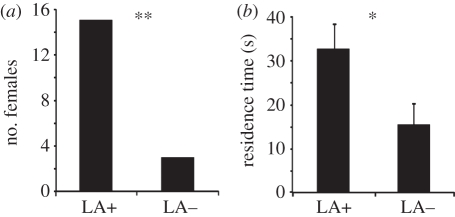
(e). Pheromone markings of males from LA+ hosts are more attractive for virgin females
In our olfactometer bioassays, virgin females were attracted more often to cavities marked by males from LA+ hosts (two-tailed binominal test: p = 0.0075, figure 4a) and spent significantly more time in these cavities (Wilcoxon matched-pairs test: Z = −2.306, d.f. = 30, p = 0.021, figure 4b) when compared with cavities marked by males from LA− hosts.
Figure 4.
Response of virgin N. vitripennis females in the olfactometer bioassay to pheromone deposits released by males from LA+ and LA− hosts, respectively. (a) First choice for and (b) mean residence time (±s.e.m.) in the marked cavities within an observation time of 5 min. Asterisks indicate significant differences at p < 0.01 (**) and p < 0.05 (*) (first choice: two-tailed binominal test, residence time: Wilcoxon matched-pairs test).
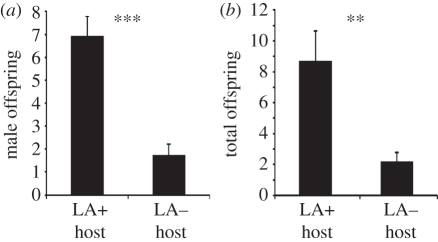
(f). Female parasitoids prefer LA+ hosts for oviposition
In our two-choice oviposition experiments, both virgin females (producing only male offspring owing to the haplodiploid sex determination of parasitic wasps) and mated females (producing offspring of either sex) laid more eggs into LA+ than in LA− hosts (Wilcoxon matched-pairs test: virgin females: Z = −3.550, d.f. = 40, p < 0.001; mated females: Z = 3.079, d.f. = 30, p = 0.0021, figure 5a,b). However, mated females did not lay a higher proportion of male offspring into LA+ than in LA− hosts (χ2-test: χ2 = 0.73, d.f. = 1, p = 0.394).
Figure 5.
Oviposition preference of (a) virgin and (b) mated N. vitripennis females for LA+ and LA− hosts in a two-choice bioassay. Data represent mean number (±s.e.m.) of (a) male pupae produced within 24 h and (b) adult offspring of mixed sex produced within 72 h by a single female. Asterisks indicate significant differences at p < 0.001 (***) and p < 0.01 (**) (Wilcoxon matched-pairs test).
4. Discussion
The present study clearly demonstrates that LA taken up by the host during feeding is directly incorporated into the abdominal sex pheromone of N. vitripennis males and thus functions as a precursor of the chemical signal. Furthermore, the fatty acid composition of the host is of crucial importance for both the fertility and the pheromone-mediated sexual attractiveness of N. vitripennis males. Males developing in hosts containing higher amounts of unsaturated fatty acids including LA possess much higher numbers of spermatozoa and release more of the sex pheromone than males whose dietary lipids contain higher proportions of saturated fatty acids. Since N. vitripennis females are monandrous in natural populations [46] and typically mate with the first male they encounter, LA+ males have clearly enhanced mating chances by bringing more virgin females to orient towards their pheromone markings. Furthermore, the threefold higher number of spermatozoa found in males from LA+ hosts should translate into significant fitness benefits because of delayed sperm depletion. Nasonia vitripennis is a haplodiploid species with male offspring developing from unfertilized eggs only. In a recent study, we found a correlation between the mating history of males and the proportion of sons in the offspring they fathered, and significant sperm depletion was already measurable after seven successive copulations [41]. Our data suggest that sperm depletion might occur even earlier in males of poor nutritional condition. Sex ratios in species under local mate competition like Nasonia are typically strongly female-biased [47,48]. Females mating with males from hosts poor in LA are more likely to run out of sperm and get constrained to produce suboptimal offspring sex ratios. However, females may avoid these costs by using the male sex pheromone to discriminate against these males of lower quality.
The link between dietary lipids and sexual attractiveness on the one hand and fertility on the other makes the abdominal sex pheromone of N. vitripennis a reliable signal indicating male mate quality. However, the signal might not be completely insusceptible to cheating because males of inferior quality might invest more of the available LA into the pheromone at the cost of decreased sperm production to get mating opportunities at all. Males of superior nutritional condition can afford to produce high amounts of the sexual signal without facing this trade-off. Thus, our study is one of the very few explaining why male signals provide reliable information about direct benefit quality and supports both the handicap hypothesis [3] and the phenotype-linked fertility hypothesis ([49] and references therein; [50]).
Our results demonstrate furthermore that foraging N. vitripennis females prefer those hosts for oviposition that contain higher amounts of nutrients making their sons more fertile and more attractive for sexual mates. This suggests that they are able to evaluate the fatty acid composition of their hosts during or already prior to oviposition. But females did not allocate a higher proportion of sons to LA+ hosts. This indicates that the female offspring also benefits from the increased availability of PUFAs as has been shown for several other animals [21].
An important question is whether the choice situation arranged in our experiments is a realistic one and reflects what female wasps might actually encounter in the field. We are confident that this is the case although data on the variability of fatty acid compositions in field populations of N. vitripennis host pupae are missing. However, a comparative investigation of adult insects from seven insect orders suggested a high variability of fatty acid composition in cyclorraphous flies, with LA proportions ranging between 0 and 25 per cent [45]. Our results showed that the fatty acid composition of the host is strongly influenced by its diet. Therefore, variability in the fatty acid composition of the natural host diets should result in variability of host quality as well. As for the N. vitripennis birds' nest ecotype, a food web has been modelled for European populations showing that females parasitized mainly four dipteran hosts either sucking the blood of live nestlings (Protocalliphora azurea, Protocalliphora falcozi), feeding on dead nestlings (Calliphora vicina) or on faeces and other organic materials in the nests (Potamia littoralis) [51]. It is reasonable to assume that these diets differ clearly in the availability and composition of dietary lipids and, thus, result in differing host qualities within an individual birds' nest. The same can be predicted for hosts of the N. vitripennis carcass ecotype because fat is not equally distributed within a dead animal. Rabbit liver, for instance, contains 60 per cent more LA than lean muscle tissue or blood plasma [52,53]. Hence, it is likely that host larvae feeding on different tissues and organs of a carcass differ in their fatty acid composition. Chemical analyses are in progress to test this hypothesis and to determine the natural variability of fatty acid compositions of N. vitripennis hosts from both habitat types.
Our findings add another facet to the ‘mother knows best’ principle by demonstrating a causal link between the oviposition preference of the mother and the mating chances of her sons. This is particularly interesting in the light of Nasonia being a haplodiploid species. Here, the classical ‘sexy son’ scenario of sexual selection theory [54,55] does not work because any trait of sexual attractiveness owned by the father cannot be inherited directly to the male offspring. However, by making the right oviposition decisions, a female may nevertheless influence the reproductive success of her sons.
The results of the present study point emphatically to LA as a crucial dietary resource of juvenile N. vitripennis males influencing both their fertility and their pheromone-mediated sexual attractiveness as adults. While the causality of this conclusion was supported for the pheromone biosynthesis in our 13C-labelling experiments, also other differences between safflower and coconut oil (e.g. the oleic acid content) might have been responsible for the different numbers of spermatozoa found in males from LA+ and LA− hosts, respectively. However, there is ample literature demonstrating for many taxa of animals and humans that LA and other PUFAs influence the fertility of both males and females (reviewed by [21]). PUFAs are essential components of all cell membranes and, in particular, male spermatozoa require high PUFA contents to provide the plasma membrane with the essential fluidity for gamete fusion [21]. Furthermore, animals including many insects are able to elongate LA to C20-PUFAs, which can be further metabolized to prostaglandins [20]. This hormonally active class of molecules is also known to have an impact on the reproduction of many animals, including insects [56].
It has long been a paradigm that animals are unable to biosynthesize LA by themselves and thus, depend on the dietary uptake of this nutrient [20]. Today we know that this is not true for all animals because at least some have been shown to possess Δ-12-desaturases, enabling them to synthesize LA from oleic acid by introducing a second double bond at position 12 [20,57,58]. Nothing, however, is known about this ability in Nasonia and other Hymenoptera. This aspect needs further investigation.
Acknowledgements
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, grant RU-717/10-1). The authors thank Alexandra Schrempf for her help in performing the sperm count, Daniela Pothmann for technical assistance and two anonymous reviewers for helpful comments.
References
- 1.Anderson M. E. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Maynard Smith J., Harper D. 2003. Animal signals. Oxford, UK: Oxford University Press [Google Scholar]
- 3.Zahavi A. 1975. Mate selection—a selection for a handicap. J. Theor. Biol. 53, 205–214 10.1016/0022-5193(75)90111-3 (doi:10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 4.Cotton S., Fowler K., Pomiankowski A. 2004. Do sexual ornaments demonstrate heightened condition dependent expression as predicted by the handicap hypothesis? Proc. R. Soc. Lond. B 271, 771–783 10.1098/rspb.2004.2688 (doi:10.1098/rspb.2004.2688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grafen A. 1990. Biological signals as handicaps. J. Theor. Biol. 144, 517–546 10.1016/S0022-5193(05)80088-8 (doi:10.1016/S0022-5193(05)80088-8) [DOI] [PubMed] [Google Scholar]
- 6.Johansson B. J., Jones T. M. 2007. The role of chemical communication in mate choice. Biol. Rev. 82, 265–289 10.1111/j.1469-185X.2007.00009.x (doi:10.1111/j.1469-185X.2007.00009.x) [DOI] [PubMed] [Google Scholar]
- 7.Eisner T., Smedley S. R., Young D. K., Eisner M., Roach B., Meinwald J. 1996. Chemical basis of courtship in a beetle (Neopyrochroa flabellata): Cantharidin as precopulatory ‘enticing’ agent. Proc. Natl Acad. Sci. USA 93, 6494–6498 10.1073/pnas.93.13.6494 (doi:10.1073/pnas.93.13.6494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray D. A. 1996. Carotenoids and sexual dichromatism in North American passerine birds. Am. Nat. 148, 453–480 10.1086/285935 (doi:10.1086/285935) [DOI] [Google Scholar]
- 9.Hill G. E., Inouye C. Y., Montgomerie R. 2002. Dietary carotenoids predict plumage coloration in wild house finches. Proc. R. Soc. Lond. B 269, 1119–1124 10.1098/rspb.2002.1980 (doi:10.1098/rspb.2002.1980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodric-Brown A. 1989. Dietary carotenoids and male mating success in the guppy—an environmental component to female choice. Behav. Ecol. Sociobiol. 25, 393–401 10.1007/BF00300185 (doi:10.1007/BF00300185) [DOI] [Google Scholar]
- 11.Conner W. E., Roach B., Benedict E., Meinwald J., Eisner T. 1990. Courtship pheromone production and body size as correlates of larval diet in males of the arctiid moth, Utetheisa ornatrix. J. Chem. Ecol. 16, 543–552 10.1007/BF01021785 (doi:10.1007/BF01021785) [DOI] [PubMed] [Google Scholar]
- 12.Eisner T., Meinwald J. 1995. Defense-mechanisms of arthropods. 129. The chemistry of sexual selection. Proc. Natl Acad. Sci. USA 92, 50–55 10.1073/pnas.92.1.50 (doi:10.1073/pnas.92.1.50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landolt P. J., Phillips T. W. 1997. Host plant influences on sex pheromone behavior of phytophagous insects. Annu. Rev. Entomol. 42, 371–391 10.1146/annurev.ento.42.1.371 (doi:10.1146/annurev.ento.42.1.371) [DOI] [PubMed] [Google Scholar]
- 14.Shelly T. E. 2000. Flower-feeding affects mating performance in male oriental fruit flies Bactrocera dorsalis. Ecol. Entomol. 25, 109–114 10.1046/j.1365-2311.2000.00231.x (doi:10.1046/j.1365-2311.2000.00231.x) [DOI] [Google Scholar]
- 15.Shelly T. E., Nishida R. 2004. Larval and adult feeding on methyl eugenol and the mating success of male oriental fruit flies, Bactrocera dorsalis. Entomol. Exp. Appl. 112, 155–158 10.1111/j.0013-8703.2004.00180.x (doi:10.1111/j.0013-8703.2004.00180.x) [DOI] [Google Scholar]
- 16.Shelly T. E., Edu J., Pahio E. 2007. Condition-dependent mating success in male fruit flies: ingestion of a pheromone precursor compensates for a low-quality diet. J. Insect Behav. 20, 347–365 10.1007/s10905-007-9082-3 (doi:10.1007/s10905-007-9082-3) [DOI] [Google Scholar]
- 17.Jurenka R. 2004. Insect pheromone biosynthesis. Topics Curr. Chem. 239, 97–131 10.1007/b95450 (doi:10.1007/b95450) [DOI] [PubMed] [Google Scholar]
- 18.Martin J., Lopez P. 2010. Condition-dependent pheromone signaling by male rock lizards: more oily scents are more attractive. Chem. Senses 35, 253–262 10.1093/chemse/bjq009 (doi:10.1093/chemse/bjq009) [DOI] [PubMed] [Google Scholar]
- 19.Tillman J. A., Seybold S. J., Jurenka R. A., Blomquist G. J. 1999. Insect pheromones—an overview of biosynthesis and endocrine regulation. Insect Biochem. Mol. Biol. 29, 481–514 10.1016/S0965-1748(99)00016-8 (doi:10.1016/S0965-1748(99)00016-8) [DOI] [PubMed] [Google Scholar]
- 20.Blomquist G. J., Borgeson C. E., Vundla M. 1991. Polyunsaturated fatty acids and eicosanoids in insects. Insect Biochem. 21, 99–106 10.1016/0020-1790(91)90069-Q (doi:10.1016/0020-1790(91)90069-Q) [DOI] [Google Scholar]
- 21.Wathes D. C., Abayasekara D. R. E., Aitken R. J. 2007. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 77, 190–201 10.1095/biolreprod.107.060558 (doi:10.1095/biolreprod.107.060558) [DOI] [PubMed] [Google Scholar]
- 22.Fisher H. S., Rosenthal G. G. 2006. Female swordtail fish use chemical cues to select well-fed mates. Anim. Behav. 72, 721–725 10.1016/j.anbehav.2006.02.009 (doi:10.1016/j.anbehav.2006.02.009) [DOI] [Google Scholar]
- 23.Giaquinto P. C., Berbert C. M. D., Delicio H. C. 2010. Female preferences based on male nutritional chemical traits. Behav. Ecol. Sociobiol. 64, 1029–1035 10.1007/s00265-010-0918-z (doi:10.1007/s00265-010-0918-z) [DOI] [Google Scholar]
- 24.Meikle D. B., Kruper J. H., Browning C. R. 1995. Adult male house mice born to undernourished mothers are unattractive to oestrous females. Anim. Behav. 50, 753–758 10.1016/0003-3472(95)80135-9 (doi:10.1016/0003-3472(95)80135-9) [DOI] [Google Scholar]
- 25.Jaenike J. 1978. On optimal oviposition behavior in phytophagous insects. Theor. Popul. Biol. 14, 350–356 10.1016/0040-5809(78)90012-6 (doi:10.1016/0040-5809(78)90012-6) [DOI] [PubMed] [Google Scholar]
- 26.Gripenberg S., Mayhew P. J., Parnell M., Roslin T. 2010. A meta-analysis of preference-performance relationships in phytophagous insects. Ecol. Lett. 13, 383–393 10.1111/j.1461-0248.2009.01433.x (doi:10.1111/j.1461-0248.2009.01433.x) [DOI] [PubMed] [Google Scholar]
- 27.Awmack C. S., Leather S. R. 2002. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 47, 817–844 10.1146/annurev.ento.47.091201.145300 (doi:10.1146/annurev.ento.47.091201.145300) [DOI] [PubMed] [Google Scholar]
- 28.Vinson S. B., Iwantsch G. F. 1980. Host suitability for insect parasitoids. Annu. Rev. Entomol. 25, 397–419 10.1146/annurev.en.25.010180.002145 (doi:10.1146/annurev.en.25.010180.002145) [DOI] [Google Scholar]
- 29.Godfray H. C. J. 1994. Parasitoids: behavioral and evolutionary ecology. Princeton, NJ: Princeton University Press [Google Scholar]
- 30.Godfray H. C. J. 2010. Nasonia: a jewel among wasps. Heredity 104, 235–236 10.1038/hdy.2010.3 (doi:10.1038/hdy.2010.3) [DOI] [PubMed] [Google Scholar]
- 31.Quicke D. L. J. 1997. Parasitic wasps. London, UK: Chapman & Hall [Google Scholar]
- 32.Whiting A. R. 1967. Biology of parasitic wasp Mormoniella vitripennis (=Nasonia brevicornis) (Walker). Q. Rev. Biol. 42, 333–406 10.1086/405402 (doi:10.1086/405402) [DOI] [Google Scholar]
- 33.Schröder H. 1997. Differenzierung zweier Ökotypen bei Nasonia vitripennis (Walker 1836) (Hymenoptera: Pteromalidae). Aachen, Germany: Shaker Verlag [Google Scholar]
- 34.Ruther J., Stahl L. M., Steiner S., Garbe L.-A., Tolasch T. 2007. A male sex pheromone in a parasitic wasp and control of the behavioral response by the female's mating status. J. Exp. Biol. 210, 2163–2169 10.1242/jeb.02789 (doi:10.1242/jeb.02789) [DOI] [PubMed] [Google Scholar]
- 35.Ruther J., Thal K., Blaul B., Steiner S. 2010. Behavioural switch in the sex pheromone response of Nasonia vitripennis females is linked to receptivity signaling. Anim. Behav. 80, 1035–1040 10.1016/j.anbehav.2010.09.008 (doi:10.1016/j.anbehav.2010.09.008) [DOI] [Google Scholar]
- 36.Steiner S., Ruther J. 2009. How important is sex for females of a haplodiploid species under local mate competition? Behav. Ecol. 20, 574. 10.1093/beheco/arp033 (doi:10.1093/beheco/arp033) [DOI] [Google Scholar]
- 37.Ruther J., Steiner S., Garbe A. 2008. 4-Methylquinazoline is minor component of the male sex pheromone in Nasonia vitripennis. J. Chem. Ecol. 34, 1–4 10.1007/s10886-007-9411-1 (doi:10.1007/s10886-007-9411-1) [DOI] [PubMed] [Google Scholar]
- 38.Abdel-Latief M., Garbe L.-A., Koch M., Ruther J. 2008. An epoxide hydrolase involved in the biosynthesis of an insect sex attractant and its use to localize the production site. Proc. Natl Acad. Sci. USA 105, 8914–8919 10.1073/pnas.0801559105 (doi:10.1073/pnas.0801559105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruther J., Thal K., Steiner S. 2011. Pheromone Communication in Nasonia vitripennis: abdominal sex attractant mediates site fidelity of releasing males. J. Chem. Ecol. 37, 161–165 10.1007/s10886-010-9898-8 (doi:10.1007/s10886-010-9898-8) [DOI] [PubMed] [Google Scholar]
- 40.Steiner S., Ruther J. 2009. Mechanism and behavioral context of male sex pheromone release in Nasonia vitripennis. J. Chem. Ecol. 35, 416–421 10.1007/s10886-009-9624-6 (doi:10.1007/s10886-009-9624-6) [DOI] [PubMed] [Google Scholar]
- 41.Ruther J., Matschke M., Garbe L.-A., Steiner S. 2009. Quantity matters: male sex pheromone signals mate quality in the parasitic wasp Nasonia vitripennis. Proc. R. Soc. B 276, 3303–3310 10.1098/rspb.2009.0738 (doi:10.1098/rspb.2009.0738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moran J. H., Mitchell L. A., Bradbury J. A., Qu W., Zeldin D. C., Schnellmann R. G., Grant D. F. 2000. Analysis of the cytotoxic properties of linoleic acid metabolites produced by renal and hepatic P450s. Toxicol. Appl. Pharmacol. 168, 268–279 10.1006/taap.2000.9053 (doi:10.1006/taap.2000.9053) [DOI] [PubMed] [Google Scholar]
- 43.Steiner S., Hermann N., Ruther J. 2006. Characterization of a female-produced courtship pheromone in the parasitoid Nasonia vitripennis. J. Chem. Ecol. 32, 1687–1702 10.1007/s10886-006-9102-3 (doi:10.1007/s10886-006-9102-3) [DOI] [PubMed] [Google Scholar]
- 44.Garbe L.-A., Tressl R. 2004. Metabolism of deuterated threo-dihydroxy fatty acids in Saccharomyces cerevisiae: enantioselective formation and characterization of hydroxylactones and gamma-lactones. Helv. Chim. Acta. 87, 180–196 10.1002/hlca.200490007 (doi:10.1002/hlca.200490007) [DOI] [Google Scholar]
- 45.Thompson S. N. 1973. A review and comparative characterization of fatty acid compositions of seven insect orders. Comp. Biochem. Physiol. B 53, 467–482 [Google Scholar]
- 46.Grillenberger B. K., Koevoets T., Burton-Chellew M. N., Sykes E. M., Shuker D. M., van de Zande L., Bijlsma R., Gadau J., Beukeboom L. W. 2008. Genetic structure of natural Nasonia vitripennis populations: validating assumptions of sex-ratio theory. Mol. Ecol. 17, 2854–2864 10.1111/j.1365-294X.2008.03800.x (doi:10.1111/j.1365-294X.2008.03800.x) [DOI] [PubMed] [Google Scholar]
- 47.Shuker D. M., West S. A. 2004. Information constraints and the precision of adaptation: sex ratio manipulation in wasps. Proc. Natl Acad. Sci. USA 101, 10 363–10 367 10.1073/pnas.0308034101 (doi:10.1073/pnas.0308034101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Werren J. H. 1980. Sex-ratio adaptations to local mate competition in a parasitic wasp. Science 208, 1157–1159 10.1126/science.208.4448.1157 (doi:10.1126/science.208.4448.1157) [DOI] [PubMed] [Google Scholar]
- 49.Reynolds J. D., Gross M. R. 1990. Costs and benefits of female mate choice—is there a lek paradox? Am. Nat. 136, 230–243 10.1086/285093 (doi:10.1086/285093) [DOI] [Google Scholar]
- 50.Sheldon B. C. 1994. Male phenotype, fertility, and the pursuit of extra-pair copulations by female birds. Proc. R. Soc. Lond. B 257, 25–30 10.1098/rspb.1994.0089 (doi:10.1098/rspb.1994.0089) [DOI] [Google Scholar]
- 51.Peters R. S., Abraham R. 2010. The food web of parasitoid wasps and their non-phytophagous fly hosts in birds' nests (Hymenoptera: Chalcidoidea, and Diptera: Cyclorrapha). J. Nat. Hist. 44, 625–638 10.1080/00222930903437317 (doi:10.1080/00222930903437317) [DOI] [Google Scholar]
- 52.Tres A., Bou R., Codony R., Guardiola F. 2008. Influence of different dietary doses of n-3- or n-6-rich vegetable fats and α-tocopheryl acetate supplementation on raw and cooked rabbit meat composition and oxidative stability. J. Agric. Food Chem. 56, 7243–7253 10.1021/jf800736w (doi:10.1021/jf800736w) [DOI] [PubMed] [Google Scholar]
- 53.Tres A., Bou R., Codony R., Guardiola F. 2009. Dietary n-6 or n-3-rich vegetable fats and α-tocopheryl acetate: effects on fatty acid composition and stability of rabbit plasma, liver and meat. Animal 3, 1408–1419 10.1017/S1751731109990334 (doi:10.1017/S1751731109990334) [DOI] [PubMed] [Google Scholar]
- 54.Quarnström A., Price T. D. 2001. Maternal effects, paternal effects and sexual selection. Trends Ecol. Evol. 16, 95–100 10.1016/S0169-5347(00)02063-2 (doi:10.1016/S0169-5347(00)02063-2) [DOI] [PubMed] [Google Scholar]
- 55.Weatherhead P. J., Robertson R. J. 1979. Offspring quality and the polygyny threshold—sexy son hypothesis. Am. Nat. 113, 201–208 10.1086/283379 (doi:10.1086/283379) [DOI] [Google Scholar]
- 56.Stanley D. 2006. Prostaglandins and other eicosanoids in insects: biological significance. Annu. Rev. Entomol. 51, 25–44 10.1146/annurev.ento.51.110104.151021 (doi:10.1146/annurev.ento.51.110104.151021) [DOI] [PubMed] [Google Scholar]
- 57.Borgeson C. E., Kurtti T. J., Munderloh U. G., Blomquist G. J. 1991. Insect tissues, not microorganisms, produce linoleic acid in the house cricket and the American cockroach. Experientia 47, 238–241 10.1007/BF01958146 (doi:10.1007/BF01958146) [DOI] [PubMed] [Google Scholar]
- 58.Weinert J., Blomquist G. J., Borgeson C. E. 1993. De-novo biosynthesis of linoleic-acid in two non-insect invertebrates-the land slug and the garden snail. Experientia 49, 919–921 10.1007/BF01952610 (doi:10.1007/BF01952610) [DOI] [Google Scholar]