Abstract
Competition elevates plasma testosterone in a wide variety of vertebrates, including humans. The ‘challenge hypothesis’ proposes that seasonal peaks in testosterone during breeding are caused by social challenges from other males. However, during experimentally induced male–male conflicts, testosterone increases only in a minority of songbird species tested so far. Why is this so? Comparative evidence suggests that species with a short breeding season may not elevate testosterone levels during territory defence. These species may even be limited in their physiological capability to increase testosterone levels, which can be tested by injecting birds with gonadotropin-releasing hormone (GnRH). We studied two populations of black redstarts that differ in breeding altitude, morphology and the length of their breeding season. Unexpectedly, males of neither population increased testosterone in response to a simulated territorial intrusion, but injections with GnRH resulted in a major elevation of testosterone. Thus, black redstarts would have been capable of mounting a testosterone response during the male–male challenge. Our data show, for the first time, that the absence of an androgen response to male–male challenges is not owing to physiological limitations to increase testosterone. Furthermore, in contrast to comparative evidence between species, populations of black redstarts with a long breeding season do not show the expected elevation in testosterone during male–male challenges.
Keywords: GnRH, challenge hypothesis, number of broods hypothesis, breeding season length, territorial aggression, androgen responsiveness
1. Introduction
The steroid hormone testosterone is one of the main hormones involved in the modulation of social and sexual behaviour. For example, studies in a variety of taxa have shown that castration reduces sexual behaviour in males and that subsequent administration of testosterone generally restores it [1,2]. Likewise, testosterone levels are often elevated during periods of intense competition for mates and/or territories (e.g. [3–5]), suggesting that this hormone promotes male–male competition. However, this is only one side of the coin, as behaviour can also feed back on hormone levels on a short-term and a long-term basis [6]. Androgens are, for example, elevated after competition in humans [7] and after territorial fights in cichlid fish [8] or Siberian hamsters (Phodopus sungorus) [9] (for a review on vertebrates see [10]). Whereas many of these studies were done in captivity, the relationship between competitive behaviour and testosterone has also been studied extensively in the field, mostly by testing territorial male songbirds with a simulated intruder into their territories—so-called simulated territorial intrusion (STI) experiments. These studies were based on the ‘challenge hypothesis’ [5], which argues that males of monogamous biparental bird species should show an elevation in testosterone only during male–male conflicts, but maintain lower breeding baseline levels of testosterone during other times. These brief elevations in testosterone may promote a male's reproductive success by enhancing territorial and sexual behaviours, but prolonged high levels of testosterone should be avoided as they may interfere with paternal behaviour [11,12] and may incur other costs [13]. Thus, testosterone is thought to balance the trade-off between investment in territory defence and paternal care, and should only increase during times of social instability (i.e. when challenged by other males). As competitive interactions between males usually occur during territory establishment in early spring, these surges in testosterone have been considered to result in breeding season testosterone profiles typical for many temperate zone species; namely high testosterone levels (close to the physiological maximum, referred to as ‘level C’) at the beginning of the breeding season and lower testosterone levels during incubation and feeding of young. However, the experimental evidence for the challenge hypothesis has been mixed. While males of some songbird species respond with an increase in testosterone above breeding baseline levels after an STI (e.g. [14–16]), many others do not (e.g. [17–20]; reviewed in [21,22]). This difference between species may be related to differences in their life history. So far, three ecological hypotheses—the essential paternal care hypothesis, the short season hypothesis and the number of broods hypothesis—have been put forward to explain between-species differences in androgen responsiveness to male–male interactions [22]. These hypotheses are not mutually exclusive and are all based on the general idea that biparental species living in environments with strong time or resource limits for breeding should not increase testosterone during STIs, because high levels of testosterone may interfere with paternal care. In general, populations with short breeding seasons breed relatively synchronously. As a consequence, mating opportunities after egg-laying are rare, and males may maximize fitness by allocating most of their time and energy to paternal care of their current clutch rather than investing in male competition or extra-pair matings. This framework emerged from studies in arctic birds that have a very short and highly synchronized breeding season, and that do not modulate testosterone during STIs [23] (‘short season hypothesis’). Building upon this short season hypothesis for arctic birds, Landys et al. [18] and Goymann et al. [21] observed in a comparative study that males of temperate zone species that raise only one brood per season also did not increase testosterone during STIs, whereas males of multiple-brooded species did (‘number of broods hypothesis’). Goymann [22] generalized the short season hypothesis (originally proposed for arctic birds) and showed that the likelihood that males show a rise in testosterone during STIs increases with the length of the breeding season (i.e. males of species with a short breeding season do not increase testosterone during STIs, whereas males of species with a long breeding season show this androgen responsiveness to male–male interactions).
Life-history differences between species may not only translate into distinct hormonal responses to STIs, but may be related to further endocrinological differences in how individuals deal with social cues. Of particular importance is the idea that males of some species may not show an increase in testosterone during STIs because their levels of testosterone are already maximal [21,22]. This may be either because they are frequently challenged by other males or because they are physiologically constrained to further increase testosterone. This can be tested by injecting birds with gonadotropin-releasing hormone (GnRH). GnRH induces the pituitary to release luteinizing hormone (LH), which then stimulates the testes to secrete testosterone. Not many studies have tested the androgen response to GnRH in songbirds, but it is already evident that there are differences between species [24–29]. To the best of our knowledge, no study has yet tested the androgen response to STIs and GnRH in the same individual. This is, however, of paramount importance to find out whether individuals who do not mount an androgen response to male–male interactions would be capable of mounting such a response at all. If not, their testosterone levels may have been already maximal and they would not be able to respond to any social cues, including interactions between males or with receptive females.
Accordingly, in this study, we first tested the short season and the number of broods hypotheses by comparing populations of the same species that differ in the length of their breeding season and in the number of clutches. Following the predictions of the two hypotheses, we expected that the population with a short breeding season would not show an increase in testosterone during STIs, whereas the population with the long breeding season should do so. Second, by injecting GnRH after the STI, we tested whether testosterone does not increase during STIs because testosterone levels were already maximally elevated. We conducted STI experiments and GnRH challenges during territory establishment (when male–male interactions occur frequently and testosterone levels are expected to be high) and during the parental phase of the first clutch (when interactions between males are less frequent and testosterone levels are expected to be lower). This approach allowed us to differentiate whether the hypothalamic-pituitary-gonadal (HPG) axis of the tested males is not sensitive to territorial challenges at all and whether they therefore do not increase testosterone during any breeding stage or sensitivity changes during the breeding season.
Our study species was the black redstart (Phoenicurus ochruros), a highly territorial, socially monogamous songbird that covers a large breeding range from high to low altitudes within Europe [30]. High-altitude populations have a short breeding season with typically one brood, whereas low-altitude populations have a long breeding season and raise two or more broods [30]. To test whether different life histories led to distinct androgen responses to male–male interactions and to differences in HPG axis physiology in general, we challenged males of a low- and a high-altitude population with STIs and injections with GnRH during two breeding stages.
Black redstarts show a resurgence of territorial behaviour in autumn after moult, before they leave their breeding grounds. This behaviour offered an additional opportunity to assess seasonal changes in androgen responsiveness to STIs and GnRH. Therefore, we also collected data in autumn for the low-altitude population and compared the intensity of territorial behaviour and androgen responsiveness between the breeding season and autumn.
2. Methods
Low-altitude male black redstarts were caught in 2008 between 1 and 30 April (territory establishment), between 19 May and 12 June (feeding 1st clutch) and between 19 September and 6 October (autumn territoriality) in Upper Barbaria (47° N, 11° E; 500–600 m above sea level). High-altitude male black redstarts were caught in the Southern Tyrolean Alps (46° N, 11° E; 1800–2500 m above sea level) between 5 and 15 May (territory establishment) and again between 18 June and 4 July (feeding young). Overall, we collected samples from 107 birds that were subjected to different experimental treatments.
(a). Simulated territorial intrusion
To elicit a territorial response, we placed a stuffed decoy into the centre of a territory and played back black redstart song using five different playbacks in random order (wav files, each repeated at a rate of eight strophes per minute). This procedure has been successfully used to elicit a territorial response in male black redstarts in another study [31]. As decoys, we used three different stuffed males in full adult plumage that were protected by an inconspicuous cage made of a wire frame and a mist net. We recorded the following behaviours of the territory owner for 10 min: (i) latency to respond to the STI either by singing or approaching the decoy; (ii) the first time the male was in a 5 m radius around the decoy; (iii) the time the male spent in this 5 m radius; (iv) the time the territory owner was fluffed; and (v) the number of head noddings, which are typical threat postures of male black redstarts [31]. Furthermore, we noted whether the male attacked the decoy or sang at any time during the STI. After 10 min, mealworm-baited ground traps and spring traps were opened and the playback continued until the bird was caught (mean ± s.d.: 35 ± 20 min, range: 13.5–94 min).
Control males were caught either passively while searching for food or similar to STI males, with playback and presentation of a decoy within 10 min of the onset of the STI, and with the traps open from the beginning (mean ± s.d.: 246 ± 129 s, range: 60–480 s), following Wingfield & Wada [14], who demonstrated that an increase in testosterone during an STI can be observed only after 10 min of stimulation.
Immediately upon capture, a blood sample (approx. 120 µl) was taken after venipuncture from the wing vein (178 ± 112 s) and collected into heparinized capillaries. The bird was then injected with 50 µl chicken GnRH-I (Bachem H 3106; 1.25 µg dissolved in 50 µl isotonic saline; see [26]) into the pectoralis major muscle and kept in a holding bag. Another blood sample (approx. 120 µl) was taken after 30 min following procedures described by Moore et al. [27] and Wingfield et al. [29]. Control and experimental groups did not differ significantly in body mass (t = 1.1, d.f. = 67, p = 0.3), length of the right tarsus (t = 1.2, d.f. = 67, p = 0.2), length of the right wing (t = 1.5, d.f. = 68, p = 0.1) and cloacal protuberance (CP) volume (estimated by expressing it as a cylindrical shape: V = π × (CP width/2)2 × CP height; t = −1.8, d.f. = 67, p = 0.08; see electronic supplementary material for detailed results on morphological measurements). In addition, each male was banded with a numbered aluminium ring (Vogelwarte Radolfzell) and a combination of two colour rings.
(b). Plasma separation and hormone analysis
Plasma was immediately separated by centrifugation with a Compur Minicentrifuge (Bayer Diagnostics). The amount of plasma was measured with a Hamilton syringe and stored in 500 µl ethanol [32]. After returning from the field, samples were stored at −80°C. Testosterone concentration was determined by direct radioimmunoassay (RIA) following Goymann et al. [33]. For details of the extraction, see the electronic supplementary material. Mean ± s.d. efficiency of the extraction with dichloromethane was 96 ± 9 per cent. The lower limit of detection of the assay was determined as the first value outside the 95 per cent confidence interval (CI) for the zero standard (Bmax) and was 6.7 pg tube−1. Samples were measured in duplicates in two assays, each containing samples from both populations. The intra-assay coefficients of variation were 4.7 and 4.2 per cent, respectively. The inter-assay variation was 5.5 per cent. As the testosterone antibody shows significant cross-reactions with 5a-dihydrotestosterone (44%), our measurements may include a minor fraction of this additional androgen.
(c). Statistical analysis
Data analysis was done with R v. 2.9.1 [34]. We used general linear models to analyse the influence of altitude and age (yearling or adult) on body mass, wing length, tarsus length and CP (electronic supplementary material). Behavioural data were first analysed including data from the low- and high-altitude populations without data from autumn. Second, we analysed behavioural data including only the low-altitude population to compare breeding season with autumn data. Behavioural data also include data from individuals that we were not able to catch within 2 h. We analysed whether the latency to first approach within 5 m (seconds), the time spent within 5 m (seconds) and agitation-related behaviours (head nodding, feather fluffing) differed between altitudes and life-cycle stages. We set territory establishment a priori as reference level in all models. Head nodding (factor loading: 0.70) and feather fluffing (factor loading: 0.70) were combined to one variable by using principle component analysis. The first principle component factor explained 60 per cent of the total variance and we used it as a score for agitation (agitation score).
Log-transformed post-capture testosterone levels, log-transformed GnRH-induced testosterone levels, and the absolute and relative (electronic supplementary material) increase from post-capture to GnRH-induced testosterone levels were analysed with general linear models for effects of treatment, altitude, breeding stage and age. We calculated combined models for the low- and high-altitude populations excluding low-altitude autumn data. In addition, we calculated models with data from the low-altitude population only, including testosterone data from the breeding season and autumn period. Furthermore, we used general linear models to check whether post-capture testosterone levels, GnRH-induced testosterone levels and the absolute increase in testosterone of STI males after GnRH injection were correlated with any of the behaviours shown during the STI. Not all males captured during an STI were injected with GnRH, resulting in different sample sizes for the various analyses.
To estimate androgen responsiveness to the STI and GnRH, we calculated effect sizes (Cohen's d; see [21,22]) using the program ESCI [35]. Significance was accepted at α < 0.05 (two-tailed) and data are presented as (back-transformed) means and 95 per cent CIs.
3. Results
(a). Behaviour
The STIs elicited similar behavioural responses as intrusions by real males or territorial conflicts with neighbours ([36]; B. Apfelbeck 2008–2010, personal observations).
(b). Low-altitude population in Upper Bavaria
Males responded to an STI similarly across life-cycle stages. Only the first approach within 5 m differed significantly between stages (F2,56 = 5.0, p = 0.01; table 1); males approached the decoy faster during territory establishment than when they were feeding the young of their first brood (t = 2.61, d.f. = 56, p = 0.012; table 1). But they approached the decoy as fast in autumn as during territory establishment (t = −0.396, d.f. = 56, p = 0.69; table 1). They spent similar amounts of time within 5 m of the decoy during all life-cycle stages (F2,56 = 0.8, p = 0.4; table 2). Likewise, the agitation score did not differ between territory establishment and the parental phase (t = −0.54, d.f. = 39, p = 0.6; table 1; because of differences in recording techniques, autumn agitation-related behaviours were not included). Also, the number of individuals that attacked the decoy did not differ between life-cycle stages (first 10 min of STI: Fisher's test, p = 0.7; STI until capture: Fisher's test, p = 0.9). However, fewer individuals sang during the STI in autumn than during the STIs during breeding (first 10 min of STI: Fisher's test, p = 0.0003; STI until capture: Fisher's test, p = 0.007).
Table 1.
Mean (±95% CI) intensity of behaviours measured during the STIs in relation to altitude, breeding stage and season.
| low altitude |
high altitude |
||||
|---|---|---|---|---|---|
| territory establishment n = 31 | parental phase n = 11 | autumn n = 17 | territory establishment n = 13 | parental phase n = 25 | |
| approach (s) | 186 ± 45 | 335 ± 77 | 241 ± 75 | 263 ± 104 | 262 ± 74 |
| time within 5 m (s) | 317 ± 54 | 251 ± 80 | 285 ± 78 | 212 ± 89 | 207 ± 65 |
| fluffing (%) | 70 ± 10 | 76 ± 14 | 81 ± 14 | 60 ± 13 | |
| head nods per minute | 2.9 ± 0.6 | 3.2 ± 1.7 | 3.7 ± 1.1 | 2.7 ± 1 | |
Table 2.
Results of the general linear models for post-capture and GnRH-induced testosterone levels, and the increase in testosterone caused by GnRH with respect to behavioural measurements during the STIs. Significant effects are printed in bold.
| post-capture testosterone |
GnRH-induced testosterone |
testosterone increase |
||||
|---|---|---|---|---|---|---|
| test statistic | p-value | test statistic | p-value | test statistic | p-value | |
| approach | F1,37 = 1.3 | 0.3 | F1,25 = 0.3 | 0.6 | F1,24 = 2.18 | 0.1 |
| time within 5 m | F1,37 = 0.7 | 0.4 | F1,25 = 0.7 | 0.4 | F1,24 = 1.6 | 0.2 |
| agitation | F1,37 = 0.3 | 0.6 | F1,25 = 0.7 | 0.4 | F1,24 = 0.2 | 0.6 |
| attacks (yes, no) | F1,37 = 0.04 | 0.9 | F1,25 = 0.4 | 0.5 | F1,24 = 0.3 | 0.6 |
| song (yes, no) | F1,37 = 2.2 | 0.1 | F1,25 = 0.05 | 0.8 | F1,24 = 0.01 | 0.9 |
| STI duration | F1,39 = 9.7 | 0.003 | F1,27 = 0.6 | 0.4 | F1,26 = 0.2 | 0.7 |
| attacks (yes, no) | F1,39 = 0.8 | 0.4 | F1,27 = 1.4 | 0.24 | F1,26 = 0.02 | 0.9 |
| STI duration × attacks | F1,39 = 5.5 | 0.01 | F1,27 = 1.5 | 0.23 | F1,26 = 5.6 | 0.02 |
(c). High-altitude population in Southern Tyrol
Males approached the decoy as fast and stayed as long within 5 m of the decoy during the parental phase as during territory establishment (approach: F1,36 = 0.004, p = 0.9; time within 5 m: Mann–Whitney U-test, U = 159.5, p = 0.9; table 1). However, males were more agitated during the STI in the territory establishment phase than during the parental phase (F1,36 = 6.3, p = 0.02; table 1).
(d). Comparison of the low-altitude and high-altitude populations
The latency to approach within 5 m of the decoy did not differ between breeding stages (F1,76 = 3.1, p = 0.08) and altitude (F1,76 = 0.5, p = 0.5; stage × altitude: F1,76 = 3.5, p = 0.07). Males of the high-altitude population spent slightly less time within 5 m than males of the low-altitude population (F1,77 = 4.1, p = 0.05). But there was no difference between breeding stages (F1,77 = 3.5, p = 0.07). Males from the high-altitude population were less agitated during the STIs in the parental phase than in the territory establishment phase (stage × altitude: F1,76 = 4.2, p = 0.04; breeding stage: F1,76 = 2.2, p = 0.1; altitude: F1,76, p = 0.8).
(e). Hormones
(i). Hormonal response to simulated territorial intrusions and gonadotropin-releasing hormone with respect to altitude
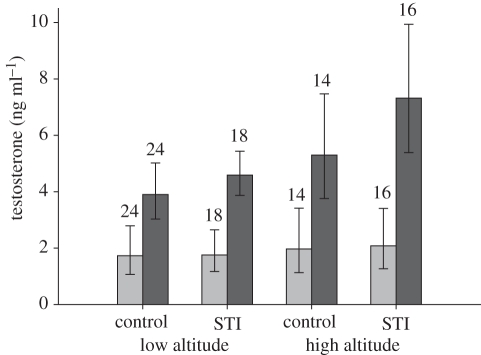
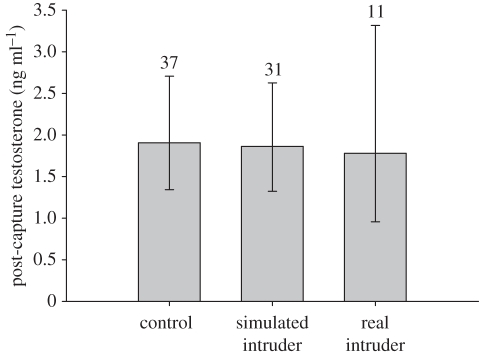
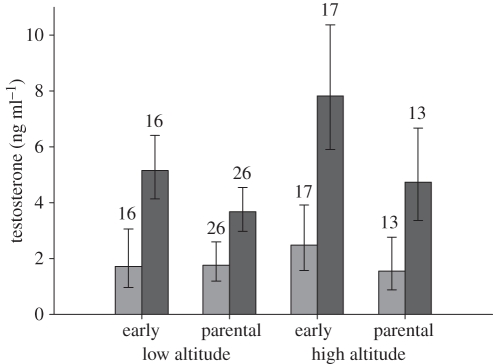
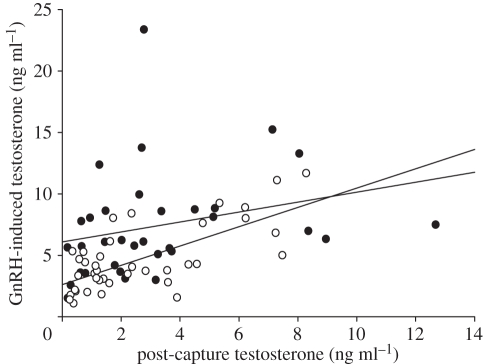
Male black redstarts did not increase plasma testosterone in response to an STI (F1,65 = 0.04, p = 0.84; figure 1) regardless of altitude (F1,65 = 0.14, p = 0.71; figure 1) and breeding stage (F1,65 = 0.09, p = 0.76). This means their androgen responsiveness to male–male interactions was very low (mean (±95% CI) effect sizes: overall: d = −0.07 (−0.54; 0.4); low-altitude population: d = −0.18 (−0.79; 0.43); high-altitude population: d = 0.03 (−0.7; 0.75)). In a small subset of the STI experiments, the STIs induced real intrusions by neighbouring or other males. Post-capture testosterone levels of males facing a real intruder also did not significantly differ from those of controls or STI-only males (F2,73 = 0.03, p = 0.9; figure 2). GnRH-induced testosterone levels were significantly higher than testosterone levels before the injection (paired t-test: t = −9.72, p < 0.0001, d = 1.1 (0.8; 1.4); figures 1 and 3). Furthermore, testosterone levels before and after the GnRH injection were significantly positively correlated (r = 0.61, p < 0.0001; figure 4). The GnRH-induced increase in testosterone did not differ between control and STI-challenged males (F1,66 = 1.6, p = 0.2, d = 0.36 (−0.11; 0.83)). Thus, despite the fact that male black redstarts did not increase testosterone during STIs, they would have had the potential to do so because they were not physiologically limited. Post-capture testosterone levels did not differ between breeding stages (F1,65 = 0.7, p = 0.41, d = 0.23 (−0.24; 0.7); figure 3) nor between altitudes (F1,65 = 0.2, p = 0.66, d = −0.14 (−0.62; 0.33); figure 3). However, the physiological potential to release androgens was significantly higher during the territory establishment phase than during the parental phase (F1,68 = 9.7, p = 0.003, d = 0.74 (0.25; 1.22); figure 3). Correspondingly, the increase in testosterone was higher during territory establishment than during the parental phase (F1,66 = 6.4, p = 0.01, d = 0.58 (0.1; 1.05)). Furthermore, GnRH-induced testosterone levels were significantly lower in males from the low-altitude than from the high-altitude population (F1,68 = 9.9, p = 0.002, d = −0.78 (−1.7; −0.28); figures 1 and 3). Thus, the increase in testosterone was also significantly lower in males of the low-altitude than of the high-altitude population (F1,66 = 6.7, p = 0.01, d = −0.71 (−1.2; −0.22)). Between age classes there was no significant difference in post-capture testosterone levels (F1,65 = 3.0, p = 0.08), GnRH-induced testosterone levels (F1,68 = 3.0, p = 0.088) nor the increase in testosterone (F1,66 = 0.63, p = 0.4).
Figure 1.
Back-transformed means (±95% CI) of post-capture testosterone levels did not differ between control and simulated territorial intrusion (STI) males of the low- and the high-altitude populations. However, testosterone levels increased after a GnRH injection within the same individual. Sample sizes are given above bars. Light grey bars, post-capture; dark grey bars, GnRH induced.
Figure 2.
Back-transformed means (±95% CI) of post-capture testosterone levels did not differ between control males and males who were challenged with a simulated intruder or a real intruder. Sample sizes are given above bars.
Figure 3.
Back-transformed means (±95% CI) of post-capture testosterone levels were similar during the early and the parental breeding phase, and between the low- and the high-altitude population. However, the physiological potential to mount an androgen response to GnRH was significantly higher during the early breeding than during the parental phase, and higher in the high than in the low-altitude population. Sample sizes are given above the bars. Light grey bars, post-capture; dark grey bars, GnRH induced.
Figure 4.
Post-capture testosterone levels were highly correlated with GnRH-induced levels (r = 0.61, p < 0.0001). Data are shown for both populations taken together for the early (black circles) and the parental breeding phase (white circles).
(ii). Hormonal response to simulated territorial intrusions and gonadotropin-releasing hormone for the low-altitude population in relation to life-cycle stages
Post-capture testosterone levels significantly differed with life-cycle stage (F2,49 = 73.3, p < 0.0001). A priori contrasts revealed that they did not differ between territory establishment and the parental phase (t = 0.005, d.f. = 49, p = 0.99, d = 0.06 (−0.56; 0.68)), but were significantly higher during territory establishment than in autumn (mean ±95% CI: 40 ± 8 pg ml−1; t = −6.73, d.f. = 49, p < 0.0001, d = 1.4 (0.6; 2.2)). Testosterone levels did not increase during the STIs (F1,49 = 1.6, p = 0.2), and this was consistent between life-cycle stages (non-significant interaction: F2,49 = 0.07, p = 0.9).
GnRH-induced testosterone levels also differed with life-cycle stage (F2,52 = 482, p < 0.0001). They were significantly higher during territory establishment than during the parental phase (t = −2.2, d.f. = 52, p = 0.03, d = 0.85 (0.4; 1.29); figure 3) and autumn (mean ±95% CI: 41 ± 7 pg ml−1; t = −27.5, d.f. = 52, p < 0.0001, d = 3.7 (2.4; 4.9)).
Likewise, the increase in testosterone caused by GnRH depended on life-cycle stage (F2,49 = 7.0, p = 0.002). The increase was higher during territory establishment than in autumn (t = −2.4, d.f. = 49, p = 0.02, d = 1.4 (0.6; 2.3)), but did not differ between territory establishment and the parental phase (t = −1.5, d.f. = 49, p = 0.1). The increase did not differ between control and STI males (F1,49 = 0.54, p = 0.47), and this was independent of life-cycle stage (F2,49 = 0.15, p = 0.9).
(iii). Testosterone and behaviour
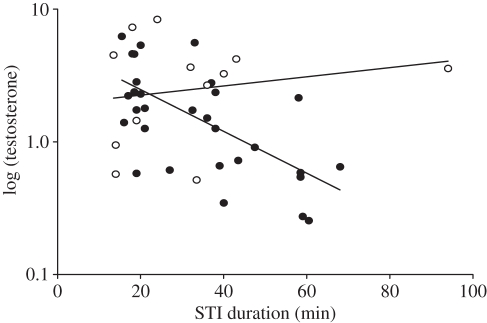
Post-capture and GnRH-induced testosterone levels and the increase in testosterone caused by GnRH did not covary with the behaviours measured during the STIs (table 2). A linear model fitted for testosterone in relation to the duration of the territorial intrusion and to whether the territory owner attacked the decoy revealed that post-capture testosterone declined with the duration of the intrusion but remained high if males attacked the decoy (table 2 and figure 5). This result remains robust even when removing the ‘outlier’ in the upper right corner of figure 5 (STI duration: F1,38 = 9.7, p = 0.003; attacks: F1,38 = 0.7, p = 0.4; interaction: F1,38 = 5.5, p = 0.02). GnRH-induced testosterone levels did not show a significant relationship with STI duration, attacks nor the interaction between the two (table 2). In accordance with this, STI duration was positively correlated with the GnRH-induced testosterone increase in non-attacking males but not in males that attacked the decoy (table 2).
Figure 5.
Post-capture testosterone levels plotted against the duration of the simulated territorial intrusion (STI). In males that attacked the decoy (white circles, n = 12), testosterone levels stayed high, while in males that did not attack the decoy, testosterone levels declined with STI duration (black circles, n = 31).
4. Discussion
In contrast to our prediction, the androgen responsiveness of male black redstarts to an STI (Rmale–male sensu [21]) did not depend on the number of broods or the length of the breeding season: both populations studied did not increase testosterone when challenged with STIs, neither during the early breeding season nor when they were feeding the young of their first brood. However, in both populations injection of GnRH led to an increase in testosterone levels in control and STI males, demonstrating that all males would have had the physiological potential (Rpotential sensu [21]) to further increase testosterone during STIs. To our knowledge, this is the first study that measured the androgen responsiveness to an STI and the physiological potential to release androgens in the same individuals. Thus, the black redstart is the first species for which it has been shown that a lacking increase in testosterone during STIs is not caused by a restriction in the potential to increase testosterone. Furthermore, males facing a real intruder showed behaviours similar to those of males experiencing a simulated intrusion (see also [36]) and also lacked the expected increase in testosterone, suggesting that the simulated intrusion mimicked a real territorial threat.
(a). Testosterone in relation to simulated territorial intrusions and gonadotropin-releasing hormone
Interspecific comparisons of the challenge hypothesis confirm the influence of mating system on seasonal testosterone profiles (Rseason sensu [21]) in birds [21,37], and of sexual and parental behaviour on testosterone levels in vertebrates in general [10]. However, in a situational rather than a seasonal context, the predictions of the challenge hypothesis often do not hold [21]. A central prediction is that in socially monogamous bird species with biparental care, aggressive interactions between males lead to short-term increases in testosterone above breeding baseline levels. These surges in testosterone are expected to cause a seasonal peak in testosterone at the beginning of the breeding season, when competition between males is particularly high [5]. On this seasonal basis, our data are in line with the challenge hypothesis as testosterone levels in black redstarts were highest during territory establishment and before a second clutch was initiated. During incubation, testosterone levels were low (B. Apfelbeck & W. Goymann 2008–2009, unpublished data). However, in a situational rather than a seasonal context, the predictions of the challenge hypothesis do not hold in black redstarts. Aggressive encounters between males do not seem to be the direct cause of seasonal peaks in testosterone, because black redstarts did not elevate testosterone levels during simulated or real territorial intrusions. Thus, in this species, the seasonal androgen response (Rseason) is no proxy for, and does not predict the androgen responsiveness to, male–male interactions (Rmale–male) [21,22]. Because this was also the case in various other species, several hypotheses have been proposed to relate the lack of testosterone response following male–male interactions to life-history differences between species (see §1). We tested one set of these hypotheses: the short breeding season and number of broods hypotheses [22,23] in two populations of black redstarts. But we found no evidence for a differential testosterone response to STIs depending on the length of the breeding season and number of broods: males of both populations did not increase testosterone when challenged with an STI. For the high-altitude population, this conforms to the idea that species breeding in severe environments should not elevate testosterone during male–male interactions in order to minimize interference with parental care. However, males of the low-altitude population would have been expected to elevate testosterone during male–male interactions, because their breeding season is long and they raise multiple clutches. Thus, the comparison of two populations of black redstarts is not consistent with the pattern predicted by the short season or number of broods hypotheses. Hence, other (so far unconsidered) factors may influence whether some species increase testosterone during territorial challenges, while others do not. In this context, the actual function of short-term modulations of testosterone after territorial conflicts may be important. Especially, studies in mammals and fish suggest that the androgen release after defence of the home territory may be crucial for the formation of the winner effect, and thus may promote territoriality [38,39]. Black redstarts of the low-altitude population defend territories with low testosterone levels in autumn (i.e. after having finished moult and before they migrate). When they return the following spring, they typically breed on these territories [40]. In autumn, they show no androgen response to territorial or GnRH challenges. Thus, in black redstarts residency effects might be decoupled from androgen release after territorial conflicts. This may be in contrast to some other temperate zone songbirds, such as the song sparrow, in which testosterone increases during male–male interactions during the breeding season [14]. However, the relationship between the winner effect, territoriality and short-term testosterone release has not been studied in songbirds yet.
Until now, it was unknown whether any of the bird species that did not increase testosterone during STIs had the capacity to further increase testosterone: if testosterone levels were already maximal, the lack of a testosterone response to the challenge would not be too surprising [22]. The black redstart is the first species for which we can now exclude this explanation: although males of both populations did not increase testosterone during STIs, both populations responded with a substantial increase in testosterone after injections with GnRH. Hence, males did not have maximum testosterone levels during any breeding stage and would have had the capacity to modulate circulating testosterone during a social challenge. Similarly, male rufous-collared sparrows (Zonotrichia capensis) and Gambel's white-crowned sparrows (Zonotricha leucophrys gambelii) also increase testosterone after a GnRH challenge [27], but do not modulate testosterone levels after a territorial dispute [41,42]. STI and GnRH challenges were, however, performed on different sets of individuals in these studies and thus could not exclude the possibility that testosterone levels were already maximal in STI-challenged individuals. These and our data indicate that, with respect to the effects of GnRH, species that do not increase testosterone when challenged with an intruder are similar to species that increase testosterone during STIs: the latter also respond to GnRH with an increase in testosterone [15,16,26,27].
In species that are responsive to GnRH, but not to male–male interactions, other social cues, such as sexual interactions with females [43,44], may modulate plasma testosterone levels [21,22]. Often it is difficult to distinguish whether the seasonal peak in testosterone coincides with the time period of frequent male–male interactions or the time period when females are most fertile, or both. Accordingly, in the low-altitude black redstart population, we observed a peak in testosterone during the parental phase of the first brood when many females had already started a second clutch (B. Apfelbeck 2008–2010, personal observations). During this time, overt male–male competition does not seem to be particularly strong, and any socially induced peak in testosterone may be more probably related to male–female interactions. In contrast to black redstarts, both male–male and male–female interactions may shape seasonal testosterone patterns in males of species that mount an androgen response both to STIs and GnRH. Song sparrows (Melospiza melodia), for example, show a peak in testosterone upon arrival at their breeding grounds when they establish territories and again when their mates start egg-laying [45].
If the amount of testosterone produced is relevant for the processing of social cues from other males or females, then the capability to do so probably changes during the life cycle. Similar to dark-eyed juncos [26], the capacity of male black redstarts to mount a testosterone response after a GnRH injection differed between life-cycle stages: GnRH-induced testosterone levels were significantly higher during the early breeding season than when they were feeding nestlings or fledglings (and during autumn, GnRH did not induce higher testosterone at all). In combination with studies that also measured LH release after GnRH injection [26,29], our data also suggest that the seasonal differences in testosterone release are, at least to some extent, regulated at the level of the testes. Possibly, the lower capacity to raise testosterone during the parental phase might be an adaptation to avoid interference of high testosterone levels with paternal care [5]. These data also demonstrate that seasonal peaks in testosterone do not necessarily reflect maximum physiological levels (‘level C’) as postulated by the challenge hypothesis [5], and that ‘level C’ is not fixed throughout the breeding season but changes depending on the breeding sub-stage.
Although testosterone levels before the GnRH injection were similar in black redstarts of the high- and the low-altitude populations, the increase after a GnRH injection was higher in males of the high-altitude population. Thus, the morphological differences between the two populations (electronic supplementary material) correspond with physiological differences in the regulation of the HPG axis. Similarly, Moore and colleagues [27] found the highest increase in testosterone after a GnRH injection in a high-altitude tropical species when comparing closely related Zonotrichia species in their physiological response to GnRH. Furthermore, this is in line with a comparative study in tropical birds demonstrating that seasonal androgen maxima are highest in species with short breeding seasons and at high altitudes [46]. Our study on black redstarts suggests that such patterns may also exist in temperate zone species and that such relationships may not only exist between species, but also within populations of the same species breeding at different altitudes.
(b). Testosterone and behaviour
GnRH-induced maximum testosterone levels were highly positively correlated with post-capture testosterone levels and showed large inter-individual differences. Jawor et al. [26] found that in dark-eyed juncos, the androgen response to GnRH was repeatable within individuals and While et al. [47] found repeatable baseline testosterone levels in individuals of the Australian lizard (Egernia whitii). These findings raise the possibility that differences in baseline and/or maximum testosterone levels are related to individual differences in behaviour. However, individual correlations between testosterone and behaviour are rarely found (e.g. [48,49]). In our study, differences in post-capture and GnRH-induced testosterone levels were not related to differences in the behavioural response to a simulated intruder. Unexpectedly, post-capture testosterone levels declined with STI duration so that males that were caught at a later time point during the intrusion had lower testosterone levels than males caught earlier. However, this was not true for males that attacked the decoy: in these males, testosterone levels remained high. During STIs, territory owners cannot evict the intruder and, therefore, testosterone levels might start to decline during long-lasting intrusions [50] (but see [51]). Alternatively, during STIs, males are likely to be more active than during other periods, resulting in an increase in their metabolism: If the secretion of testosterone from the testes remains constant, but clearance increases owing to a higher blood flow through the liver, this may result in declining testosterone concentrations. But why did males that attacked the decoy maintain higher testosterone concentrations? Although there is little evidence for a relationship between coping style or personality and testosterone [52], a recent study of European stonechats by C. Muck & W. Goymann [53] showed that individuals that attacked their mirror image had higher baseline testosterone levels than individuals that did not attack the mirror. Thus, our observation in black redstarts is consistent with a situation in which individuals with a bold personality express higher levels of testosterone than individuals with a more shy personality, regardless of the situation.
5. Conclusions
Although differences in the androgen physiology of two black redstart populations exist, the fact that males of both populations do not mount an androgen response to STIs but to GnRH indicates that the regulation of territorial defence is similar in both populations. Thus, the length of the breeding season or the number of broods cannot universally explain between-species differences in androgen responsiveness to territorial challenges. Because black redstarts increased testosterone after injections with GnRH, they would have had the physiological capacity (Rpotential) to increase testosterone during STIs. In other vertebrate taxa, short-term testosterone increases after male–male conflicts have been suggested to be responsible for the winner effect, and thereby reinforce territoriality. However, in species like the black redstart, which defend breeding territories in autumn, territory defence may be decoupled from short-term testosterone modulations. We suggest that the extent to which the winner effect and territoriality are coupled to short-term testosterone release might be a promising factor to explain the existing variation in androgen responsiveness to male–male interactions.
Acknowledgements
All experimental procedures were approved by the governmental authorities of Upper Bavaria and Southern Tyrol.
We thank Dieter Schmidl, Felix Pustal, Johanna Stegherr, Matthias Schneider and Agnes Türk for their tireless assistance in the field, Ingrid Schwabl and Monika Trappschuh for their experienced help in the laboratory, and the Max Planck Gesellschaft and Manfred Gahr for funding. Barbara Helm, Manfred Gahr, Redouan Bshary and two anonymous referees gave valuable comments on previous versions of the article.
References
- 1.Valenstein E. S., Young W. C. 1955. An experiential factor influencing the effectiveness of testosterone propionate in eliciting sexual behavior in male guinea-pigs. Endocrinology 56, 173–177 10.1210/endo-56-2-173 (doi:10.1210/endo-56-2-173) [DOI] [PubMed] [Google Scholar]
- 2.Balthazart J., Reid J., Absil P., Foidart A., Ball G. F. 1995. Appetitive as well as consummatory aspects of male sexual behavior in quail are activated by androgens and estrogens. Behav. Neurosci. 109, 485–501 10.1037/0735-7044.109.3.485 (doi:10.1037/0735-7044.109.3.485) [DOI] [PubMed] [Google Scholar]
- 3.Lincoln G. A., Guinness F., Short R. V. 1972. The way in which testosterone controls the social and sexual behavior of the red deer stag (Cervus elaphus). Horm. Behav. 3, 375–396 10.1016/0018-506X(72)90027-X (doi:10.1016/0018-506X(72)90027-X) [DOI] [Google Scholar]
- 4.Moore M. C., Marler C. A. 1987. Effects of testosterone manipulations on nonbreeding season territorial aggression in free-living male lizards, Sceloporus jarrovi. Gen. Comp. Endocrinol. 65, 225–232 10.1016/0016-6480(87)90170-5 (doi:10.1016/0016-6480(87)90170-5) [DOI] [PubMed] [Google Scholar]
- 5.Wingfield J. C., Hegner R. E., Dufty A. M., Jr, Ball G. F. 1990. The ‘challenge hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846 10.1086/285134 (doi:10.1086/285134) [DOI] [Google Scholar]
- 6.Harding C. F. 1981. Social modulation of circulating hormone levels in the male. Am. Zool. 21, 223–231 [Google Scholar]
- 7.Archer J. 2006. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci. Biobehav. Rev. 30, 319–345 10.1016/j.neubiorev.2004.12.007 (doi:10.1016/j.neubiorev.2004.12.007) [DOI] [PubMed] [Google Scholar]
- 8.Hirschenhauser K., Taborsky M., Oliveira T., Canario A. V. M., Oliveira R. F. 2004. A test of the ‘challenge hypothesis’ in cichlid fish: simulated partner and territory intruder experiments. Anim. Behav. 68, 741–750 10.1016/j.anbehav.2003.12.015 (doi:10.1016/j.anbehav.2003.12.015) [DOI] [Google Scholar]
- 9.Scotti M.-A. L., Schmidt K. L., Newman A. E. M., Bonu T., Soma K. K., Demas G. E. 2009. Aggressive encounters differentially affect serum dehydroepiandrosterone and testosterone concentrations in male Siberian hamsters (Phodopus sungorus). Horm. Behav. 56, 376–381 10.1016/j.yhbeh.2009.07.004 (doi:10.1016/j.yhbeh.2009.07.004) [DOI] [PubMed] [Google Scholar]
- 10.Hirschenhauser K., Oliveira R. F. 2006. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim. Behav. 71, 265–277 10.1016/j.anbehav.2005.04.014 (doi:10.1016/j.anbehav.2005.04.014) [DOI] [Google Scholar]
- 11.Ketterson E. D., Val Nolan J., Wolf L., Ziegenfus C. 1992. Testosterone and avian life histories: effects of experimentally elevated testosterone on behavior and correlates of fitness in the dark-eyed junco (Junco hyemalis). Am. Nat. 140, 980–999 10.1086/285451 (doi:10.1086/285451) [DOI] [Google Scholar]
- 12.De Ridder E., Pinxten R., Eens M. 2000. Experimental evidence of a testosterone-induced shift from paternal to mating behaviour in a facultatively polygynous songbird. Behav. Ecol. Sociobiol. 49, 24–30 10.1007/s002650000266 (doi:10.1007/s002650000266) [DOI] [Google Scholar]
- 13.Wingfield J. C., Lynn S. E., Soma K. K. 2001. Avoiding the costs of testosterone: ecological bases of hormone-behavior interactions. Brain Behav. Evol. 57, 239. 10.1159/000047243 (doi:10.1159/000047243) [DOI] [PubMed] [Google Scholar]
- 14.Wingfield J. C., Wada M. 1989. Changes in plasma levels of testosterone during male–male interactions in the song sparrow, Melospiza melodia: time course and specificity of response. J. Comp. Physiol. A 166, 189–194 10.1007/BF00193463 (doi:10.1007/BF00193463) [DOI] [Google Scholar]
- 15.Wingfield J. C., Hahn T. P. 1994. Testosterone and territorial behaviour in sedentary and migratory sparrows. Anim. Behav. 47, 77–89 10.1006/anbe.1994.1009 (doi:10.1006/anbe.1994.1009) [DOI] [Google Scholar]
- 16.McGlothlin J. W., Jawor J. M., Greives T. J., Casto J. M., Philips J. L., Ketterson E. D. 2008. Hormones and honest signals: males with larger ornaments elevate testosterone more when challenged. J. Evol. Biol. 21, 39–48 10.1111/j.1420-9101.2007.01471.x (doi:10.1111/j.1420-9101.2007.01471.x) [DOI] [PubMed] [Google Scholar]
- 17.Van Duyse E., Pinxten R., Darras V. M., Arckens L., Eens M. 2004. Opposite changes in plasma testosterone and corticosterone levels following a simulated territorial challenge in male great tits. Behaviour 141, 451–467 10.1163/156853904323066739 (doi:10.1163/156853904323066739) [DOI] [Google Scholar]
- 18.Landys M. M., Goymann W., Raess M., Slagsvold T. 2007. Hormonal responses to male–male social challenge in the blue tit, Cyanistes caeruleus: single-broodedness as an explanatory variable. Physiol. Biochem. Zool. 80, 228–240 10.1086/510564 (doi:10.1086/510564) [DOI] [PubMed] [Google Scholar]
- 19.Landys M. M., Goymann W., Schwabl I., Trapschuh M., Slagsvold T. 2010. Impact of season and social challenge on testosterone and corticosterone levels in a year-round territorial bird. Horm. Behav. 58, 317–325 10.1016/j.yhbeh.2010.02.013 (doi:10.1016/j.yhbeh.2010.02.013) [DOI] [PubMed] [Google Scholar]
- 20.Lynn S. E., Hahn T. P., Breuner C. W. 2007. Free-living male mountain white-crowned sparrows exhibit territorial aggression without modulating total or free plasma testosterone. Condor 109, 173–180 10.1650/0010-5422(2007)109[173:FMMWSE]2.0.CO;2 (doi:10.1650/0010-5422(2007)109[173:FMMWSE]2.0.CO;2) [DOI] [Google Scholar]
- 21.Goymann W., Landys M. M., Wingfield J. C. 2007. Distinguishing seasonal androgen responses from male–male androgen responsiveness: revisiting the challenge hypothesis. Horm. Behav. 51, 463–476 10.1016/j.yhbeh.2007.01.007 (doi:10.1016/j.yhbeh.2007.01.007) [DOI] [PubMed] [Google Scholar]
- 22.Goymann W. 2009. Social modulation of androgens in male birds. Gen. Comp. Endocrinol. 163, 149–157 10.1016/j.ygcen.2008.11.027 (doi:10.1016/j.ygcen.2008.11.027) [DOI] [PubMed] [Google Scholar]
- 23.Wingfield J. C., Hunt K. E. 2002. Arctic spring: hormone–behavior interactions in a severe environment. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 132, 275–286 10.1016/S1096-4959(01)00540-1 (doi:10.1016/S1096-4959(01)00540-1) [DOI] [PubMed] [Google Scholar]
- 24.Busch D. S., Robinson T. R., Hahn T. P., Wingfield J. C. 2008. Sex hormones in the song wren: variation with time of year, molt, gonadotropin releasing hormone, and social challenge. Condor 110, 125–133 10.1525/cond.2008.110.1.125 (doi:10.1525/cond.2008.110.1.125) [DOI] [Google Scholar]
- 25.Goymann W., Wingfield J. C. 2004. Competing females and caring males. Sex steroids in African black coucals, Centropus grillii. Anim. Behav. 68, 733–740 10.1016/j.anbehav.2003.12.012 (doi:10.1016/j.anbehav.2003.12.012) [DOI] [Google Scholar]
- 26.Jawor J. M., McGlothlin J. W., Casto J. M., Greives T. J., Snajdr E. A., Bentley G. E., Ketterson E. D. 2006. Seasonal and individual variation in response to GnRH challenge in male dark-eyed juncos (Junco hyemalis). Gen. Comp. Endocrinol. 149, 182–189 [DOI] [PubMed] [Google Scholar]
- 27.Moore I. T., Perfito N., Wada H., Sperry T. S., Wingfield J. C. 2002. Latitudinal variation in plasma testosterone levels in birds of the genus Zonotrichia. Gen. Comp. Endocrinol. 129, 13–19 10.1016/S0016-6480(02)00563-4 (doi:10.1016/S0016-6480(02)00563-4) [DOI] [PubMed] [Google Scholar]
- 28.Spinney L. H., Bentley G. E., Hau M. 2006. Endocrine correlates of alternative phenotypes in the white-throated sparrow (Zonotrichia albicollis). Horm. Behav. 50, 762–771 10.1016/j.yhbeh.2006.06.034 (doi:10.1016/j.yhbeh.2006.06.034) [DOI] [PubMed] [Google Scholar]
- 29.Wingfield J. C., Hegner R. E., Lewis D. M. 1991. Circulating levels of luteinizing hormone and steroid hormones in relation to social status in the cooperatively breeding white-browed sparrow weaver, Plocepasser mahali. J. Zool. 225, 43–58 10.1111/j.1469-7998.1991.tb03800.x (doi:10.1111/j.1469-7998.1991.tb03800.x) [DOI] [Google Scholar]
- 30.Landmann A. 1996. Der Hausrotschwanz: vom Fels zum Wolkenkratzer: Evolutionsbiologie eines Gebirgsvogels. Wiesbaden, Germany: AULA-Verlag [Google Scholar]
- 31.Landmann A., Kollinsky C. 1995. Territory defence in black redstarts, Phoenicurus ochruros: effects of intruder and owner age? Ethology 101, 121–129 10.1111/j.1439-0310.1995.tb00351.x (doi:10.1111/j.1439-0310.1995.tb00351.x) [DOI] [Google Scholar]
- 32.Goymann W., Schwabl I., Trappschuh M., Hau M. 2007. Use of ethanol for preserving steroid and indoleamine hormones in bird plasma. Gen. Comp. Endocrinol. 150, 191–195 10.1016/j.ygcen.2006.09.014 (doi:10.1016/j.ygcen.2006.09.014) [DOI] [PubMed] [Google Scholar]
- 33.Goymann W., Geue D., Schwabl I., Flinks H., Schmidl D., Schwabl H., Gwinner E. 2006. Testosterone and corticosterone during the breeding cycle of equatorial and European stonechats (Saxicola torquata axillaris and S. t. rubicola). Horm. Behav. 50, 779–785 10.1016/j.yhbeh.2006.07.002 (doi:10.1016/j.yhbeh.2006.07.002) [DOI] [PubMed] [Google Scholar]
- 34.R Development Core Team 2009. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 35.Cumming G., Finch S. 2001. A primer on the understanding, use, and calculation of confidence intervals that are based on central and noncentral distributions. Educ. Psychol. Meas. 61, 532–574 10.1177/0013164401614002 (doi:10.1177/0013164401614002) [DOI] [Google Scholar]
- 36.Nesenhoener H. 1956. Beobachtungen, insbesondere brutbiologischer Art, am Hausrotschwanz (Phoenicurus ochruros). Ber. naturw. Ver. Bielefeld 14, 128–156 [Google Scholar]
- 37.Hirschenhauser K., Winkler H., Oliveira R. F. 2003. Comparative analysis of male androgen responsiveness to social environment in birds: the effects of mating system and paternal incubation. Horm. Behav. 43, 508–519 10.1016/S0018-506X(03)00027-8 (doi:10.1016/S0018-506X(03)00027-8) [DOI] [PubMed] [Google Scholar]
- 38.Oliveira R. F., Silva A., Canario A. V. M. 2009. Why do winners keep winning? Androgen mediation of winner but not loser effects in cichlid fish. Proc. R. Soc. B 276, 2249–2256 10.1098/rspb.2009.0132 (doi:10.1098/rspb.2009.0132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuxjager M. J., Marler C. A. 2010. How and why the winner effect forms: influences of contest environment and species differences. Behav. Ecol. 21, 37–45 10.1093/beheco/arp148 (doi:10.1093/beheco/arp148) [DOI] [Google Scholar]
- 40.Weggler M. 2000. Reproductive consequences of autumnal singing in black redstarts (Phoenicurus ochruros). Auk 117, 65–73 10.1642/0004-8038(2000)117[0065:RCOASI]2.0.CO;2 (doi:10.1642/0004-8038(2000)117[0065:RCOASI]2.0.CO;2) [DOI] [Google Scholar]
- 41.Meddle S. L., Romero L. M., Astheimer L. B., Buttemer W. A., Moore I. T., Wingfield J. C. 2002. Steroid hormone interrelationships with territorial aggression in an Arctic-breeding songbird, Gambel's white-crowned sparrow (Zonotrichia leucophrys gambelii). Horm. Behav. 42, 212–221 10.1006/hbeh.2002.1813 (doi:10.1006/hbeh.2002.1813) [DOI] [PubMed] [Google Scholar]
- 42.Moore I. T., Wada H., Perfito N., Busch D. S., Hahn T. P., Wingfield J. C. 2004. Territoriality and testosterone in an equatorial population of rufous-collared sparrows, Zonotrichia capensis. Anim. Behav. 67, 411–420 10.1016/j.anbehav.2003.03.021 (doi:10.1016/j.anbehav.2003.03.021) [DOI] [Google Scholar]
- 43.Moore M. C. 1982. Hormonal response of free-living male white-crowned sparrows to experimental manipulation of female sexual behavior. Horm. Behav. 16, 323–329 10.1016/0018-506X(82)90030-7 (doi:10.1016/0018-506X(82)90030-7) [DOI] [PubMed] [Google Scholar]
- 44.Moore M. 1983. Effect of female sexual displays on the endocrine physiology and behaviour of male white-crowned sparrows, Zonotrichia leucophrys. J. Zool. Lond. 199, 137–148 10.1111/j.1469-7998.1983.tb02085.x (doi:10.1111/j.1469-7998.1983.tb02085.x) [DOI] [Google Scholar]
- 45.Wingfield J. C. 1984. Environmental and endocrine control of reproduction in the song sparrow, Melospiza melodia: I. Temporal organization of the breeding cycle. Gen. Comp. Endocrinol. 56, 406–416 10.1016/0016-6480(84)90083-2 (doi:10.1016/0016-6480(84)90083-2) [DOI] [PubMed] [Google Scholar]
- 46.Goymann W., Moore I. T., Scheuerlein A., Hirschenhauser K., Grafen A., Wingfield J. C. 2004. Testosterone in tropical birds: effects of environmental and social factors. Am. Nat. 164, 327–334 10.1086/422856 (doi:10.1086/422856) [DOI] [PubMed] [Google Scholar]
- 47.While G. M., Isaksson C., McEvoy J., Sinn D. L., Komdeur J., Wapstra E., Groothuis T. G. G. 2010. Repeatable intra-individual variation in plasma testosterone concentration and its sex-specific link to aggression in a social lizard. Horm. Behav. 58, 208–213 10.1016/j.yhbeh.2010.03.016 (doi:10.1016/j.yhbeh.2010.03.016) [DOI] [PubMed] [Google Scholar]
- 48.Johnsen T. S. 1998. Behavioural correlates of testosterone and seasonal changes of steroids in red-winged blackbirds. Anim. Behav. 55, 957–965 10.1006/anbe.1997.0642 (doi:10.1006/anbe.1997.0642) [DOI] [PubMed] [Google Scholar]
- 49.McGlothlin J., Jawor J., Ketterson E. 2007. Natural variation in a testosterone mediated trade-off between mating effort and parental effort. Am. Nat. 170, 864–875 10.1086/522838 (doi:10.1086/522838) [DOI] [PubMed] [Google Scholar]
- 50.Kempenaers B., Peters A., Foerster K. 2008. Sources of individual variation in plasma testosterone levels. Phil. Trans. R. Soc. B 363, 1711–1723 10.1098/rstb.2007.0001 (doi:10.1098/rstb.2007.0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wikelski M., Hau M., Wingfield J. C. 1999. Social instability increases plasma testosterone in a year-round territorial neotropical bird. Proc. R. Soc. Lond. B 266, 551–556 10.1098/rspb.1999.0671 (doi:10.1098/rspb.1999.0671) [DOI] [Google Scholar]
- 52.Koolhaas J. M., de Boer S. F., Coppens C. M., Buwalda B. 2010. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front. Neuroendocrinol. 31, 307–321 10.1016/j.yfrne.2010.04.001 (doi:10.1016/j.yfrne.2010.04.001) [DOI] [PubMed] [Google Scholar]
- 53.Muck C., Goymann W. Submitted. Hormones mirror personalty: a mirror image stimulation test in male stonechats. [Google Scholar]