Abstract
Costs and benefits of foraging have been studied in predatory animals. In nematodes, ambushing or cruising behaviours represent adaptations that optimize foraging strategies for survival and host finding. A behaviour associated with host finding of ambushing nematode dauer juveniles is a sit-and-wait behaviour, otherwise known as nictation. Here, we test the function of nictation by relating occurrence of nictation in Pristionchus pacificus dauer juveniles to the ability to attach to laboratory host Galleria mellonella. We used populations of recently isolated and mutagenized laboratory strains. We found that nictation can be disrupted using a classical forward genetic approach and characterized two novel nictation-defective mutant strains. We identified two recently isolated strains from la Réunion island, one with a higher proportion of nictating individuals than the laboratory strain P. pacificus PS312. We found a positive correlation between nictation frequencies and host attachment in these strains. Taken together, our combination of genetic analyses with natural variation studies presents a new approach to the investigation of behavioural and ecological functionality. We show that nictation behaviour in P. pacificus nematodes serves as a host-finding behaviour. Our results suggest that nictation plays a role in the evolution of new life-history strategies, such as the evolution of parasitism.
Keywords: ambush, behavioural genetics, body-waving, host attachment, nictation, Pristionchus pacificus
1. Introduction
Behavioural adaptations have been important in enabling nematodes to successfully exploit diverse habitats [1,2]. But little is known of the extent to which changes in behaviour influence the evolution of new life-history strategies in organisms.
One model used in foraging theory separates predatory and parasitic animals into two categories: cruisers and ambushers [3–5]. Cruisers are in constant movement and actively search for food (high energy cost), whereas ambushers tend to stand still and wait for their prey/host to approach (low energy cost) [3–5]. Infective juveniles of some ambusher nematode species show a specific search behaviour in which the animals stand on their tail and wave. This standing behaviour has been termed ‘winken’ [6], ‘nictation’ [7–9], ‘standing’ [10–12] and most recently ‘body waving’ [13]. For a complete revision about the terminology of this behaviour, see Kruitbos & Wilson [14]; for the sake of simplicity, we refer to this behaviour as nictation.
Nictation consists of raising the anterior and middle body regions of the juvenile off the ground, supported only by the tip of its tail [8,15]. In different species of nictating nematodes, juveniles can either stay in an erect pose or wave their bodies in three-dimensional spirals and loops [7,8,15]. In the laboratory, nictation behaviour is only observed when the nematodes are exposed to irregular substrates [13]. Foraging strategies of host finding in nematodes from the order Rhabditida have been extensively studied, e.g. in the parasitic Steinernema spp., Heterorhabditis spp. and Phasmarhabditis hermaphrodita because of their importance to pest management. The slug-parasitic nematode P. hermaphrodita attaches to hosts by crawling and is devoid of nictation, whereas insect parasitic Steinernema spp. attach by nictating [13,16]. Nictation behaviour has been also described in the animal-parasitic nematodes Heligmosomoides polygyros [17] and Strongyloides ratti [18]. By contrast, foraging strategies in the Diplogastridae, which are often associated with insects but do not necessarily parasitize them, have been poorly investigated. In this context, multiple questions can be addressed: do non-parasitic insect-associated nematodes show cruiser and/or ambusher behaviour? Is there a nictation-like behaviour? Such questions could best be addressed using laboratory model species in which behavioural assays could be complemented with genetic analyses.
Nematodes of the genus Pristionchus have a necromenic association with scarab beetles, in which arrested dauer-stage nematodes invade the insect, wait for the host to die and then resume development by feeding on growing micro-organisms on the carcass [19,20]. Pristionchus pacificus, a satellite model nematode for evolutionary and developmental studies, is known to live in association with scarab beetles in nature [21,22].
The P. pacificus community has up-to-date genetic and genomic tools available as well as transgenic techniques and the nematode is also amenable to studies of behaviour and neurobiology owing to the relative simplicity and detailed description of its nervous system (D. Bumbarger & R. J. Sommer 2011, personal communication). Therefore, the features of P. pacificus allow us to address the question of whether nictation behaviour provides a selective advantage for nematode–host associations.
Molecular and morphological similarities support the hypothesis that the infective juvenile stage of parasitic nematodes is homologous to the dauer stage of non-parasitic nematodes, such as Pristionchus [23–26]. Harsh environmental conditions, such as high temperature, low food availability and high population density, induce many non-parasitic nematodes to develop into an alternative developmental juvenile stage referred to as ‘dauer’. The dauer stage of Pristionchus species is responsible for host finding and attachment to host [20], and it is the only stage of development in which nictation occurs.
Nictation is proposed to provide a selective advantage that allows dauer juveniles to attach to passing hosts. On this basis, nictation could serve as an adaptation for the dauer juveniles to make contact with a host, in most cases an insect, for transportation, i.e. a phoretic interaction [27]. A consequence of a phoretic interaction is the permanent adherence of the dauer to the cuticle of its host, and in some cases cuticle penetration into body cavities of the host [28]. Therefore, phoresis has been suggested to serve as a pre-adaptation for the evolution of parasitism [9,23,24,26].
Herein by the use of an experimental set-up with P. pacificus strains, we tested the hypothesis that nictation behaviour favours the ability to attach to its beetle host with recent isolated and laboratory-generated mutant strains. Using forward genetics, we screened for mutants lacking nictation behaviour in P. pacificus. We isolated two mutants that can develop into proper dauers but are nictation-defective. We compared these two mutant strains with recently isolated strains from la Réunion island, with variable nictation rates, to test their ability to attach to artificial hosts under laboratory conditions.
2. Material and methods
(a). Nematode culture
Breeding and maintenance of P. pacificus follow Caenorhabditis elegans standard culture methods that have been described previously [29,30]. Nematode strains used in this study were: (i) P. pacificus reference PS312 strain; (ii) P. pacificus RS5401 and RS5386, which are recent isolates from isogenic lines of Oryctes borbonicus scarab beetles or from soil samples from la Réunion island as described by Herrmann et al. [31]; and (iii) mutagenized P. pacificus tu426 and tu427, which are nictation-defective strains isolated using a classic forward genetics approach [32]. In this study, we adopt the definition of strain described in Herrmann et al. [19]. Strain stocks were maintained on NGM plates with Escherichia coli OP50 lawns [29,30]. All P. pacificus strains used in this study are available upon request.
To generate dauers, we performed dauer inductions using the ‘wet-plate method’. We resuspended three 6 cm NGM plates with fully grown mixed-stage worms into 1 ml of OP50 liquid medium and added it onto 10 cm NGM plates. Worms were grown at 20–25°C for approximately 14 days or until a sufficient number of dauers were found on the plate (A. Weller, 2009, personal communication). Timing of worms to dauer formation was investigated in P. pacificus PS312 and the two nictation-defective mutants tu426 and tu427. We scored total hours necessary to reach the highest amount of dauers in the dauer induction plates.
Steinernema feltiae, Steinernema carpocapsae and P. hermaphrodita were supplied by Becker Underwood, UK. Dauer juveniles of S. feltiae and S. carpocapsae were cultured following Kaya & Stock [33]. Briefly, approximately 1000 S. feltiae or S. carpocapsae dauer juveniles were added to moistened Whatman filter paper in a 10 cm Petri dish and 5 to 10 Galleria mellonella were added. Plates were sealed with Parafilm and incubated at room temperature for 4 days. Once dead, then G. mellonella were transferred to White traps and dauers collected in the surrounding water. Nematodes were washed three times in M9 buffer before use and were stored at 4°C in 200 ml tissue culture flasks. Phasmarhabditis hermaphrodita (Nemaslug) were mixed with tap water (1 g in 100 ml) and stored similarly to Steinernema spp.
(b). Nictation and attachment tests
Nictation test arenas consisted of 6 cm NGM plates sparsely covered with sterile sand grains evenly spread with a shaker. Either 1000 or 5000 dauers, previously washed and resuspended in distilled water, were applied to the centre of the Petri dish and left to dry. The dishes were left covered (to preserve environmental conditions within the plate) at room temperature and nictation was recorded after the first hour and every 12 h thereafter for a period of 5 days. We used the first time point (1 h) to test for nictation differences between P. pacificus strains. To obtain the proportion of nictating dauers and dauers attached to host, we scored nictation activity for individual dauers that lifted their body off the substrate. For the host attachment studies, a single host was applied onto the plate already containing dauers and sand, prepared as described above. In two independent assays, we used 1000 and 5000 dauers, respectively, per plate and placed one G. mellonella moth larva (commercially available from HW Terra, Germany) on each plate; i.e. each replicate consisted of either 1000 or 5000 dauers/host/plate for each assay. All replicates contained the same stage, and the same size hosts. Nematodes were exposed to the host for a 2 hour period in covered plates. The hosts were removed, dissected and resuspended in water to allow release of dauers from the carcass. We counted the number of resuspended dauers.
(c). Mutant screen
We screened for nictation-defective mutant strains using EMS mutagenesis [32] in P. pacificus PS312. We screened approximately 350 gametes (1300 homozygous F2 lines) in two mutagenic screens over a six-month period. We isolated homozygous F2 single worm clones in 96-well plates each containing 40 µl OP50 solution per well and allowed the generation of enough dauers (modified wet-plate method for large-scale screenings). We screened for nictation depletion after approximately 14 days in 30 µl containing more than 300 dauers per well; the remaining 5–10 µl was used to recover the homozygous lines. Candidate strains were confirmed by multiple searches for the defective phenotype in at least three independent dauer inductions (with 6000–60 000 dauers per induction) using the wet-plate method described above.
(d). Nictation and attachment calculations
We counted the total number of dauers in nictation from n = 1000 dauers, and calculated means and standard errors from three to 12 independent replicates. Standard errors of the mean were corrected for small sample size (n < 20) [34]. We tested the normality of our data using the Shapiro–Wilk test. We performed an ANOVA between the groups, and a Tukey's honestly significant difference (HSD) test to assess significant differences in nictation between the strains. To assess for differences in the time to dauer formation between P. pacificus PS312 and the mutant strains, we used a non-parametric Mann–Whitney U-test because some of the data did not follow a normal distribution.
To study the relationship between nictation and attachment, we used multiple replicates of single assays. We calculated the number of dauers in nictation on each plate immediately before host exposure and the number of dauers on the host 2 h later. We tested nictation and attachment to host relationship in two independent assays, with two replicates of 1000 dauers and three replicates of 5000 dauers, respectively. The correlation coefficient (r) and coefficient of determination (r2) were calculated. ANOVAs and Tukey's HSD tests were also performed on these data.
3. Results
(a). Morphological characterization of Pristionchus pacificus dauer juveniles
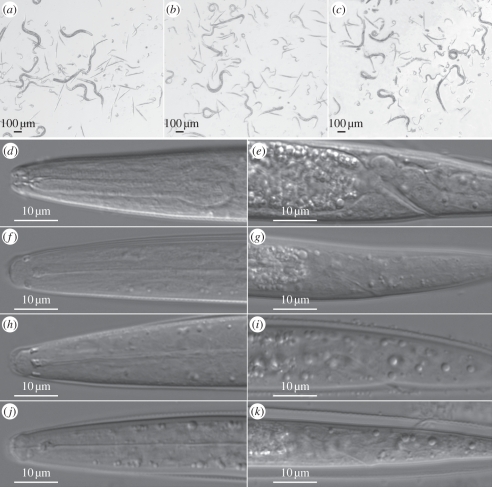
The dauer stage is an alternative developmental stage to the J3 stage of directly developing nematodes (figure 1). Under stressful conditions or high densities, P. pacificus juveniles enter the dauer stage (figure 1a). Specialized morphological and physiological characteristics, such as high lipid storage, strong and impermeable cuticle, no food intake and extended lifespan, among others, develop in the dauers for endurance (table 1). Several characteristics distinguish arrested dauer juveniles from the active J3 juveniles (table 1 and figure 1d–k). Specifically, P. pacificus (PS312) dauers possess thin bodies and a dark intestine, which indicates their non-functional gut (figure 1f,g and table 1). Dauers contain a constricted pharynx and a closed oral orifice with an internal plug (figure 1f,h,j and table 1) and closed anus (figure 1g). Dauers also show a specialized cuticle and a stereotypical gonadal arrest with fewer germ cells than J3 juveniles (not shown) as has been previously described for C. elegans (table 1) [47–49]. The mid-body region of dauers is characterized by a high density of lipid droplets, which is absent in the J3 stage.
Figure 1.
Pristionchus pacificus dauer juveniles after 15 days of induction in liquid culture: (a) PS312 strain, (b) tu426 strain, and (c) tu427 strain. Partial dauers are observed only in the nictation-defective mutants: (b) tu426 and (c) tu427. A dorsal view of the head region shows closed oral orifices and constricted pharynges of (f) PS312, (h) tu426, and (j) tu427 dauer juveniles when compared with reference PS312 third juvenile stage (d). Lateral view of PS312 third juvenile stage with an open anus is shown (e). By contrast, lateral views of strains PS312 (g), tu426 (i), tu427 (k) display a closed anus of dauer juveniles. The dauer juvenile stage (f–k) shows higher amounts of lipid droplets throughout the body than the third juvenile stage (d,e).
Table 1.
Dauer characteristics in P. pacificus. Most of these characteristics have been described previously (see references); differences found in C. elegans are shown in bold.
| category | dauer characteristic | references |
|---|---|---|
| (a) morphology | thin and dense body; axial ratio for P. pacificus is 16:1 (length:width), for C. elegans it is 30:1. | [35–37] |
| remodelled foregut pharynx | [38] | |
| dark sealed intestine, generally darker than corresponding J3 stage, or L2 for C. elegans | [35–37] | |
| closed mouth and constricted pharynx | [35–37,39] | |
| gonadal arrest | [35–37] | |
| strengthened, specialized cuticle with lateral ridges: peripheral ridges become more pronounced in P. pacificus, whereas conspicuous lateral alae become visible for C. elegans | [35–37,39] | |
| fat bodies in intestinal and hypodermal cells | [40] | |
| remodelled neurons in C. elegans; has not been characterized for P. pacificus | [41] | |
| (b) physiology | developmental arrest | [42] |
| increase in lifespan reduced metabolic activity and dependence on internal energy storage; work in progress for P. pacificus (M. Mayer, A. Ogawa & R. Sommer, 2009, personal communication) | [37,43,44] | |
| resistance to environmental stress heat, cold, desiccation, oxidative stress and detergents such as SDS in C. elegans; has not been tested for P. pacificus | [35–37,43–46] | |
| (c) behaviour | lethargic needle-like pose with reduced activity | [35–37] |
| nictation | [35] |
(b). Nictation behaviour in Pristionchus pacificus dauers and other nematode species
We induced nictation behaviour in dauers by adding sand grains to the substrate of the worms. As previously described for other nematode species [8], P. pacificus dauers lift all of the body off the substrate except the posterior tip. The nematodes keep a primarily straight posture (figure 2a) and occasionally wave their bodies in three-dimensional spirals and loops (electronic supplementary material, movie S1). Thus, P. pacificus dauer juveniles show a nictation behaviour typical of other nematodes.
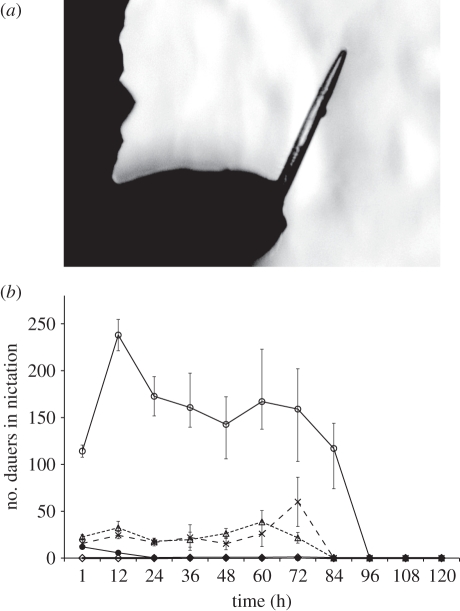
Figure 2.
Nictation in P. pacificus. (a) A representative dauer in nictation. Its posterior end remains attached to the substrate, whereas its anterior stands erect with occasional waving. Note a lipid droplet often observed on the side of the pharynx region of dauers in nictation. (b) Number of dauers in nictation in populations (n = 1000) of two entomopathogenic Steinernema species (S. feltiae (dashed lines with triangles) and S. carpocapsae (dashed lines with crosses)), the slug parasitic P. hermaphrodita (double line with rhombi), and two P. pacificus strains (PS312 (solid lines with filled circles) and RS5386 (solid lines with open circles)). Error bars represent standard errors of the mean (s.e.m.) corrected for small sample size n < 20.
We scored nictation and compared P. pacificus with other nematodes species (figure 2b). Initially (after 1 h), the free-living P. pacificus (PS312) exhibited nictation behaviours similar to that of the entomopathogenic nematodes S. carpocapsae and S. feltiae. Species began to exhibit differences after 12 h. Pristionchus pacificus PS312 started at around 15 dauers in nictation, and dropped to nearly zero after 24 h (figure 2b). The entomopathogenic S. carpocapsae and S. feltiae maintained 20–30 dauers in nictation until 36 h. Interestingly, S. carpocapsae reached a peak of nictation of around 60 dauers at 72 h, whereas S. feltiae reached a peak of around 40 dauers at 60 h (figure 2b). The slug parasitic P. hermaphrodita did not show any nictation throughout the whole period. By contrast, only the recently isolated P. pacificus strain RS5386 showed a higher proportion of nictation from the beginning and up to 96 h after-sand addition. Over 100 P. pacificus RS5386 dauers displayed nictation after 1 h, followed by a sharp increase at 12 h (over 200 dauers in nictation), followed by a drop to ca 150 dauers after 24 h, which persisted up to 4 days post-sand addition.
(c). Nictation-defective Pristionchus pacificus mutant strains
To test the hypothesis that nictation behaviour affects attachment abilities for host association, we used forward genetics to generate strains lacking this behaviour in the P. pacificus PS312 strain. In total, we isolated 11 nictation-defective mutants. Most of these mutant strains showed dauer entry defects, referred to as ‘partial dauers’ (table 2) [42,49,50]. To reduce the probability that severe anatomical defects are the cause of nictation-defective phenotypes, we compared the morphological characteristics of dauer juveniles between mutants and reference animals (table 2 and figure 1). We focused on conspicuous morphological characteristics (table 1) that define the dauer stage by assigning a similarity score to reference dauers, ranging from low to high (1–3) (table 2). We used the nictation-defective mutants with the highest similarity index to reference dauers for the rest of the study, i.e. tu427 (score of 20) and tu426 (score of 21). The nictation-defective mutants tu426 (figure 1b,h,i) and tu427 (figure 1c,j,k) showed all dauer characteristics described for P. pacificus PS312 (figure 1a,f,g and electronic supplementary material, tables S1 and S2). Specifically, the oral orifices are closed and contain an internal plug [48] and their pharynges are constricted (figure 1f,h,j) [49]. In all three strains, the intestine is reduced and the mid-body region is characterized by a high density of lipid droplets. In figure 1g,i,k, the closed anus of the dauers is shown.
Table 2.
Resemblance of dauer characteristics in nictation-depleted P. pacificus mutants to reference PS312 dauers: 1, low; 2, medium; 3, high. Most dauer-like mutants (highest similarity index) are shown in bold.
| dauer characteristics | tu427 | tu428 | tu429 | tu430 | tu431 | tu432 | tu433 | tu426 | tu434 | tu435 | tu436 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| dark body | 3 | 1 | 3 | 3 | 2 | 2 | 3 | 3 | 1 | 3 | 2 |
| thin body | 3 | 1 | 3 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | 2 |
| constricted pharynx (does not pump) | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 |
| thin dark intestine | 3 | 1 | 3 | 2 | 3 | 3 | 2 | 3 | 2 | 2 | 2 |
| lipid droplet accumulation | 3 | 1 | 2 | 2 | 3 | 3 | 3 | 3 | 2 | 3 | 3 |
| gonad arrest | 3 | 1 | 2 | 2 | 3 | 3 | 2 | 3 | 2 | 3 | 2 |
| absence of intermediate or partial dauers | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 |
| similarity index (total) | 20 | 7 | 18 | 15 | 17 | 17 | 16 | 21 | 12 | 17 | 15 |
We observed an asynchrony in dauer formation between the reference and nictation-defective mutant strains (electronic supplementary material, figure S1). Pristionchus pacificus PS312 developed dauer juveniles approximately 120 h after eggs laying begins, whereas tu427 mutant animals developed dauer juveniles after 125 h. The tu426 mutant animals were significantly slower (Mann–Whitney test, U = 2967.5, tu426 n = 53, PS312 n = 56, p < 0.001 two-tailed) in developing dauers; they spent more than 200 h in liquid culture before dauers appeared (electronic supplementary material, figure S1). Age differences observed in dauer formation between the different strains have not been shown to affect nictation behaviour. Previous observations in our laboratory comparing same dauer strains 12 or 25 days after induction showed no relevant differences in nictation numbers (data not shown).
(d). Relationship between nictation in Pristionchus pacificus strains and attachment to the laboratory host Galleria mellonella
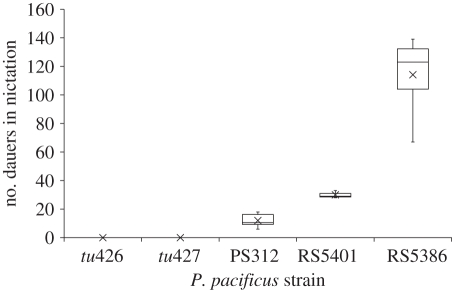
To resolve the role of nictation in P. pacificus, we measured nictation rates of different strains and studied their attachment abilities to the larva of the moth G. mellonella (figures 3 and 4). The two mutant strains, tu427 and tu426, show a complete absence of nictation during the nictation induction assays (figures 3 and 4). By contrast, one recent isolate from la Réunion island, which was kept in the laboratory for less than six months, showed a significantly higher nictation rate than the reference strain PS312 (ANOVA: F = 142.17, d.f. = 4, p < 0.0001; Tukey's HSD test: p < 0.01; electronic supplementary material, tables S1 and S3). RS5386 exhibits a two- to 10-fold higher nictation rate than the reference strain PS312 (figures 3 and 4). RS5401 showed a slightly higher nictation rate than PS312 (Tukey's HSD test: p < 0.05; table 3).
Figure 3.
Number of dauers in nictation in populations (n = 1000) of P. pacificus nictation-defective mutant strains tu426 and tu427, the reference laboratory strain PS312 and two strains from la Réunion island: RS5401 and RS5386. Maximum and minimum values are shown by whiskers, upper (75%) and lower (25%) quartiles are shown by the upper and lower limits of each box, medians and means are shown by the straight line and cross within each box, respectively.
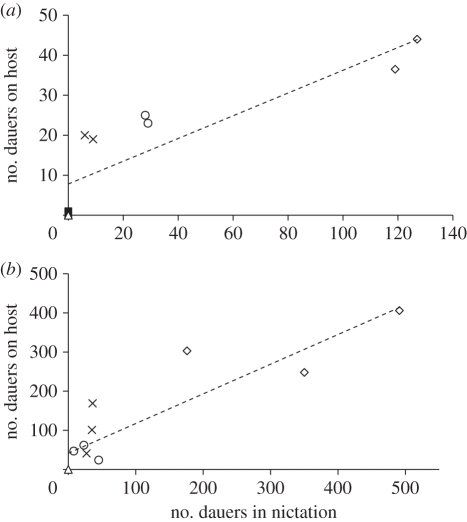
Figure 4.
Relationship between nictation and attachment to the laboratory host G. mellonella for two nictation-defective mutant strains (tu426 in solid squares, and tu427 in open triangles), the reference laboratory strain (PS312 shown in crosses) and two recently isolated strains of la Réunion island (RS5401 in open circles, and RS5386 in open rhombi) in two different dauer concentrations assays: (a) total dauers on plate n = 1000. Correlation coefficient r = 0.87, and coefficient of determination r2 = 0.76. (b) Total dauers on plate n = 5000. Correlation coefficient r = 0.90, and coefficient of determination r2 = 0.80.
Table 3.
Pairwise comparisons of nictation in P. pacificus strains using Tukey's honestly significant difference (HSD) test (cf. electronic supplementary material, table S1 and figure S3). n.s., non-significant differences.
| strain A | strain B | p |
|---|---|---|
| tu426 | tu427 | n.s. |
| tu426 | PS312 | n.s. |
| tu426 | RS5401 | <0.01 |
| tu426 | RS5386 | <0.01 |
| tu427 | PS312 | n.s. |
| tu427 | RS5401 | <0.01 |
| tu427 | RS5386 | <0.01 |
| PS312 | RS5401 | <0.05 |
| PS312 | RS5386 | <0.01 |
| RS5401 | RS5386 | <0.01 |
We found a proportional increase in the attachment to the host, which is directly related to the increase in nictation behaviour previously recorded for the different strains. The ability to attach to hosts increases dramatically when P. pacificus strains are able to nictate, as shown for reference and la Réunion strains (figure 4). In a sample containing 1000 dauers, we found absence of dauers attached to the hosts after 2 h of exposure for tu427 and tu426 (figure 4a). By contrast, we found approximately 20 P. pacificus PS312 dauer juveniles on host grubs after a similar exposure (figure 4a). The strains from la Réunion, RS5401 and RS5386, showed a relative increase in average attachment. However, despite the generally higher attachment of RS5401 to host grubs, this increase is not significantly higher than that of PS312 (ANOVA: F = 106.25, d.f. = 4, p < 0.0001; Tukey's HSD test: p > 0.05 (non-significant); figure 4a and electronic supplementary material, tables S2 and S4). By contrast, RS5386 showed nearly 40 worms attached to host and showed significantly higher numbers than the reference strain PS312, which only showed about 20 worms attached to the host (Tukey's HSD test: p < 0.01; figure 4a and electronic supplementary material, tables S2 and S4). The two nictation-defective mutant strains showed no significant difference in attachment ability when compared with each other (Tukey's HSD test: p > 0.05 (non-significant); figure 4a and table 4). In summary, a positive correlation (r = 0.87, r2 = 0.76, p ≤ 0.001) was found between nictation and attachment to hosts in P. pacificus strains.
Table 4.
Pairwise comparisons of nictation (bold) and attachment (italics) results of P. pacificus strains at different densities (1000 and 5000 worms in single assays) using Tukey's HSD test (cf. electronic supplementary material, table S2 and figure S4). n.s., non-significant differences.
| 1000 dauers | tu426 | tu427 | PS312 | RS5401 | RS5386 |
|---|---|---|---|---|---|
| tu426 | — | n.s. | p < 0.01 | p < 0.01 | p < 0.01 |
| tu427 | n.s. | — | p < 0.01 | p < 0.01 | p < 0.01 |
| PS312 | n.s. | n.s. | — | n.s. | p < 0.01 |
| RS5401 | p < 0.01 | p < 0.01 | p < 0.01 | — | p < 0.01 |
| RS5386 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | — |
| 5000 dauers | tu426 | tu427 | PS312 | RS5401 | RS5386 |
| tu426 | — | — | — | — | — |
| tu427 | — | — | n.s. | n.s. | p < 0.01 |
| PS312 | — | n.s. | — | n.s. | p < 0.01 |
| RS5401 | — | n.s. | n.s. | — | p < 0.01 |
| RS5386 | — | p < 0.01 | p < 0.01 | p < 0.01 | — |
To test whether population density had any effect on nictation or attachment to insect hosts, we repeated these experiments with a fivefold higher initial number of dauers (n = 5000 dauers) (figure 4b and electronic supplementary material, tables S2 and S4). We found that the relationship was maintained with relationship values that were almost identical (r = 0.9, r2 = 0.8, p < 0.001). We conclude that density does not play a role in the positive relationship observed between nictation and host attachment. Taken together, we found a high positive correlation between the number of worms in nictation and the number of worms attached to host moth larvae. Coefficient of determination (r2) and correlation coefficient (r) of nictation and attachment approach a value of 0.8 and 0.9, respectively, suggesting a relationship between both variables (figure 4). Thus, populations of nematodes with higher proportions of nictation are found to be more effective at attaching to mobile insects.
4. Discussion
(a). Use of mutant strains in combination with wild strains to study behavioural phenotypes in laboratory settings
We took advantage of laboratory tools to combine, for the first time, mutagenesis-generated strains defective in a specific behaviour with recently isolated wild strains showing variable degrees of the specific behaviour. Classical forward genetic screens in C. elegans have proved to be a powerful tool for studying behaviours relevant to the species survival, such as mechanosensation mutants [51], chemosensation mutants [52], thermotaxis [53] and egg laying [54]. Populations of P. pacificus strains used in our study show a gradient in the proportions of nictation of each strain, which range from complete disruption of nictation in tu427 and tu426 mutants to high nictation in RS5386. Such a gradient of mutants, together with recently isolated strains, is required to correlate this behaviour to a particular function, such as host attachment. The low gamete number required to generate the nictation-defective strains tu427 and tu426 in our mutant screen shows that this behaviour may be complex and regulated by multiple genetic loci.
Nictation is absent in all stages of development, except in dauers; therefore programmes that act upstream of dauer formation, such as dauer induction or entry, may also affect behavioural phenotypes of the dauer. Presence of partial dauers in our nictation-defective mutants (table 2) suggests that nictation in some of our mutants may be affected owing to dauer formation defects. Previous studies in C. elegans of dauer formation mutants (daf), such as daf-2, have shown that these genes act independently on different aspects of development, such as entry and exit to dauer, intestinal pigmentation and reproduction [42]. Furthermore, previous studies have reported partial or intermediate dauer formation by mutations in daf-9, daf-15, daf-16, daf-18, daf-20, daf-12 and unmapped sy5315 X-linked mutation [49,55–58]. It remains to be tested whether any of these mutants also show defects in nictation behaviour. Pristionchus pacificus nictation-defective mutant strains that form partial dauers might contain mutations in the orthologues of any of the formerly mentioned genes. Further experiments are necessary to determine how genes that regulate dauer entry also affect behaviour.
In our study, we isolated and characterized mutants with a complete absence of nictation behaviour, but we did not screen for mutants with defects in specific individual steps and events of nictation. Nictation most probably consists of a number of individual events that are controlled by independent regulatory genetic units. Such regulatory units might in turn have different effects on host finding and/or attachment. Other behaviours in C. elegans have been observed to have different sensitivities. Mutant strains such as unc-97 (uncoordinated) and mec-3 (mechanosensation defective) showed different degrees of sensitivities in behavioural mechanosensory responses [58]; whereas male mating behaviour also contained multiple independent sub-behaviours controlled by different neuronal and genetic inputs [59,60]. For example, it is known that vulva location by the males is mediated by the neurons HOA and HOB, and that the genes lov-1 and possibly klp-6 and pkd-2 mediate these responses [59,60]. Therefore, nictation may also be divided into sub-behaviours regulated independently as described for previous behaviours.
(b). Nictation behaviour is relevant for host finding
The evolution of parasitism involves a series of events, including an initial association with a host. Previous comparisons of nictating species and insect associations of different entomopathogenic nematodes suggested that nictation may provide a higher chance of contact with a host owing to a higher body surface area exposure to the transitory insect [9,13,61]. Comparative studies in Caenorhabditis species also suggested that nictation may be associated with the attachment of transitory animals [47,62]. However, few studies so far have investigated host finding or nictation behaviour in the diplogastrids [6,63], although these nematodes often show specific insect associations. Pristionchus pacificus is a necromenic nematode, i.e. uses its dead host as a source of food, and shows a life history tightly associated with beetles [19,20]. Previous comparisons of nictation in different nematode species suggested a relationship to host attachment, but the present study is the first to show a relationship of the evolutionary history of foraging behaviour in nematodes at the population level by the use of different P. pacificus strains.
Many aspects of nematode host finding are still unclear. Nematodes perceive their environment primarily by chemosensation, thermosensation and mechanosensation. Rhabditid nematodes are commonly described as ‘cruisers’ if they spend most of their time crawling and searching for resource-associated cues, such as insect host chemicals. The slug parasitic nematode P. hermaphrodita shows minimal nictation in our study, as has been previously reported [13], and may therefore apply a cruiser strategy. In P. pacificus, interception of the chemical communication system of the insect is likely to be involved in host preferences [64]. ‘Ambushers’ are instead more sedentary. It was initially assumed that ambush foragers were not as responsive to chemical cues as cruise foragers. However, it has since become apparent that they do respond to chemical cues, although their response is fundamentally different from cruise foragers [65]. Steinernema species, both cruiser and ambusher, respond strongly to volatile cues [66]. Our experiments show that Pristionchus species first have the ability to recognize and move towards host-associated volatiles by chemotaxis, which typically applies to a cruiser strategy [22,64]. Second, P. pacificus show nictation behaviour that applies to a typical ambusher behaviour as well. For other ambush foragers, stimuli from the environment have been demonstrated to be important for host finding [67], and environmental cues are used to assess patch quality [68,69] and select ambush sites [70,71]. Therefore, we propose that P. pacificus dauers may also have the ability to scan the surrounding environment, as shown for some Steinernema species [11]. We speculate that the differences in the variability observed within each strain may be a consequence of environmental differences across replicates and unidentified strain-specific traits related to host attachment, e.g. host sensing. It should be noted, however, that other differences between the genotypes/strains also affect these traits. Furthermore, we propose that nictation behaviour may also facilitate scanning and detecting host-associated cues by the dauer, such as volatile chemicals [12].
In conclusion, we provide evidence at the intraspecific level that nictation is associated with attachment. It is tempting to speculate that nictation or nictation-like host finding behaviours are crucial during the initial steps of the evolution of parasitism. The specificity of this behaviour to the host-finding stage of nematodes, both in parasitic and non-parasitic species, reveals the relevance of nictation to understanding the origins of parasitism. Future studies should aim to understand the genetic and sensory regulation of this behaviour.
Acknowledgements
We thank Dr Robbie Rae for the S. carpocapsae dauers and helpful discussions. We thank Dr Akira Ogawa for ideas and suggestions and Dr Dan Bumbarger for discussion. We are grateful to Metta Riebesell for help on the figures and movies; Simone Kienle for providing the ‘high nictators’; and Andreas Weller for help on initial beetle attachment experiments, as well as for help on the ‘wet-plate’ dauer induction protocol. We thank Dr Erik Ragsdale for comments on this manuscript. We are grateful to Dr Andrew Crawford at the U. de los Andes for assistance on the statistics, and Dr Andrew Brown at Becker Underwood, UK, for supplying Steinernema and Phasmarhabditis nematodes. Thanks to Mónica Rodriguez and Diego Rubio for proof-reading the manuscript. Funds for research came from the Max Planck Society, and a three month DAAD Study and Research Fellowship granted to F.D.B.
References
- 1.Croll N. A. 1970. Sensory basis of activation in nematodes. Exp. Parasitol. 27, 350–356 10.1016/0014-4894(70)90038-X (doi:10.1016/0014-4894(70)90038-X) [DOI] [PubMed] [Google Scholar]
- 2.Dusenbery D. B. 1980. Responses of the nematode Caenorhabditis elegans to controlled chemical stimulation. J. Compar. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 136, 327–331 10.1007/BF00657352 (doi:10.1007/BF00657352) [DOI] [Google Scholar]
- 3.Pianka E. R. 1966. Convexity, desert lizards, and spatial heterogeneity. Ecology 47, 1055–1059 10.2307/1935656 (doi:10.2307/1935656) [DOI] [Google Scholar]
- 4.Schoener T. W. 1971. Theory of feeding strategies. Annu. Rev. Ecol. Syst. 2, 369–404 10.1146/annurev.es.02.110171.002101 (doi:10.1146/annurev.es.02.110171.002101) [DOI] [Google Scholar]
- 5.Campbell J. F., Gaugler R. 1997. Inter-specific variation in entomopathogenic nematode foraging strategy: dichotomy or variation along a continuum? Fundam. Appl. Nematol. 20, 393–398 [Google Scholar]
- 6.Völk J. 1950. Die Nematoden der Regenwürmer und aasbesuchenden Käfer. Zoologische Jahrbücher (Abteilung für Systematik), 79, 1–70 [Google Scholar]
- 7.Croll N. A., Matthews B. E. 1977. Survival of nematodes. In Biology of nematodes, pp. 152–165 New York, NY: Wiley [Google Scholar]
- 8.Ishibashi N., Kondo E. 1990. Behaviour of infective juveniles. In Entomopathogenic nematodes in biological control, pp. 139–150 Boca Raton, FL: CRC Press [Google Scholar]
- 9.Campbell J. F., Gaugler R. 1993. Nictation behaviour and its ecological implications in the host search strategies of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae). Behaviour 126, 155–169 10.1163/156853993X00092 (doi:10.1163/156853993X00092) [DOI] [Google Scholar]
- 10.Campbell J. F., Kaya H. K. 1999. How and why a parasitic nematode jumps. Nature 397, 485–486 10.1038/17254 (doi:10.1038/17254) [DOI] [Google Scholar]
- 11.Campbell J. F., Kaya H. K. 1999. Mechanism, kinematic performance, and fitness consequences of jumping behavior in entomopathogenic nematodes (Steinernema spp.). Can. J. Zool. 77, 1947–1955 10.1139/cjz-77-12-1947 (doi:10.1139/cjz-77-12-1947) [DOI] [Google Scholar]
- 12.Campbell J. F., Kaya H. K. 2000. Influence of insect associated cues on the jumping behavior of entomopathogenic nematodes (Steinernema spp.). Behaviour 137, 591–609 10.1163/156853900502231 (doi:10.1163/156853900502231) [DOI] [Google Scholar]
- 13.Kruitbos L. M., Heritage S., Hapca S., Wilson M. J. 2009. Influence of substrate on the body-waving behaviour of nematodes. Nematology 11, 917–925 10.1163/156854109X443433 (doi:10.1163/156854109X443433) [DOI] [Google Scholar]
- 14.Kruitbos L. M., Wilson M. J. 2010. Is it time to ‘wave’ goodbye to ‘nictating’ nematodes? Nematology 2, 309–310 10.1163/138855409X12506855979794 (doi:10.1163/138855409X12506855979794) [DOI] [Google Scholar]
- 15.Reed E. M., Wallace H. R. 1965. Leaping locomotion by an insect-parasitic nematode. Nature 206, 210–211 10.1038/206210a0 (doi:10.1038/206210a0) [DOI] [Google Scholar]
- 16.Rae R., Robertson J. F., Wilson M. J. 2006. The chemotactic response of Phasmarhabditis hermaphrodita (Nematoda: Rhabditida) to cues of Deroceras reticulatum (Mollusca: Gastropoda). Nematology 8, 197–200 10.1163/156854106777998746 (doi:10.1163/156854106777998746) [DOI] [Google Scholar]
- 17.Hernandez A. D., Sukdeo M. V. K. 1995. Host grooming and the transmission strategy of Heligmosomoides polygyros. J. Parasitol. 81, 865–869 10.2307/3284031 (doi:10.2307/3284031) [DOI] [PubMed] [Google Scholar]
- 18.Viney M., Lok J. B. 2007. Strongyloides spp. WormBook. 10.1895/wormbook.1.141.1 (doi:10.1895/wormbook.1.141.1) [DOI] [Google Scholar]
- 19.Herrmann M., Mayer W. E., Sommer R. J. 2006. Nematodes of the genus Pristionchus are closely associated with scarab beetles and the Colorado potato beetle in Western Europe. Zoology (Jena) 109, 96–108 10.1016/j.zool.2006.03.001 (doi:10.1016/j.zool.2006.03.001) [DOI] [PubMed] [Google Scholar]
- 20.Weller A. M., Mayer W. E., Rae R., Sommer R. J. 2010. Quantitative assessment of the nematode fauna present on Geotrupes dung beetles reveals species-rich communities with a heterogeneous distribution. J. Parasitol. 96, 525–531 10.1645/GE-2319.1 (doi:10.1645/GE-2319.1) [DOI] [PubMed] [Google Scholar]
- 21.Hong R. L., Sommer R. J. 2006. Pristionchus pacificus: a well-rounded nematode. Bioessays 28, 651–659 10.1002/bies.20404 (doi:10.1002/bies.20404) [DOI] [PubMed] [Google Scholar]
- 22.Herrmann M., Mayer W. E., Hong R. L., Kienle S., Minasaki R., Sommer R. J. 2007. The nematode Pristionchus pacificus (Nematoda: Diplogastridae) is associated with the oriental beetle Exomala orientalis (Coleoptera: Scarabaeidae) in Japan. Zool. Sci. 24, 883–889 10.2108/zsj.24.883 (doi:10.2108/zsj.24.883) [DOI] [PubMed] [Google Scholar]
- 23.Osche G. 1956. Die Präadaptation freilebender Nematoden an den Parasitismus. Zool. Anz., 19, 391–396 [Google Scholar]
- 24.Sudhaus W. 2008. Evolution of insect parasitism in Rhabditid and Diplogastrid nematodes (eds Makarov S. E., Dimitrijevic R. N.). Belgrade: Institute of Zoology and SASA, Sofia: BAS and UNESCO MAB, Vienna: Faculty of Life Sciences [Google Scholar]
- 25.Ogawa A., Streit A., Antebi A., Sommer R. 2009. A conserved endocrine mechanism controls the formation of dauer and infective larvae in nematodes. Curr. Biol. 19, 67–71 10.1016/j.cub.2008.11.063 (doi:10.1016/j.cub.2008.11.063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dieterich C., Sommer R. J. 2009. How to become a parasite—lessons from the genomes of nematodes. Trends Genet. 25, 203–209 10.1016/j.tig.2009.03.006 (doi:10.1016/j.tig.2009.03.006) [DOI] [PubMed] [Google Scholar]
- 27.Kiontke K., Sudhaus W. 2006. Ecology of Caenorhabditis species. WormBook. 10.1895/wormbook.1.37.1 (doi:10.1895/wormbook.1.37.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee D. L. 2002. Life cycles. In The biology of nematodes, pp. 61–72 London, UK: Taylor & Francis [Google Scholar]
- 29.Brenner S. 1974. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sommer R. J., Sternberg P. W. 1996. Apoptosis and change of competence limit the size of the vulva equivalence group in Pristionchus pacificus: a genetic analysis. Curr. Biol. 6, 52–59 10.1016/S0960-9822(02)00421-9 (doi:10.1016/S0960-9822(02)00421-9) [DOI] [PubMed] [Google Scholar]
- 31.Herrmann M., Kienle S., Rochat J., Mayer W. E., Sommer R. J. 2010. Haplotype diversity of the nematode Pristionchus pacificus on Reunion in the Indian Ocean suggests multiple independent invasions. Biol. J. Linn. Soc. 100, 170–179 10.1111/j.1095-8312.2010.01410.x (doi:10.1111/j.1095-8312.2010.01410.x) [DOI] [Google Scholar]
- 32.Pires da Silva A. 2006. Pristionchus pacificus genetic protocols. WormBook. 10.1895/wormbook.1.114.1 (doi:10.1895/wormbook.1.114.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaya H. K., Stock P. S. 1997. Techniques in insect nematology. In Manual of techniques in insect pathology, pp. 281–322 New York, NY: Academic Press [Google Scholar]
- 34.Sokal R. R. 1981. Biometry: the principles and practice of statistics in biological research, 2nd ed. New York: W.H. Freeman & Co [Google Scholar]
- 35.Cassada R. C., Russell R. L. 1975. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46, 326–342 10.1016/0012-1606(75)90109-8 (doi:10.1016/0012-1606(75)90109-8) [DOI] [PubMed] [Google Scholar]
- 36.Riddle D. L. 1988. The dauer larva. In The nematode Caenorhabditis elegans (ed. Wood W. B.), pp. 393–412 Cold Spring Harbor, NY: Cold Spring Harbor Laboratory [Google Scholar]
- 37.Riddle D. L., Albert P. S. 1997. Genetic and Environmental Regulation of Dauer Larva Development. (eds Riddle D. L., Blumenthal T., Meyer B. J., Priess J. R.), pp. 739–768 Cold Spring Harbor, New York: Cold Spring Harbor Laboratory; 10.1101/087969532.33.739 (doi:10.1101/087969532.33.739). [DOI] [PubMed] [Google Scholar]
- 38.Ao W., Gaudet J., Kent W. J., Muttumu S., Mango S. E. 2004. Environmentally Induced Foregut Remodeling by PHA-4/FoxA and DAF-12/NHR. Science 305, 1743–1746 10.1126/science.1102216 (doi:10.1126/science.1102216) [DOI] [PubMed] [Google Scholar]
- 39.Popham J. D., Webster J. M. 1978. An alternative interpretation of the fine structure of the basal zone of the cuticle of the dauerlarva of the nematode Caenorhabditis elegans (Nematoda). Can. J. Zool. 56, 1556–1563 10.1139/z78-217 (doi:10.1139/z78-217) [DOI] [PubMed] [Google Scholar]
- 40.Burnell A. M., Houthoofd K., O'Hanlon K., Vanfleteren J. R. 2005. Alternate metabolism during the dauer stage of the nematode Caenorhabditis elegans. Exp. Gerontol. 40, 850–856 10.1016/j.exger.2005.09.006 (doi:10.1016/j.exger.2005.09.006) [DOI] [PubMed] [Google Scholar]
- 41.Albert P. S., Riddle D. L. 1983. Developmental alterations in sensory neuroanatomy of the Caenorhabditis elegans dauer larva. J. Comp. Neurol. 219, 461–481 10.1002/cne.902190407 (doi:10.1002/cne.902190407) [DOI] [PubMed] [Google Scholar]
- 42.Apfeld J., Kenyon C. 1998. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell 95, 199–210 10.1016/S0092-8674(00)81751-1 (doi:10.1016/S0092-8674(00)81751-1) [DOI] [PubMed] [Google Scholar]
- 43.Larsen P. L. 1993. Aging and resistance to oxidative damage in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 90, 8905–8909 10.1073/pnas.90.19.8905 (doi:10.1073/pnas.90.19.8905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lithgow G. J., White T. M., Melov S., Johnson T. E. 1995. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc. Natl Acad. Sci. USA 92, 7540–7544 10.1073/pnas.92.16.7540 (doi:10.1073/pnas.92.16.7540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holt S. J., Riddle D. L. 2003. SAGE surveys C. elegans carbohydrate metabolism: evidence for an anaerobic shift in the long-lived dauer larva. Mech. Ageing Dev. 124, 779–800 10.1016/S0047-6374(03)00132-5 (doi:10.1016/S0047-6374(03)00132-5) [DOI] [PubMed] [Google Scholar]
- 46.Vanfleteren J. R., De Vreese A. 1996. Rate of aerobic metabolism and superoxide production rate potential in the nematode Caenorhabditis elegans. J. Exp. Zool. 274, 93–100 (doi:10.1002/(SICI)1097-010X(19960201)274:2<93::AID-JEZ2>3.0.CO;2-8) [DOI] [PubMed] [Google Scholar]
- 47.Cassada R. C., Russell R. L. 1975. The dauer larva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46, 326–342 10.1016/0012-1606(75)90109-8 (doi:10.1016/0012-1606(75)90109-8) [DOI] [PubMed] [Google Scholar]
- 48.Riddle D. L., Swanson M. M., Albert P. S. 1981. Interacting genes in nematode dauer larva formation. Nature 290, 668–671 10.1038/290668a0 (doi:10.1038/290668a0) [DOI] [PubMed] [Google Scholar]
- 49.Vowels J. J., Thomas J. H. 1992. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics 130, 105–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antebi A., Culotti J., Hedgecock E. 1998. daf-12 regulates developmental age and the dauer alternative in Caenorhabditis elegans. Development 125, 1191–1205 [DOI] [PubMed] [Google Scholar]
- 51.Chalfie M., Au M. 1989. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science 243, 1027–1033 10.1126/science.2646709 (doi:10.1126/science.2646709) [DOI] [PubMed] [Google Scholar]
- 52.Wicks S. R., de Vries C. J., van Luenen H. G., Plasterk R. H. 2000. CHE-3, a cytosolic dynein heavy chain, is required for sensory cilia structure and function in Caenorhabditis elegans. Dev. Biol. 221, 295–307 10.1006/dbio.2000.9686 (doi:10.1006/dbio.2000.9686) [DOI] [PubMed] [Google Scholar]
- 53.Hedgecock E. M., Russell R. L. 1975. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 72, 4061–4065 10.1073/pnas.72.10.4061 (doi:10.1073/pnas.72.10.4061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trent C., Tsuing N., Horvitz H. R. 1983. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics 104, 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albert P. S., Riddle D. L. 1988. Mutants of Caenorhabditis elegans that form dauer-like larvae. Dev. Biol. 126, 270–293 10.1016/0012-1606(88)90138-8 (doi:10.1016/0012-1606(88)90138-8) [DOI] [PubMed] [Google Scholar]
- 56.Antebi A., Yeh W., Tait D., Hedgecock E. M., Riddle D. L. 2000. daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans. Genes Dev. 14, 1512–1527 10.1101/gad.14.12.1512 (doi:10.1101/gad.14.12.1512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inoue T., Ailion M., Poon S., Kim H. K., Thomas J. H., Sternberg P. W. 2007. Genetic analysis of dauer formation in Caenorhabditis briggsae. Genetics 177, 809–818 10.1534/genetics.107.078857 (doi:10.1534/genetics.107.078857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hobert O., Moerman D. G., Clark K. A., Beckerle M. C., Ruvkun G. 1999. A conserved LIM protein that affects muscular adherens junction integrity and mechanosensory function in Caenorhabditis elegans. J. Cell Biol. 144, 45–57 10.1083/jcb.144.1.45 (doi:10.1083/jcb.144.1.45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barr M. M., Sternberg P. W. 1999. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 401, 386–389 [DOI] [PubMed] [Google Scholar]
- 60.Peden E. M., Barr M. M. 2005. The KLP-6 kinesin is required for male mating behaviors and polycystin localization in Caenorhabditis elegans. Curr. Biol. 15, 394–404 10.1016/j.cub.2004.12.073 (doi:10.1016/j.cub.2004.12.073) [DOI] [PubMed] [Google Scholar]
- 61.Lacey L. A., Kaya H. K., Bettencourt R. 1995. Dispersal of Steinernema glaseri (Nematoda: Steinernematidae) in adult Japanese beetles, Popillia japonica (Coleoptera: Scarabaeidae). Biocontrol. Sci. Tech. 5, 121–130 10.1080/09583159550040060 (doi:10.1080/09583159550040060) [DOI] [Google Scholar]
- 62.Baird S. E. 1999. Natural and experimental associations of Caenorhabditis remanei with Trachelipus rathkii and other terrestrial isopods. Nematology 1, 471–475 10.1163/156854199508478 (doi:10.1163/156854199508478) [DOI] [Google Scholar]
- 63.Bovien P. 1937. Some types of association between nematodes and insects. Vidensk. Medd. fra Dansk Naturh. Foren. 101 [Google Scholar]
- 64.Hong R. L., Svatoš A., Herrmann M., Sommer R. J. 2008. Species-specific recognition of beetle cues by the nematode Pristionchus maupasi. Evol. Dev. 10, 273–279 10.1111/j.1525-142X.2008.00236.x (doi:10.1111/j.1525-142X.2008.00236.x) [DOI] [PubMed] [Google Scholar]
- 65.Lewis E., Gaugler R., Harrison R. 1992. Entomopathogenic nematode host finding: response to host contact cues by cruise and ambush foragers. Parasitology 105, 309–315 10.1017/S0031182000074230 (doi:10.1017/S0031182000074230) [DOI] [Google Scholar]
- 66.Campbell J. F., Lewis E. E., Stock S. P., Nadler S., Kaya H. K. 2003. Evolution of host search strategies in entomopathogenic nematodes. J. Nematol. 35, 142–145 [PMC free article] [PubMed] [Google Scholar]
- 67.Lewis E. E., Campbell J., Griffin C., Kaya H., Peters A. 2006. Behavioral ecology of entomopathogenic nematodes. Available at: http://eprints.nuim.ie/900/
- 68.O'Brien W. J., Browman H. I., Evans B. I. 1990. Search strategies of foraging animals. See http://adsabs.harvard.edu/abs/1990AmSci..78..152O
- 69.Sonerud G. A. 1992. Search tactics of a pause-travel predator: adaptive adjustments of perching times and move distances by hawk owls (Surnia ulula). Behav. Ecol. Sociobiol. 30, 207–217 10.1007/BF00166705 (doi:10.1007/BF00166705) [DOI] [Google Scholar]
- 70.Greco C. F., Kevan P. G. 1994. Contrasting patch choosing by anthophilous ambush predators: vegetation and Xoral cues for decisions by a crab spider (Misumena vatia) and males and females of an ambush bug (Phymata americana). Can. J. Zool. 72, 1583–1588 10.1139/z94-210 (doi:10.1139/z94-210) [DOI] [Google Scholar]
- 71.Greco C. F., Kevan P. G. 1995. Patch choice in the anthophilous ambush predator Phymata americana: improvement by switching hunting sites as part of the initial choice. Can. J. Zool. 73, 1912–1917 10.1139/z95-224 (doi:10.1139/z95-224) [DOI] [Google Scholar]