Abstract
When social animals engage in inter-group contests, the outcome is determined by group sizes and individual masses, which together determine group resource-holding potential (‘group RHP’). Individuals that perceive themselves as being in a group with high RHP may receive a motivational increase and increase their aggression levels. Alternatively, individuals in lower RHP groups may increase their aggression levels in an attempt to overcome the RHP deficit. We investigate how ‘group RHP’ influences agonistic tactics in red wood ants Formica rufa. Larger groups had higher total agonistic indices, but per capita agonistic indices were highest in the smallest groups, indicating that individuals in smaller groups fought harder. Agonistic indices were influenced by relative mean mass, focal group size, opponent group size and opponent group agonistic index. Focal group attrition rates decreased as focal group relative agonistic indices increased and there was a strong negative influence of relative mean mass. The highest focal attrition rates were received when opponent groups were numerically large and composed of large individuals. Thus, fight tactics in F. rufa seem to vary with both aspects of group RHP, group size and the individual attributes of group members, indicating that information on these are available to fighting ants.
Keywords: Formica rufa, aggression, resource-holding potential, body size, group size, Lanchester's attrition laws
1. Introduction
Fighting animals are faced both with ‘strategic’ decisions, such as whether to give up and withdraw from a contest, and ‘tactical’ decisions about ‘how’ to fight. The importance of these two types of decision will depend on the type of agonistic encounter. Strategic decisions determine the outcome of ritualized contests where agonistic behaviour consists of non-injurious activities, such as signals and trials of strength. When fights involve dangerous activities, leading to injuries or fatalities, strategic decisions will play a more limited role. In contests that are only resolved when one party is killed, ‘giving up decisions’ may even be entirely absent. Tactical decisions, by contrast, must be made in both types of contest. These decisions may be influenced by a combination of fighting ability ‘resource-holding potential’ (RHP); [1–3], experience [4–6], resource value [7,8] and motivational state [8–10]. For example, in hermit crabs [11] and swordtail fish [12], rates of agonistic behaviour increase with the value of the contested resource. Fight tactics may vary with RHP in one of two ways. First, if an opponent has access to information on its own RHP and that of its rival, the individual of greater RHP may receive a motivational increase and fight harder [13]. Alternatively, the weaker opponent might fight harder in an attempt to compensate for its low RHP or because an individual's future fitness is approximately zero if it does not fight (desperado effect [14]).
While the influence of motivation and RHP on fight tactics have been investigated in pairwise encounters, it is not known whether these factors exert a similar influence on the tactics employed during ‘multi party’ contests or ‘battles’ between rival groups. These occur in social animals, such as humans [15], other primates [16–18], lions [19] and ants [3,20–23]. Similar to aggression in pairwise contests between two individuals, the fighting strength of a group, referred to as ‘group RHP’ [3], will be influenced by the morphological and physiological attributes of the group members [3]. In addition, it is clear that group RHP should also be influenced by the number of individuals in the group [17,22,24]. However, there are two distinct ways in which the benefit of group size could accrue. Lanchester's Linear and Square Attrition Laws [15,24] predict the attrition rates experienced by competing groups of different sizes. The Linear Law is based on the assumption that in the group comprising the greater number of individuals it is not possible, or there is no advantage, to concentrating attacks on individuals of the less numerous group. Thus, inter-group contests may comprise one-against-one encounters between members of each group [25]. In such encounters, the ‘extra’ individuals of the larger group do not fight until they are needed to replace others that have been removed from the battle (owing to injuries or fatalities). The key prediction of the Linear Law is that individual fighting abilities and number of individuals exert an equal influence on group RHP [15,24,25]; that is, the RHP of the larger group will increase as a linear function of its size advantage. The Square Law assumes that all individuals from the larger group will participate simultaneously, such that members of this group are able to concentrate their attacks on members of the smaller group [15]. The key prediction of the Square Law is therefore that group size will exert a greater influence over group RHP than the individual fighting abilities of the group members; in this case, the RHP of the larger group will increase as a square function of its size advantage.
Under both Attrition Laws, larger groups are expected to win as a result of enhanced group RHP. As in the case of pairwise contests between individuals, however, fighting groups may adjust their tactics on the basis of group RHP. First, analogous to the motivational increase received by the individual of greater RHP in a pairwise contest, members of the group with greater RHP may ‘fight harder’ by performing agonistic behaviours at a greater rate than members of the weaker group. This would lead to a positive relationship between group RHP and aggression rates. Alternatively, members of the smaller group might increase their aggression rates in an attempt to compensate for inferior group RHP. This would lead to a negative relationship between group RHP and aggression rate. If rates of agonistic behaviour increase with group RHP (individuals in stronger groups fight harder) this could have an additive effect for the stronger group, further enhancing the benefits of superior group RHP. If rates of agonistic behaviour decline with group RHP, (individuals in weaker groups fight harder) this could have a compensatory effect for the weaker group, reducing the benefits of superior group RHP that accrue to the stronger group. Thus, if rates of agonistic behaviour increase with group RHP, this would elevate the chance of finding data that support the Square Law. If rates of agonistic behaviour decrease with group RHP this would elevate the chance of obtaining data that support the Linear Law.
Two mechanisms through which fight tactics may be adjusted during battles between rival groups of ants have been previously suggested. First, elevated rates of aggression may occur in the larger group as a result of ‘social facilitation’, where individuals respond positively to the immediate presence of aggressive nest-mates [26,27]. Secondly, in addition to detecting the immediate presence of nest-mates it has also been suggested that ants may use some form of ‘numerical assessment’ [22,28] where individuals have access to information on the size of the group to which they belong. Presumably, this could allow aggression rates to be adjusted either up or down. Southern red wood ants, Formica rufa, engage in inter-group contests over ownership of foraging territories, when workers emerge from colonies in the spring [29,30]. A previous study has shown that attrition rates in this species are described better by the Linear Law than by the Square Law [3]. In that study, group RHP appeared to be more closely linked to the individual correlates of RHP (e.g. body size) of group members than group sizes [3]. The aim of this study is to determine how group RHP influences the agonistic tactics used by members of rival groups during encounters in F. rufa. If social facilitation allows members of the stronger group to fight harder then we would expect to see a positive relationship between correlates of group RHP (number of individuals and average body mass) and the aggression rates of group members. If, however, there is a stronger trend for weaker groups attempting to compensate by fighting harder, we would expect to see a negative relationship between group RHP and aggression rates. We also aim to determine the effect of differences in aggression rates on the outcome of these encounters by examining their effect on attrition rates.
2. Methods
(a). Collection and maintenance of animals
We collected adult female workers of F. rufa (L) weekly between 18 March and 28 May 2009 from two sites 10 miles apart (grid references SX 82013 76270 and SX 71407 67595), on East Dartmoor, Devon, UK. Three independent nests were selected from each site and each was allocated to one of three between-site dyads, within which agonistic encounters were staged. Approximately, equal-sized nests were matched within dyads to reduce any effects of colony size on outcome [3]. Ants were maintained in fluon-coated plastic containers (19.5 × 12 × 13.5 cm) with their own nesting material and sprayed with water daily. Laboratory conditions for maintenance and contests were 20 ± 0.5°C and day length 12 L : 12 D.
We selected workers that were displaying the typical aggressive responses of flared mandibles and gaster flexion when a pair of entomologist's forceps was introduced into the nest container. Ants were used in staged encounters between 0 and 2 days after collection. Contests took place in a plastic arena (17 × 11 × 5 cm), with fluon-coated sides. Prior to contests, arenas were divided in half with a fluon-coated barrier placed centrally across the width of the arena. All ants used in contests were marked immediately prior to contests with a single dot of yellow or light blue paint, to enable colony identification during interactions. Ants were then simultaneously placed into opposite sides of the arena and allowed to acclimatize for 15 min before the central barrier was removed and the contest began. The colour mark was alternated between colonies and replicates to remove any correlation between colour and colony identity.
(b). Staging contests and scoring fight tactics
Contests were staged between groups of ants containing 5, 10 or 20 nestmates, providing nine possible combinations in a fully orthogonal design. Each combination was repeated five times for each colony pair, providing 45 encounters for each colony and 135 encounters in total.
Contests were initiated by barrier removal. Agonistic behaviour by each worker was recorded using scan samples at 5 min intervals for the 30 min contest [31]. Agonistic behaviour was recorded as (i) threat to an opponent, (ii) seizing of an opponent's leg or antenna, or (iii) attack on an opponent. ‘Threats’ were non-contact postures where the mandibles were flared and the gaster was brought forward underneath the thorax in a threat display, but without the release of formic acid. ‘Seizings’ involved workers grasping an opponent by the leg or antenna using the mandibles, but did not include gaster flexion. ‘Attacks’ were defined as occasions when workers seized an opponent with their mandibles while performing gaster flexion, which often results in the attacker spraying formic acid on the seized opponent. These behaviours were weighted on a numbered scale according to escalation intensity [31–33]; threat was scored ‘1’ < seizing, scored ‘2’ < attack, scored ‘3’). If more than one individual attacked or seized the same opponent their scores were summed.
We summed all scores over the 30 min contest period to calculate a cumulative index for agonistic responses for each group during the entire encounter [31,34,35]. We termed this the total agonistic index (TAI). Since TAI could increase simply as a function of group size, we also divided TAI by group size to gain a per capita agonistic index (PCAI) to determine whether aggression levels vary with group size at the individual level.
After the 30 min contest period, water was provided on cotton wool and the contest was allowed to continue overnight. After 24 h had elapsed since the start of the contests, the number of dead and surviving individuals in each group was counted [21]. Groups as a whole were then weighed to the nearest 0.0001 g. The mean body mass of individuals in each group was then calculated as a measure of average individual RHP, a measure that has previously been found to influence group contest outcome in F. rufa [3]. The mean worker mass of groups ranged between 43 and 173 mg and had an overall mean ± s.e. of 100 ± 1.3 mg.
(c). Statistical analysis
In each contest, we assigned focal group status to one group and opponent group status to the opposing group; group status was randomly assigned in each contest to remove any potential site bias towards which group had focal status [3]. All analyses conducted were generalized linear mixed models, using the lme4 package in R statistical software v. 2.11.0. To account for the potential pseudoreplication of staging multiple contests from three dyads, we included ‘dyad’ as a random factor in each model.
First, we analysed the influence of four explanatory variables on focal group TAI and focal group PCAI, which were non-normally distributed count data. Therefore, we used Poisson error distribution, log link function and Laplace parameter estimation [3,36,37]. Both analyses included the three explanatory variables (i) absolute difference in mean body mass (focal-opponent group), (ii) focal group size (5, 10 or 20), and (iii) opponent group size (5, 10 or 20). The fourth variable in the analysis of focal group TAI was opponent group TAI, and in the analysis of focal group PCAI this variable was opponent group PCAI.
Focal group TAI was influenced by all four variables and focal group PCAI was influenced by three of the four fixed factors (see results), so we then separately investigated whether absolute difference in TAI and PCAI (focal-opponent group) influenced the attrition rate of focal groups. This two-vector proportional response variable (number of dead and surviving focal group workers) was bound together into a single response, ‘Attrition’ [37]. The generalized linear mixed models included the fixed factor ‘absolute difference in TAI’ or ‘absolute difference in PCAI’ (focal-opponent group). The data were bound between 0.0 and 1.0, so we used binomial error distribution, a logit link function and Laplace parameter estimation [36,37].
We then investigated the influence of (i) absolute difference (focal-opponent group) in mean body mass of group members, (ii) focal group size (5, 10 or 20) and (iii) opponent group size (5, 10 or 20) on ‘Attrition’. We used binomial error distribution, a logit link function and Laplace parameter estimation [36,37]. Note that agonistic indices were not included in these final analyses as they were influenced by the other fixed factors (see below).
For each analysis, the maximal model adopted contained all potential two-way interactions, main effects and random factors. The strength of the effects was assessed using the parameter estimates. We then began to simplify models by sequentially removing the fixed-effects with the lowest ‘z’ values. Each simplification was inspected to determine if it improved the fit of the model by comparison of AIC scores (in lowest is best format) with previous iterations [36]. The minimum adequate models (best-fit) for each of the analyses are given in tables 1, 2 and 3 and the maximal models are provided as electronic supplementary material, S1, S2 and S3, respectively.
Table 1.
Minimum adequate model for focal group (a) total agonistic index (TAI) and (b) per capita agonistic index (PCAI).
| (a) AIC = 1262 | ||||
|---|---|---|---|---|
| random effects |
variance |
s.d. |
||
| dyad | 0.057 | 0.238 | ||
| fixed effects | estimate | s.e. | z value | p |
| intercept | 2.342 | 0.167 | 14.02 | <0.0001 |
| body mass difference | 0.120 | 0.022 | 5.55 | <0.0001 |
| focal group = 10 | −0.259 | 0.136 | −1.91 | 0.056 |
| focal group = 20 | 0.810 | 0.122 | 6.66 | <0.0001 |
| opponent group = 10 | 0.027 | 0.144 | 0.18 | 0.854 |
| opponent group = 20 | 0.487 | 0.130 | 3.76 | <0.001 |
| opponent group TAI | −0.072 | 0.012 | −6.01 | <0.0001 |
| body mass difference × focal group size = 10 | 0.064 | 0.025 | 2.60 | 0.009 |
| body mass difference × focal group size = 20 | −0.073 | 0.022 | −3.38 | <0.001 |
| body mass difference × opponent group size = 10 | −0.012 | 0.021 | −0.55 | 0.584 |
| body mass difference × opponent group size = 10 | −0.089 | 0.021 | −4.19 | <0.0001 |
| focal group = 10 × opponent group = 10 | −0.150 | 0.201 | −0.75 | 0.455 |
| focal group = 10 × opponent group = 20 | 0.480 | 0.177 | 2.72 | 0.007 |
| focal group = 20 × opponent group = 10 | −0.248 | 0.162 | −1.53 | 0.127 |
| focal group = 20 × opponent group = 10 | −0.335 | 0.163 | −2.05 | 0.041 |
| focal group = 10 × opponent group TAI | 0.022 | 0.007 | 2.93 | 0.003 |
| focal group = 20 × opponent group TAI | 0.023 | 0.007 | 3.22 | 0.001 |
| opponent group = 10 × opponent group TAI | 0.051 | 0.011 | 4.49 | <0.0001 |
| opponent group = 20 × opponent group TAI | 0.034 | 0.011 | 3.06 | 0.002 |
| (b) AIC = 174.8 | ||||
| random effects | variance | s.d. | ||
| dyad | 0.073 | 0.270 | ||
| fixed effects | estimate | s.e. | z value | p |
| intercept | 0.724 | 0.204 | 3.54 | <0.001 |
| body mass difference | 0.100 | 0.026 | 3.80 | <0.001 |
| focal group = 10 | −0.603 | 0.190 | −3.17 | 0.002 |
| focal group = 20 | −0.717 | 0.195 | −3.68 | <0.001 |
| opponent group PCAI | −0.154 | 0.083 | −1.85 | 0.065 |
Table 2.
Minimum adequate model for effect of difference (focal-opponent group) in (a) total agonistic index (TAI) and (b) per capita agonistic index (PCAI) on focal group attrition.
| (a) AIC = 578.0 | ||||
|---|---|---|---|---|
| random effects |
variance |
s.d. |
||
| dyad | 0.012 | 0.108 | ||
| fixed effects | estimate | s.e. | z value | p |
| intercept | −0.922 | 0.086 | −10.76 | <0.0001 |
| TAI difference | −0.021 | 0.002 | −10.65 | <0.0001 |
| (b) AIC = 612.4 | ||||
| random effects | variance | s.d. | ||
| dyad | 0.007 | 0.083 | ||
| fixed effects | estimate | s.e. | z value | p |
| intercept | −0.965 | 0.077 | −12.48 | <0.0001 |
| PCAI difference | −0.282 | 0.029 | −9.61 | <0.0001 |
Table 3.
Minimum adequate model for effect of group sizes and body mass difference (focal-opponent group) on focal group attrition.
| AIC = 502.1 | ||||
|---|---|---|---|---|
| random effects |
variance |
s.d. |
||
| dyad | 0.028 | 0.168 | ||
| fixed effects | estimate | s.e. | z value | p |
| intercept | −1.066 | 0.141 | −7.564 | <0.0001 |
| body mass difference | −0.176 | 0.034 | −5.213 | <0.0001 |
| opponent group = 10 | 0.090 | 0.145 | 0.622 | 0.533 |
| opponent group = 20 | 0.117 | 0.158 | 0.742 | 0.458 |
| body mass difference × opponent group = 10 | −0.030 | 0.049 | −0.612 | 0.540 |
| body mass difference × opponent group = 20 | −0.182 | 0.051 | −3.557 | <0.001 |
3. Results
(a). Do group size and mass influence agonistic behaviour?
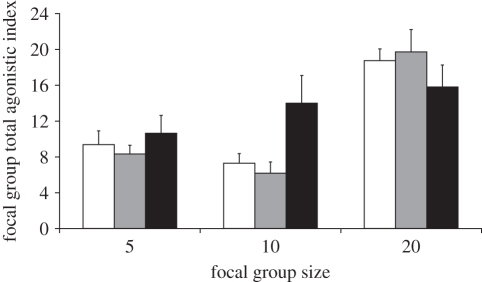
The minimum adequate model for explaining focal group TAI included interactions between mass and focal group size, mass and opponent group size, focal group size and opponent group TAI, opponent group size and opponent group TAI and focal group size and opponent group size (table 1). Overall, focal group TAI increased with mass difference, decreased as opponent group TAI increased and was highest when focal groups contained 20 individuals (figure 1) or opponent groups contained 20 individuals (figure 1). The interaction effects were driven by focal and opponent groups containing 10 individuals, which tended to have steeper or shallower predicted slopes for TAIs than focal and opponent groups that contained five or 20 individuals. The effects of mass difference, opponent group TAI and their interactions with group size are illustrated in electronic supplementary material, S4.
Figure 1.
An interaction between focal group size and opponent group size influenced focal group TAI. The estimates (+s.e.) were obtained from separate analysis, having established that this interaction was significant in the minimum adequate model. Opponent group size, open bars, 5; grey bars, 10; black bars, 20.
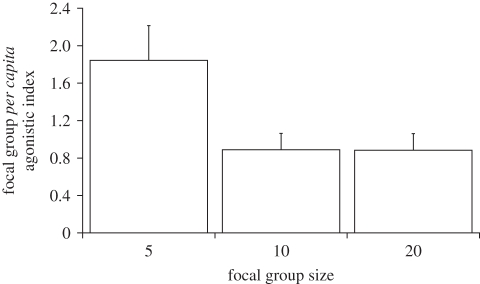
The minimum adequate model for explaining focal group PCAI included body mass difference, focal group size and opponent group PCAI (table 1). Focal group PCAI increased as body mass difference increased in favour of the focal group (electronic supplementary material, S5a). Focal groups containing five individuals had the highest mean PCAI, and groups containing 10 individuals had the lowest (figure 2). Focal group PCAI decreased as opponent group PCAI increased (electronic supplementary material, S5b). Opponent group size dropped out of the minimum adequate model, indicating that it was not an important influence on focal group PCAI.
Figure 2.
Focal group PCAI as focal group size increased. The estimates (+s.e.) for each factor were obtained from a separate generalized linear mixed model analysis, having established that this term was significant in the minimum adequate model.
(b). Do agonistic behaviour, group size and mass influence outcome?
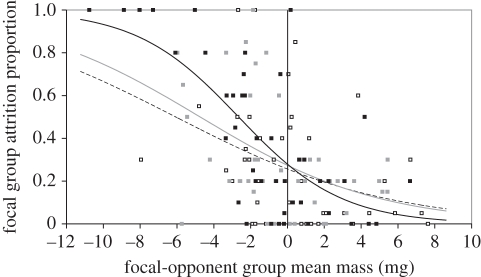
As TAI or PCAI difference increased in favour of the focal group, focal group attrition rate decreased (TAI, table 2; PCAI, table 2). When the analysis contained focal group size, opponent group size and body mass difference, the minimum adequate model for explaining focal group attrition rate included an interaction between body mass difference and opponent group size (table 3 and figure 3). Focal group attrition rate decreased as focal group mass advantage over the opponent group increased for all opponent group sizes, but the slopes of these lines differed (figure 3), with the steepest slope when opponent groups contained 20 individuals. As a result, focal group attrition rate was predicted to be very high when opponent groups contained 20 individuals and had higher mean mass than focal groups. When individuals in focal groups were heavier than individuals in opponent groups, focal group attrition rates were lowest when opponent groups contained 20 individuals, rather than five or 10 individuals (figure 3). Focal group size dropped out of the model, indicating that it did not have a significant influence on focal group attrition rate.
Figure 3.
The influence of the interaction between body mass difference (mg; focal-opponent group) and opponent group size on focal group attrition rate. The fitted lines show the predicted focal group attrition rate and markers display the observed attrition rates as body mass difference varied when opponent groups contained 5 (dashed lines and open squares), 10 (grey line and grey squares) or 20 (black line and black squares) individuals. Lines were estimated from generalized linear mixed modelling.
4. Discussion
Focal group total and per capita levels of agonistic behaviour both increased as the mean mass advantage for individuals in the focal groups increased. The largest focal groups showed the greatest total levels of agonistic behaviour, but the highest per capita levels were performed by the smallest groups. This supports the hypothesis that although larger groups may have higher group RHP, individuals in smaller groups fight harder. This indicates that social facilitation did not occur. Similarly, large individuals and smaller groups of the primate Colobus guereza are more likely to initiate aggression than smaller individuals and larger groups [18]. While social facilitation appeared to be absent, numerical assessment cannot be ruled out. If numerical assessment did occur, individuals in smaller groups reacted to an assessment of small own group size by increasing their agonistic levels relative to how individuals in larger groups reacted. This contrasts with a study on Formica xerophila, which found that individual F. xerophila workers fight more aggressively during interspecific contests against Formica integroides when they perceive themselves to be part of a large group compared with when they perceive themselves to be in a small group [22]. Similarly, in several vertebrate examples, vocal playback experiments have demonstrated that residents use numerical assessments of their own and opponent's group size. Resident lions [38,39], primates [16] and birds [40] become more willing to approach perceived conspecific intruders as the residents' numerical superiority increases. At present the cause of the differences between F. rufa and F. xerophila is not clear. However, one difference between the current study and that of Tanner [22] is that in the present study rival groups of different sizes engaged one another during the staged encounter whereas in the F. xerophila study, the ants fought in equal-sized groups taken from stocks housed at different densities. Therefore, the difference may reflect two distinct types of numerical assessment, ‘overall’ colony size in the case of Tanner [22] and ‘current size of the sub-group directly engaged in the contest’ in the case of the present study. Regardless of this difference, it appears that both species use some form of numerical assessment, a pattern that is present in taxonomically diverse examples of animals that fight in groups.
In the present study, the size of the opponent group was also important. Larger opponent groups stimulated higher total agonistic levels from focal groups, but did not influence focal group per capita agonistic indices. The total and per capita number of agonistic acts by opponents also negatively influenced the focal group's total and per capita agonistic indices. There were multiple interaction effects influencing focal group TAI, which all involved focal or opponent group sizes of 10 (table 1, electronic supplementary material, S4 and figure 1). These effects may have arisen because groups containing 10 individuals outnumbered their opponents in some contests (against five) but were also outnumbered by opponents in others (against 20). This could lead to contrasting tactics employed by groups of 10 individuals, or groups fighting against 10 individuals, as their tactical decisions may vary according to relative group size. For example, focal groups had low TAIs when focal and opponent groups contained five or 10 individuals, but when there were 20 focal individuals against 10 opponents, focal agonistic index was high (figure 1).
The measures of total and per capita agonistic behaviour of groups quantify how many agonistic acts occurred during the scan samples, but we do not know how many individuals in each group performed these acts. It may be that all of the agonistic acts were performed by a small number of individuals in each group. A further study to investigate the identity of individuals could assess the exact number of individuals from each group that were engaging in agonistic behaviour, termed functional group size [22,41]. If the majority of agonistic behaviour occurs in one-against-one interactions, functional group size may be dictated by the size of the smaller group, as excess individuals in larger groups may not take part in aggression. Future studies that investigate per capita agonistic levels relative to functional group size may also find significant differences between individuals. Certain individuals may specialize in fighting, which may be related to body size or older individuals may perform the aggression as a result of temporal polyethism [42,43]. Regardless of how the agonistic behaviours were distributed between group members, the current study shows that larger groups have higher total agonistic levels, but smaller groups have the greatest per capita agonistic levels.
There was a significant negative influence of absolute difference (focal-opponent group) in total and per capita agonistic levels on focal group attrition rates. This indicates that more aggressive groups are less likely to suffer fatalities. This could be related to the ability to kill, injure or incapacitate an opponent; in achieving this, you could avoid being killed or injured yourself. Alternatively, individuals with low RHP may simply be inferior competitors and consequently be unable to perform high levels of agonistic behaviour or escalate their attacks. While the agonistic indices observed may reflect the motivation of ants to fight, agonistic behaviour itself was strongly influenced by body mass. Body mass has previously been shown to influence fatalities in contests between F. rufa [3]. When agonistic levels were discounted, focal groups containing individuals of increasingly large relative mean body mass were found to have lower attrition rates during fatal fights, concurring with previous results on F. rufa group contests [3]. Opponent group sizes modified this influence, with the most distinct influence being when opponent groups contained 20 individuals. In particular, focal groups had high attrition rates if individuals in their opponent's group were more numerous and heavier than the individuals in the focal group. This may allow larger individuals in the opposing group to target the smaller individuals in focal groups and/or use concentrated attacks. Previous work on F. rufa has suggested that increasing focal or opponent group size does not influence the identity of which group suffers the first fatality in small group contests [3]. While the larger group sizes of the current study detected an effect of opponent group size on focal group attrition rate, there was no influence of focal group size. In contrast, numerical superiority is often an advantage in group fights or interactions across the animal kingdom (e.g. in primates [17,44]; lions [19]; wolves [45]; birds [46]; honeybees [47] and ants [21,48–50]) but does not guarantee success [3,18,51,52]. Within ants, intra and interspecific interactions between colonies may result in numerically superior colonies dominating smaller colonies. This may be through enhanced success in exploitative or interference competition [20,48,49,53–56]. For example, increased worker number has been shown to increase exploitative and interference competitive performance in the invasive Argentine ant Linepithema humile [41,57]. This species often maintains a numerical superiority relative to native species. As a result, L. humile colonies may be able to outcompete their less numerous interspecific opponents through exploitation and concentrated attacks [41,58]. In comparison, our results indicate that numerical superiority in F. rufa enabled larger groups to concentrate attacks, with up to five individuals observed attacking a single opponent. Whether these attacks are concentrated effectively, a prediction of Lanchester's Square Attrition Law is yet to be determined. Indeed, a previous study on F. rufa [3] indicates that, for small group contests at least, there may be an upper limit on the number of individuals that can effectively concentrate an attack on a single opponent. This suggests that the Square Attrition Law may be subject to an upper constraint. The fact that smaller groups fought harder while not sufficient to defeat the larger group might be an additional factor that limits the effect of superior group size.
An important factor that may differ between experiments on ant combat staged in the laboratory and in the field is that group size is fixed under laboratory conditions (e.g. [3,21]). By contrast, in the field group size can vary during the course of an agonistic encounter, owing to the process of recruitment (e.g. [20,48,59–61]). Natural wood ant battles over territory or predation [59,62] are likely to involve much larger groups. There are also likely to be asymmetries in motivation, the value of the resource under dispute and recruitment ability. This would clearly influence group RHP, per capita agonistic levels and contest escalation decisions across species. Further studies into the influence of numerical assessment and functional group size on agonistic levels and contest outcome are also required. Nevertheless, the results of the current study suggest that focal groups are more aggressive when the individuals in the group are heavier. Both focal and opponent group size may also influence the agonistic behaviour of the focal group. While smaller groups may have higher per capita agonistic levels, larger groups have the highest total agonistic levels. In addition, a focal group's attrition level will be highly dependent on the masses of the individuals in the focal and opponent group. Finally, focal group attrition will be influenced by how many individuals are contained in their opponent's group. Thus, in these inter-group battles in wood ants, individuals in smaller groups appear to fight harder but this effect is not sufficient to overcome a numerically superior rival group. Under natural conditions, however, these elevated rates of aggression in smaller groups could have a cumulative effect during repeated battles between rival colonies. Therefore, understanding the effects of group size on agonistic behaviour has the potential to yield further insights into measures of the overall success of colonies in ants (e.g. territory size) and other types of social group in the diverse range of species that engage in inter-group ‘battles’. Further, elevated aggression in smaller groups, together with upper limits on the number of individuals that can fight effectively, might constrain the advantage that accrues to larger groups.
Acknowledgements
This study was funded by the UK Biotechnology and Biological Sciences Research Council grant no. F014147/1. We are grateful to Kevin Cox, Donna Cox, Tom Stephens and the Dartington Hall estate for access to study sites.
References
- 1.Huntingford F. A., Turner A. K. 1987. Animal conflict. London, UK: Chapman & Hall [Google Scholar]
- 2.Hurd P. L. 2006. Resource holding potential, subjective resource value, and game theoretical models of aggressiveness signalling. J. Theor. Biol. 241, 639–648 10.1016/j.jtbi.2006.01.001 (doi:10.1016/j.jtbi.2006.01.001) [DOI] [PubMed] [Google Scholar]
- 3.Batchelor T. P., Briffa M. 2010. Influences on resource-holding potential during dangerous group contests between wood ants. Anim. Behav. 80, 443–449 10.1016/j.anbehav.2010.05.030 (doi:10.1016/j.anbehav.2010.05.030) [DOI] [Google Scholar]
- 4.Jutsum A. R. 1979. Interspecific aggression in leaf-cutting ants. Anim. Behav. 27, 833–838 10.1016/0003-3472(79)90020-4 (doi:10.1016/0003-3472(79)90020-4) [DOI] [Google Scholar]
- 5.Kasumovic M. M., Elias D. O., Punzalan D., Mason A. C., Andrade M. C. B. 2009. Experience affects the outcome of agonistic contests without affecting the selective advantage of size. Anim. Behav. 77, 1533–1538 10.1016/j.anbehav.2009.02.026 (doi:10.1016/j.anbehav.2009.02.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Wilgenburg E., Clemencet J., Tsutsui N. D. 2010. Experience influences aggressive behaviour in the Argentine ant. Biol. Lett. 6, 152–155 10.1098/rsbl.2009.0616 (doi:10.1098/rsbl.2009.0616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enquist M., Leimar O. 1987. Evolution of fighting behaviour: the effect of variation in resource value. J. Theor. Biol. 127, 187–206 10.1016/S0022-5193(87)80130-3 (doi:10.1016/S0022-5193(87)80130-3) [DOI] [Google Scholar]
- 8.Arnott G., Elwood R. W. 2008. Information gathering and decision making about resource value in animal contests. Anim. Behav. 76, 529–542 10.1016/j.anbehav.2008.04.019 (doi:10.1016/j.anbehav.2008.04.019) [DOI] [Google Scholar]
- 9.Arnott G., Elwood R. W. 2009. Assessment of fighting ability in animal contests. Anim. Behav. 77, 991–1004 10.1016/j.anbehav.2009.02.010 (doi:10.1016/j.anbehav.2009.02.010) [DOI] [Google Scholar]
- 10.Bergman M., Olofsson M., Wiklund C. 2010. Contest outcome in a territorial butterfly: the role of motivation. Proc. R. Soc. B 277, 3027–3033 10.1098/rspb.2010.0646 (doi:10.1098/rspb.2010.0646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnott G., Elwood R. W. 2007. Fighting for shells: how private information about resource value changes hermit crab pre-fight displays and escalated fight behaviour. Proc. R. Soc. B 274, 3011–3017 10.1098/rspb.2007.1196 (doi:10.1098/rspb.2007.1196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nijman V., Heuts B. A. 2000. Effect of environmental enrichment upon resource holding power in fish in prior residence situations. Behav. Process. 49, 77–83 10.1016/S0376-6357(00)00078-4 (doi:10.1016/S0376-6357(00)00078-4) [DOI] [PubMed] [Google Scholar]
- 13.Briffa M., Elwood R. W. 2000. The power of shell rapping influences rates of eviction in hermit crabs. Behav. Ecol. 11, 288–293 10.1093/beheco/11.3.288 (doi:10.1093/beheco/11.3.288) [DOI] [Google Scholar]
- 14.Grafen A. 1987. The logic of divisively asymmetric contests—respect for ownership and the desperado effect. Anim. Behav. 35, 462–467 10.1016/S0003-3472(87)80271-3 (doi:10.1016/S0003-3472(87)80271-3) [DOI] [Google Scholar]
- 15.Lanchester F. W. 1956. Mathematics in warfare. In The world of mathematics (ed. Newman J. R.), pp. 2137–2157 New York, NY: Simon and Schuster [Google Scholar]
- 16.Wilson M. L., Hauser M. D., Wrangham R. W. 2001. Does participation in intergroup conflict depend on numerical assessment, range location or rank for wild chimpanzees? Anim. Behav. 61, 1203–1216 10.1006/anbe.2000.1706 (doi:10.1006/anbe.2000.1706) [DOI] [Google Scholar]
- 17.Crofoot M. C., Gilby I. C., Wikelski M. C., Kays R. W. 2008. Interaction location outweighs the competitive advantage of numerical superiority in Cebus capucinus intergroup contests. Proc. Natl Acad. Sci. USA 105, 577–581 10.1073/pnas.0707749105 (doi:10.1073/pnas.0707749105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris T. R. 2010. Multiple resource values and fighting ability measures influence intergroup conflict in guerezas (Colobus guereza). Anim. Behav. 79, 89–98 10.1016/j.anbehav.2009.10.007 (doi:10.1016/j.anbehav.2009.10.007) [DOI] [Google Scholar]
- 19.Mosser A., Packer C. 2009. Group territoriality and the benefits of sociality in the African lion, Panthera leo. Anim. Behav. 78, 359–370 10.1016/j.anbehav.2009.04.024 (doi:10.1016/j.anbehav.2009.04.024) [DOI] [Google Scholar]
- 20.Hölldobler B., Lumsden C. J. 1980. Territorial strategies in ants. Science 210, 732–739 10.1126/science.210.4471.732 (doi:10.1126/science.210.4471.732) [DOI] [PubMed] [Google Scholar]
- 21.Plowes N. J. R., Adams E. S. 2005. An empirical test of Lanchester's square law: mortality during battles of the fire ant Solenopsis invicta. Proc. R. Soc. B 272, 1809–1814 10.1098/rspb.2005.3162 (doi:10.1098/rspb.2005.3162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanner C. J. 2006. Numerical assessment affects aggression and competitive ability: a team-fighting strategy for the ant Formica xerophila. Proc. R. Soc. B 273, 2737–2742 10.1098/rspb.2006.3626 (doi:10.1098/rspb.2006.3626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanner C. J. 2008. Aggressive group behaviour in the ant Formica xerophila is coordinated by direct nestmate contact. Anim. Behav. 76, 1335–1341 10.1016/j.anbehav.2008.04.022 (doi:10.1016/j.anbehav.2008.04.022) [DOI] [Google Scholar]
- 24.Adams E. S., Mesterton-Gibbons M. 2003. Lanchester's attrition models and fights among social animals. Behav. Ecol. 14, 719–723 10.1093/beheco/arg061 (doi:10.1093/beheco/arg061) [DOI] [Google Scholar]
- 25.Franks N. R., Partridge L. W. 1993. Lanchester battles and the evolution of combat in ants. Anim. Behav. 45, 197–199 10.1006/anbe.1993.1021 (doi:10.1006/anbe.1993.1021) [DOI] [Google Scholar]
- 26.Hölldobler B., Wilson E. O. 1990. The ants. Cambridge, MA: Harvard University Press [Google Scholar]
- 27.Sakata H., Katayama N. 2001. Ant defence system: a mechanism organizing individual responses into efficient collective behavior. Ecol. Res. 16, 395–403 10.1046/j.1440-1703.2001.00404.x (doi:10.1046/j.1440-1703.2001.00404.x) [DOI] [Google Scholar]
- 28.Lumsden C. J., Hölldobler B. 1983. Ritualized combat and intercolony communication in ants. J. Theor. Biol. 100, 81–98 10.1016/0022-5193(83)90093-0 (doi:10.1016/0022-5193(83)90093-0) [DOI] [Google Scholar]
- 29.Elton C. 1932. Territory among wood ants (Formica rufa L.) Picket Hill. J. Anim. Ecol. 1, 69–76 10.2307/996 (doi:10.2307/996) [DOI] [Google Scholar]
- 30.Skinner G. J. 1980. Territory, trail structure and activity patterns in the wood-ant, Formica rufa (Hymenoptera, Formicidae) in limestone woodland in Northwest England. J. Anim. Ecol. 49, 381–394 10.2307/4253 (doi:10.2307/4253) [DOI] [Google Scholar]
- 31.Roulston T. H., Buczkowski G., Silverman J. 2003. Nestmate discrimination in ants: effect of bioassay on aggressive behavior. Insect Soc. 50, 151–159 10.1007/s00040-003-0624-1 (doi:10.1007/s00040-003-0624-1) [DOI] [Google Scholar]
- 32.Carlin N. F., Hölldobler B. 1983. Nestmate and kin recognition in interspecific mixed colonies of ants. Science 222, 1027–1029 10.1126/science.222.4627.1027 (doi:10.1126/science.222.4627.1027) [DOI] [PubMed] [Google Scholar]
- 33.Tsutsui N. D., Suarez A. V., Grosberg R. K. 2003. Genetic diversity, asymmetrical aggression, and recognition in a widespread invasive species. Proc. Natl Acad. Sci. USA 100, 1078–1083 10.1073/pnas.0234412100 (doi:10.1073/pnas.0234412100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallis D. I. 1962. Aggressive behaviour in the ant, Formica fusca. Anim. Behav. 10, 267–274 10.1016/0003-3472(62)90050-7 (doi:10.1016/0003-3472(62)90050-7) [DOI] [Google Scholar]
- 35.Wenseleers T., Billen J., Hefetz A. 2002. Territorial marking in the desert ant Cataglyphis niger: does it pay to play bourgeois? J. Insect Behav. 15, 85–93 10.1023/A:1014484229639 (doi:10.1023/A:1014484229639) [DOI] [Google Scholar]
- 36.Bolker B. M., Brooks M. E., Clark C. J., Geange S. W., Poulsen J. R., Stevens M. H. H., White J.-S. S. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 10.1016/j.tree.2008.10.008 (doi:10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 37.Crawley M. J. 2007. The R book. Chichester, UK: John Wiley & Sons Ltd [Google Scholar]
- 38.McComb K., Packer C., Pusey A. 1994. Roaring and numerical assessment in contests between groups of female lions, Panthera leo. Anim. Behav. 47, 379–387 10.1006/anbe.1994.1052 (doi:10.1006/anbe.1994.1052) [DOI] [Google Scholar]
- 39.Heinsohn R. 1997. Group territoriality in two populations of African lions. Anim. Behav. 53, 1143–1147 10.1006/anbe.1996.0316 (doi:10.1006/anbe.1996.0316) [DOI] [PubMed] [Google Scholar]
- 40.Seddon N., Tobias J. A. 2003. Communal singing in the cooperatively breeding subdesert mesite Monias benschi: evidence of numerical assessment? J. Avian Biol. 34, 72–80 10.1034/j.1600-048X.2003.03067.x (doi:10.1034/j.1600-048X.2003.03067.x) [DOI] [Google Scholar]
- 41.Buczkowski G., Bennett G. W. 2008. Aggressive interactions between the introduced Argentine ant, Linepithema humile and the native odorous house ant, Tapinoma sessile. Biol. Invas. 10, 1001–1111 10.1007/s10530-007-9179-9 (doi:10.1007/s10530-007-9179-9) [DOI] [Google Scholar]
- 42.Lemoli F., Mori A. 1986. The aggression test as a possible taxonomic tool in the Formica rufa group. Aggress. Behav. 12, 93–102 (doi:10.1002/1098-2337(1986)12:2<93::AID-AB2480120203>3.0.CO;2-O) [DOI] [Google Scholar]
- 43.Tofilski A. 2002. Influence of age polyethism on longevity of workers in social insects. Behav. Ecol. Sociobiol. 51, 234–237 10.1007/s00265-001-0429-z (doi:10.1007/s00265-001-0429-z) [DOI] [Google Scholar]
- 44.Cowlishaw G. 1995. Behavioral-patterns in baboon group encounters—the role of resource competition and male reproductive strategies. Behaviour 132, 75–86 10.1163/156853995X00298 (doi:10.1163/156853995X00298) [DOI] [Google Scholar]
- 45.Harrington F. H., Mech L. D. 1979. Wolf howling and its role in territory maintenance. Behaviour 68, 207–249 10.1163/156853979X00322 (doi:10.1163/156853979X00322) [DOI] [Google Scholar]
- 46.Radford A. N., Du Plessis M. A. 2004. Territorial vocal rallying in the green woodhoopoe: factors affecting contest length and outcome. Anim. Behav. 68, 803–810 10.1016/j.anbehav.2004.01.010 (doi:10.1016/j.anbehav.2004.01.010) [DOI] [Google Scholar]
- 47.Rangel J., Griffin S. R., Seeley T. D. 2010. Nest-site defense by competing honey bee swarms during house-hunting. Ethology 116, 608–618 [Google Scholar]
- 48.Adams E. S. 1990. Boundary disputes in the territorial ant Azteca trigona—effects of asymmetries in colony size. Anim. Behav. 39, 321–328 10.1016/S0003-3472(05)80877-2 (doi:10.1016/S0003-3472(05)80877-2) [DOI] [Google Scholar]
- 49.Bhatkar A., Whitcomb W. H., Buren W. F., Callahan P., Carlysle T. 1972. Confrontation behavior between Lasius neoniger (Hymenoptera: Formicidae) and the imported fire ant. Environ. Entomol. 1, 274–279 [Google Scholar]
- 50.Hölldobler B., Wilson E. O. The multiple recruitment systems of African weaver ant Oecophylla longinoda (Latreille) (Hymenoptera Formicidae). Behav. Ecol. Sociobiol. 3, 19–60 10.1007/BF00300045 (doi:10.1007/BF00300045) [DOI] [Google Scholar]
- 51.Sugiura H., Saito C., Sato S., Agetsuma N., Takahashi H., Tanaka T., Furuichi T., Takahata Y. 2000. Variation in intergroup encounters in two populations of Japanese macaques. Int. J. Primatol. 21, 519–535 10.1023/A:1005448120967 (doi:10.1023/A:1005448120967) [DOI] [Google Scholar]
- 52.Shelley E. L., Tanaka M. Y. U., Ratnathicam A. R., Blumstein D. T. 2004. Can Lanchester's laws help explain interspecific dominance in birds? Condor 106, 395–400 10.1650/7424 (doi:10.1650/7424) [DOI] [Google Scholar]
- 53.Fellers J. H. 1987. Interference and exploitation in a guild of woodland ants. Ecology 68, 1466–1478 10.2307/1939230 (doi:10.2307/1939230) [DOI] [Google Scholar]
- 54.McGlynn T. P. 1999. Non-native ants are smaller than related native ants. Am. Nat. 154, 690–699 10.1086/303270 (doi:10.1086/303270) [DOI] [PubMed] [Google Scholar]
- 55.Davidson D. W. 1998. Resource discovery versus resource domination in ants: a functional mechanism for breaking the trade-off. Ecol. Entomol. 23, 484–490 10.1046/j.1365-2311.1998.00145.x (doi:10.1046/j.1365-2311.1998.00145.x) [DOI] [Google Scholar]
- 56.Gordon D. M., Kulig A. W. 1996. Founding, foraging, and fighting: colony size and the spatial distribution of harvester ant nests. Ecology 77, 2393–2409 10.2307/2265741 (doi:10.2307/2265741) [DOI] [Google Scholar]
- 57.Holway D. A., Case T. J. 2001. Effects of colony-level variation on competitive ability in the invasive Argentine ant. Anim. Behav. 61, 1181–1192 10.1006/anbe.2000.1698 (doi:10.1006/anbe.2000.1698) [DOI] [Google Scholar]
- 58.Human K. G., Gordon D. M. 1996. Exploitation and interference competition between the invasive Argentine ant, Linepithema humile, and native ant species. Oecologia 105, 405–412 10.1007/BF00328744 (doi:10.1007/BF00328744) [DOI] [PubMed] [Google Scholar]
- 59.Mabelis A. A. 1979. Wood ant wars—the relationship between aggression and predation in the red wood ant (Formica polyctena Forst). Neth. J. Zool. 29, 451–620 10.1163/002829679X00016 (doi:10.1163/002829679X00016) [DOI] [Google Scholar]
- 60.Powell S., Clark E. 2004. Combat between large derived societies: a subterranean army ant established as a predator of mature leaf-cutting ant colonies. Insect Soc. 51, 342–351 10.1007/s00040-004-0752-2 (doi:10.1007/s00040-004-0752-2) [DOI] [Google Scholar]
- 61.Carpintero S., Retana J., Cerda X., Reyes-Lopez J., De Reyna L. A. 2007. Exploitative strategies of the invasive Argentine ant (Linepithema humile) and native ant species in a southern Spanish pine forest. Environ. Entomol. 36, 1100–1111 10.1603/0046-225X(2007)36[1100:ESOTIA]2.0.CO;2 (doi:10.1603/0046-225X(2007)36[1100:ESOTIA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 62.Driessen G. J. J., Vanraalte A. T., Debruyn G. J. 1984. Cannibalism in the red wood ant, Formica polyctena (Hymenoptera, Formicidae). Oecologia 63, 13–22 10.1007/BF00379779 (doi:10.1007/BF00379779) [DOI] [PubMed] [Google Scholar]