Abstract
There is growing evidence of changes in the timing of important ecological events, such as flowering in plants and reproduction in animals, in response to climate change, with implications for population decline and biodiversity loss. Recent work has shown that the timing of breeding in wild birds is changing in response to climate change partly because individuals are remarkably flexible in their timing of breeding. Despite this work, our understanding of these processes in wild populations remains very limited and biased towards species from temperate regions. Here, we report the response to changing climate in a tropical wild bird population using a long-term dataset on a formerly critically endangered island endemic, the Mauritius kestrel. We show that the frequency of spring rainfall affects the timing of breeding, with birds breeding later in wetter springs. Delays in breeding have consequences in terms of reduced reproductive success as birds get exposed to risks associated with adverse climatic conditions later on in the breeding season, which reduce nesting success. These results, combined with the fact that frequency of spring rainfall has increased by about 60 per cent in our study area since 1962, imply that climate change is exposing birds to the stochastic risks of late reproduction by causing them to start breeding relatively late in the season.
Keywords: breeding phenology, climate change, Mauritius kestrel, tropical island endemic
1. Introduction
Climate change has potentially profound impacts on biodiversity—notably on population extinctions—and evidence is accumulating that such effects are already apparent in many systems [1–3]. The mechanisms through which climate change impacts on biodiversity are varied, but one potentially important mechanism concerns changes in phenology [4]. There is an increasing number of examples now showing changes in the timing of important ecological events, such as flowering in plants and reproduction in animals, in response to climate change [4–9].
Wild bird populations are important model systems for exploring how phenology, particularly the timing of breeding, is responding to climate change [4,10–12]. Recent work has shown that individual birds are remarkably flexible in their timing of breeding in a changing climate, allowing them to closely track changes in the environment [12–14]. This plasticity represents the ability of a single genotype to alter its phenotype in response to changing environmental conditions. Phenotypic plasticity is ecologically very important because it can prevent a mismatch between breeding phenology and environmental conditions [15] that would reduce individual fitness and ultimately population growth [16]. It is therefore important to understand how climate change impacts the breeding phenology of individuals within a wild population in order to comprehend long-term population-level consequences.
Current knowledge about climate impacts on breeding phenology is limited and biased towards Northern Hemisphere temperate species, which have been shown to vary their timing of breeding in relation to temperature and breed earlier in warmer springs [11,12,17]. Tropical species, especially island endemics that form an important component of global biodiversity, have yet to be explored in this context, with notable exceptions like the Darwin's finches [18]. Here, we report the response to changing climate in a tropical wild bird population, which differs notably from the temperature-induced changes observed in temperate species. Using an extraordinarily detailed long-term dataset on a formerly critically endangered tropical bird, the Mauritius kestrel (Falco punctatus), we show that the timing of breeding is delayed in response to deteriorating climatic conditions, with detrimental consequences for reproductive success.
2. Methods
(a). Study population and data
The study was conducted in the east coast mountain range of Mauritius (20.3° S, 57.6° E). Our study area covers 163 km2, encompassing a predominantly forested mountainous area bordered by agricultural land cultivating primarily sugar cane [19]. Our study population was extirpated by the 1960s, but reintroduced at the end of the 1980s as part of a recovery programme [20]. Subsequent to its reintroduction, the population has grown and has now become stabilized at approximately 42–45 breeding pairs. Since reintroduction, the population has been intensively monitored. The majority (more than 90%) of individuals entering the population are individually colour-ringed in the nest [21], and the study area is a closed system—no colour-ringed immigrants have been recorded within the population, and no colour-ringed emigrants have been recovered or resited elsewhere. Mauritius kestrels have a socially monogamous, territory-based breeding system, and their breeding season spans the Southern Hemisphere spring/summer, with the earliest eggs (clutch size two to five) being laid in early September and the latest fledgelings (brood size one to four) leaving the nest in late February [21]. Monitoring consists of locating all nesting attempts by checking all previously used sites and searching likely new areas [22]. Each breeding season the majority (more than 90%) of all breeding pairs are located and identified, and their nesting attempts are monitored, providing data on the timing of breeding (date the first egg is laid), clutch size (the number of eggs laid) and the number of chicks that subsequently fledge [21]. Individuals are marked (using a unique set of coloured leg rings) and are sexed in the nest using a biometrics-based method [23] that has been validated by genetic analysis [24]. Kestrels are single-brooded, although a second clutch is laid on occasions (usually only if the first clutch or brood is completely lost). This intensive monitoring programme means that we have, until 2005, complete, spatially referenced life histories for approximately 570 individual kestrels as the population has developed [19], with over 600 breeding attempts followed since 1987.
Daily rainfall data (millimetres) are collected using a standard rain gauge from seven stations situated throughout our study area by local sugar estates for the purposes of crop management. For our analyses, we used data from a single station at Camizard because this is the longest available time series (from 1962 onwards). We show elsewhere that the time series trends in rainfall frequency recorded at Camizard are similar to those seen in the other stations [25], implying that changes in rainfall are occurring throughout our study area.
(b). Rainfall and timing of breeding
Previous work on our study population has shown that the frequency of rainfall in the July–September (spring) period is correlated with the timing of breeding, with birds breeding earlier in dryer springs [26]. Further analysis and model selection using the Akaike information criterion (AIC; see electronic supplementary material) indicated that within the July–September period it was the frequency of rainfall in August that had a significant impact on the timing of breeding. Initially, to describe the relationship between timing of breeding and frequency of rainfall in August (R), a linear mixed model was constructed using the first egg date (i.e. the timing of breeding, TB) as the response variable, the number of rain days in August as a fixed effect and the female identity as a random effect:
where subscripts 0, i and j refer to the structuring of the data, where Rij is the R value of measurement i from subject j, β0 is the intercept, β1 the slope of the regression between breeding time and rain days in August, u0j the random intercept and e0ij the error term [27]. Male and female age groups were also used as fixed effects as these are known to influence the timing of breeding (the electronic supplementary material). The August rain days in this model, however, combines both the variance observed between individuals and that observed within an individual's life history into one predictor variable. To determine if variation seen in the timing of breeding within the population was owing to differences between individuals or owing to within-individual responses to frequency of rainfall, a methodology known as ‘within-subject centring’ was used [27,28]. Hence, a second model was constructed with two new predictor variables derived to describe the between-subject variance (βb) and within-subject variance (βw). The variable describing the between-subject variance was simply the mean number of rain days experienced by each individual and the variable describing the within-subject variance was the value obtained by subtracting the individual's mean rain value from each observation value.
A third model was constructed to determine if the estimated effects of between- and within-subject variances were statistically different. This model combined the original predictor effect (August rain days) and the new predictor effect that only combined the between-subject variation. In this model, the between-subject effect actually represents the difference between the between- and within-subject effects in the second model [27].
Finally, to determine if there was a substantial between-subject variation in the slopes of the within-subject effect, a fourth model was constructed by adding a random slope (within-subject effect, uwj) to the random intercept (female identity) of model 2.
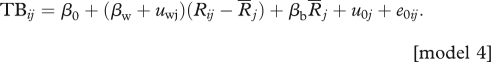 |
(c). Timing of breeding and number of fledgelings produced
To determine the relationship between reproductive success and timing of breeding, the total number of fledgelings produced by a female in year t was used as the measure of her reproductive success in year t (i.e. including first clutches and relays). This was then included as the response variable in a model framework that was similar to models 1, 2 and 3, exploring the relationship between timing of breeding and rainfall. These models were generalized linear mixed models (GLMMs) with a Poisson distribution incorporating the identity of the individual female as the random effect and the timing of breeding (i.e. first egg date, between-subject effect and/or within-subject effects, depending on the model) as a fixed predictor. The season per year of breeding was also incorporated as a fixed effect to account for seasonal variation in fledgeling production.
(d). Stochastic effects of rainfall on the seasonal decline in number of fledgelings produced
We constructed a series of models to explore how between-year variation in rainfall affects the rate at which the number of fledgelings produced declines within seasons so we could gain a more detailed understanding of the potential costs of breeding relatively late in the season. We predicted that if rainfall modifies the seasonal decline in the number of fledgelings produced, we should detect a significant interaction between the timing of breeding and rainfall. To test this, we constructed a series of models with rainfall and rain day variables from different time periods from November–January, and identified plausible models from this candidate set using AIC.
In addition, we wished to explore whether any impact of rainfall in the previous analysis occurred, because rainfall conditions interacted with the timing of breeding to affect nest survival rates. To examine this, we modelled the survival probability of eggs from laying to fledging as the response variable in a comparable model to that identified above for the number of fledgelings. Again, we predicted that if rainfall modifies the seasonal decline in egg survival, we should detect a significant interaction between the timing of breeding and rainfall. Models were constructed assuming binomial errors.
3. Results
(a). Rainfall and timing of breeding
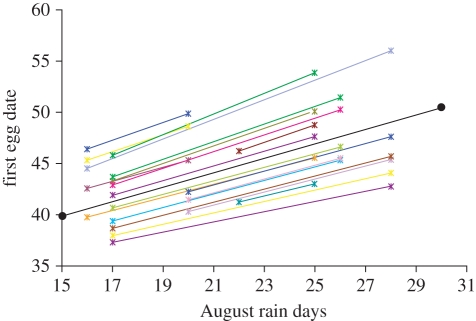
Our results indicate that individual females begin breeding progressively later as the number of rain days in August increases (figure 1). However, while there is significant evidence for within-individual response to rain (individual level plasticity), there is no statistical evidence to indicate that the population response arises from differences between individuals (table 1 and models 2 and 3); that is, the population-level pattern of plasticity only reflects individual responses. Furthermore, there is no evidence for significant differences between individuals in their within-subject slopes (table 1 and model 4), indicating that all individuals displayed similar responses to more rain days in August. This means that individual female kestrels delay breeding as the frequency of spring rainfall increases.
Figure 1.
Individual responses in the timing of breeding (first egg date) to changing rainfall conditions. Each line represents an individual response to changing rainfall conditions for a randomly selected subsample of 20 individual females bounded by the minimum and maximum rain days experienced by each individual. The black line represents the mean population response for all 61 females that bred in more than one breeding season.
Table 1.
Linear mixed models exploring the relationship between the timing of breeding (first egg date) and spring rainfall (number of August rain days). In addition to the fixed and random effects shown in the table, all models incorporated male and female age groups as fixed predictor variables to account for age-related variation in timing of breeding. n = 243 breeding attempts (first clutches of birds breeding in two or more years only).
| model | fixed effects | parameter estimate (±s.e.) | p-value | random effects | AIC | log likelihood |
|---|---|---|---|---|---|---|
| 1 | August rain days | 0.61 ± 0.21 | <0.001 | female identity | 1620.98 | −802.49 |
| 2 | within-subject effect | 0.81 ± 0.23 | <0.001 | female identity | 1617.99 | −799.99 |
| between-subject effect | −0.53 ± 0.55 | 0.343 | ||||
| 3 | August rain days | 0.81 ± 0.23 | <0.001 | female identity | 1617.99 | −799.99 |
| difference between the within- subject and between-subject effects | −1.34 ± 0.60 | <0.05 | ||||
| 4 | within-subject effect | 0.81 ± 0.23 | <0.001 | within-subject effect and female identity | 1621.99 | −799.99 |
| between-subject effect | −0.53 ± 0.55 | 0.343 |
(b). Timing of breeding and number of fledgelings produced
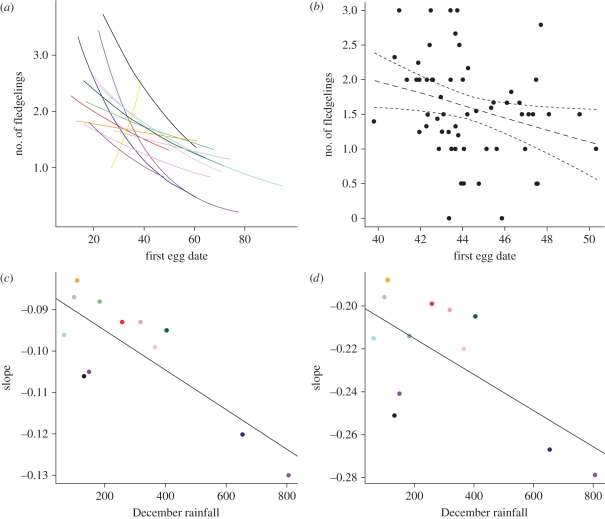
The number of fledgelings produced by a female is significantly related to her timing of breeding (r2 = 0.07, p < 0.05; figure 2a,b), indicating that early-breeding females have a higher reproductive success. While there is strong evidence for a within-subject effect (table 2 and model 2; i.e. as females breed later they fledge fewer chicks), there is only weak evidence to suggest that this is different from a between-subject effect (table 2 and model 3). Thus, it is probable that both within- and between-subject differences in the timing of breeding affect the number of fledgelings produced (i.e. while within-subject delays in timing of breeding reduce the number of fledgelings produced, subjects breeding later on average also tend to have lower numbers of fledgelings, even after controlling for age differences).
Figure 2.
Reproductive success in relation to the timing of breeding. (a) The fitted relationships between reproductive success and the timing of breeding (first egg date) for each individual breeding season (distinguished by coloured lines). (b) The relationship between the mean reproductive success and the average laying date for each individual female. (c) The relationship between the slope of the relationship in (a) (i.e. the rate of change in reproductive success with changing breeding date) and the amount of rainfall in December for each season. (d) The relationship between the rate of change of proportional reproductive success (success versus failures) and the amount of rainfall in December.
Table 2.
GLMMs (with a Poisson distribution) exploring the relationship between the reproductive success (number of fledgelings produced) and the timing of breeding (first egg date). In addition to the fixed and random effects shown in the table all models incorporated the season of breeding as fixed predictor variable, to account for seasonal variation in timing of breeding. n = 243 breeding attempts (first clutches of birds breeding in two or more years only).
| model | fixed effects | parameter estimate (±s.e.) | p-value | random effects | AIC | log likelihood |
|---|---|---|---|---|---|---|
| 1 | first egg date | −0.02 ± 0.004 | <0.001 | female identity | 281.0 | −123.51 |
| 2 | within-subject effect | −0.02 ± 0.005 | <0.001 | female identity | 280.7 | −122.34 |
| between-subject effect | −0.01 ± 0.005 | 0.099 | ||||
| 3 | first egg date | −0.02 ± 0.005 | <0.001 | female identity | 280.7 | −122.34 |
| difference between the within- subject and between-subject effects | 0.01 ± 0.008 | 0.125 |
(c). Stochastic effects of rainfall on the seasonal decline in number of fledgelings
The decline in number of fledgelings produced over time within a breeding season gets steeper as rainfall increases during the period chicks are in the nest (December, r2 = 0.63, p < 0.01; figure 2c and table 3). There is no evidence to suggest that the frequency of rainfall in spring (August) had a similar effect to December rainfall (i.e. August rain days do not have a significant impact on the number of fledgelings; r2 = 0.20, p = 0.65). The seasonal decline in number of fledgelings is therefore independent of rainfall conditions in spring that determine the timing of breeding. Similarly, when considering egg survival probability to fledging, there is also a significant negative impact of December rainfall (z = 2.36, p < 0.05), as well as an interaction between December rainfall and the timing of breeding (z = −2.72, p < 0.01; figure 2d). This implies that birds breeding relatively late in the season have fewer fledgelings than earlier-breeding individuals because of an increased risk of egg or chick mortality caused by rainfall associated with the start of the cyclone season.
Table 3.
GLMMs of reproductive success in Mauritius kestrels. Both models have an identical random effect structure, but differ in their fixed effects. The reproductive success is significantly affected by the timing of breeding (first egg date) and the interaction of December rainfall and timing of breeding. There is no further significant seasonal variation, as indicated by a χ2 test comparing models 1 and 2 (χ2 = 18.92, d.f. = 23, p = 0.71). n = 243 breeding attempts (first clutches of birds breeding in two or more years only).
| model | fixed effects | F-value | p-value | random effects | AIC | log likelihood |
|---|---|---|---|---|---|---|
| 1 | intercept | 347.14 | <0.001 | female identity | 681.16 | −335.58 |
| laying date | 29.45 | <0.001 | ||||
| December rainfall | 0.52 | 0.472 | ||||
| December rainfall × first egg date | 4.78 | 0.030 | ||||
| 2 | intercept | 380.76 | <0.001 | female identity | 708.24 | −326.12 |
| laying date | 29.14 | <0.001 | ||||
| December rainfall | 0.48 | 0.488 | ||||
| December rainfall × first egg date | 4.91 | 0.028 | ||||
| breeding season | 2.85 | 0.093 | ||||
| first egg date × breeding season | 2.64 | 0.106 |
(d). Implications of changes in rainfall
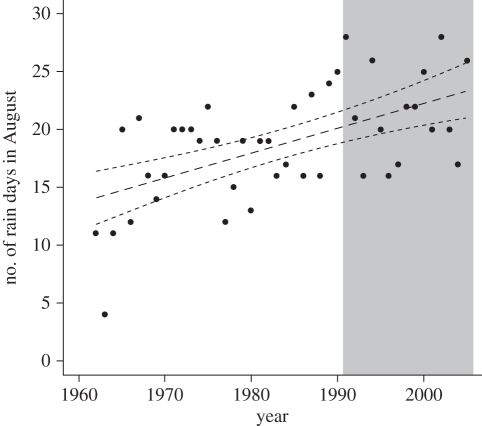
Our results have potential implications for our understanding of the impact of changes in climatic trends because the number of rain days in August has increased significantly since the 1960s (1962–2005, r2 = 0.31, p < 0.001; figure 3), implying that the timing of breeding should be getting progressively later. This change is not apparent in our data (1991–2005, r2 = 0.03, p = 0.57), but this is probably because no significant change in the number of August rain days occurred over the time period for which we have breeding data on the kestrels (1991–2005, r2 = 0.002, p = 0.23; figure 3, shaded area). However, the mean number of rain days in August is significantly greater during the period 1991–2005 than during the period 1962–1990, before kestrel data are available (1991–2005: 21.60 ± 4.19 days; 1962–1990: 17.28 ± 4.60 days; t1,42 = −3.04, p < 0.005). We found no evidence to suggest that the amount of rainfall in December has changed significantly over time (1962–2005, r2 = 0.03, p = 0.24), implying that any change in rainfall is unlikely to alter the within-season decline in number of fledgelings produced in kestrels. Consequently, while changing rainfall patterns have implications for the timing of breeding in kestrels, it seems to have a negligible impact on the numbers of fledgelings associated with the timing of breeding.
Figure 3.
Time series trend in the frequency of rainfall in spring (August) with fitted trend and 95% CI. The shaded area indicates the time period (1991–2005) for which we have kestrel breeding data available for analysis.
4. Discussion
Phenological shifts, such as changes in timing of breeding, are key processes affecting the impact of climate change on wild populations. Our study has explored the impacts of climatic conditions on the breeding phenology and reproductive success of a tropical island endemic bird, the Mauritius kestrel. Our results show that females begin breeding later as the frequency of rainfall in August (spring) increases, and all individual females within the population appear to respond to changing rainfall conditions in the same way. Birds breeding relatively late in the season have lower reproductive success (fledgeling production) compared with birds breeding earlier. This effect is apparent both within and between females. This appears to be because breeding later increases the risk that eggs and chicks in the nest are exposed to rainfall, which reduces their survival. These results are important in the context of climate change, because we also show that the frequency of spring rainfall has increased in our study area over the last 50 years [25].
There are two important mechanistic questions that arise from these results. (i) Why do female Mauritius kestrels breed progressively later as the frequency of rainfall in spring increases? (ii) Why do delays in breeding result in reduced reproductive success? The most likely cause of breeding delays in relation to the frequency of rainfall is that hunting efficiency of males who provision the females might be reduced because of lower prey detection in wetter conditions, thereby reducing the rate at which females can acquire resources immediately prior to breeding. We have no data to test this possibility directly, but there has been evidence to show that certain tropical birds have experienced weather-induced risks to resource availability [29]. Delayed breeding might cause reduced reproductive success in Mauritius kestrels owing to a mismatch between the timing of breeding and peak food abundance. It is typical for many seasonally breeding birds to time their egg laying to coincide with a subsequent peak in food abundance, which is important for chick-rearing and hence reproductive success [8,30]. While it is possible a seasonal mismatch plays a role, our data suggest a more direct mechanism: delayed breeding increases the risk that nests will be exposed to rainfall, which reduces egg survival to fledging. This reduced survival rate could occur for two main reasons—the hunting efficiency of breeding adults might be reduced because of lower prey detection in wetter conditions, or nest cavities might be flooded, increasing the risk of hypothermia in chicks [31,32]. Although we lack detailed data to distinguish between these possibilities, our results are consistent with other studies on raptor species, which show that relatively high reproductive success and the production of large broods are associated with periods of low precipitation [31–37].
Our results have implications in the context of climate change because we show that the frequency of spring rainfall has increased significantly since the 1960s in our study area. This implies that the timing of breeding in Mauritius kestrels should have become progressively later over this time period, but this pattern is not evident in our data (figure 3). This seems to be because there is no evidence of an increase in the frequency of spring rainfall within the time period (1990s onwards) covered by our data on the timing of breeding. The kestrel population is currently experiencing significantly more frequent spring rainfall than the study area experienced prior to the population being re-established. We suggest, therefore, that climate change has produced contemporary rainfall conditions in Mauritius that result in relatively late breeding and, consequently, an increased risk of breeding birds being exposed to adverse rainfall conditions during nesting.
Our results provide an interesting contrast to results emerging from studies on northern temperate bird populations. These studies have mainly focused on the impacts of temperature-related changes, with several species showing a significant advance in their timing of breeding in response to increasing spring temperatures [4,10,13,14,38–40], although some species, including the sparrow hawk (Accipiter nisus), are yet to show any such response to these changes [11,40]. Interest has focused on the extent to which these changes in timing are adaptive or result in a mismatch between the timing of breeding and food supplies, thereby reducing fitness [10,13]. Our results raise the possibility that different mechanisms might operate in sub-tropical/tropical populations in which the timing of breeding is influenced by rainfall conditions rather than temperature. Furthermore, our results suggest that the fitness cost of breeding delays (reduced fledgeling production) can be explained by increased risks associated with rainfall later in the breeding season rather than by a mismatch between timing and the food supply. There is growing evidence of systematic changes in rainfall conditions in tropical regions [41–43], and it is well recognized that tropical ecosystems are hotspots of biodiversity [44]. These general patterns and our results suggest that it is important to explore the ecological impact of climate change in wild tropical populations, and that it would be unwise to assume that these populations respond in a way that is comparable with temperate populations, which currently represent the majority of ‘model’ systems for exploring the ecology of climate change.
Acknowledgements
The Mauritius kestrel recovery programme has been sponsored by The National Parks and Conservation Service, Government of Mauritius, The Peregrine Fund, The Mauritian Wildlife Foundation, and The Durrell Wildlife and Conservation Trust. This research was supported by the Dorothy Hodgkins Postgraduate Award.
References
- 1.Crick H. Q. P. 2004. The impact of climate change on birds. Ibis 146, 48–56 10.1111/j.1474-919X.2004.00327.x (doi:10.1111/j.1474-919X.2004.00327.x) [DOI] [Google Scholar]
- 2.IPCC 2007. Climate change 2007: impacts, adaptation, and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.McLaughlin J. F., Hellmann J. J., Boggs C. L., Ehrlich P. R. 2002. Climate change hastens population extinctions. Proc. Natl Acad. Sci. USA 99, 6070–6074 10.1073/pnas.052131199 (doi:10.1073/pnas.052131199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visser M. E., Holleman L. J. M., Gienapp P. 2006. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147, 164–172 10.1007/s00442-005-0299-6 (doi:10.1007/s00442-005-0299-6) [DOI] [PubMed] [Google Scholar]
- 5.Barbraud C., Weimerskirch H. 2001. Emperor penguins and climate change. Nature 411, 183–186 10.1038/35075554 (doi:10.1038/35075554) [DOI] [PubMed] [Google Scholar]
- 6.Nordli O., Wielgolaski F. E., Bakken A. K., Hjeltnes S. H., Mage F., Sivle A., Skre O. 2008. Regional trends for bud burst and flowering of woody plants in Norway as related to climate change. Int. J. Biometeorol. 52, 625–639 10.1007/s00484-008-0156-5 (doi:10.1007/s00484-008-0156-5) [DOI] [PubMed] [Google Scholar]
- 7.Nussey D. H., Clutton-Brock T. H., Elston D. A., Albon S. D., Kruuk L. E. B. 2005. Phenotypic plasticity in a maternal trait in red deer. J. Anim. Ecol. 74, 387–396 10.1111/j.1365-2656.2005.00941.x (doi:10.1111/j.1365-2656.2005.00941.x) [DOI] [Google Scholar]
- 8.van Noordwijk A. J., McCleery R. H., Perrins C. M. 1995. Selection for the timing of great tit breeding in relation to caterpillar growth and temperature. J. Anim. Ecol. 64, 451–458 10.2307/5648 (doi:10.2307/5648) [DOI] [Google Scholar]
- 9.Thackeray S. J., et al. 2010. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Global Change Biol. 16, 3304–3313 10.1111/j.1365-2486.2010.02165 (doi:10.1111/j.1365-2486.2010.02165) [DOI] [Google Scholar]
- 10.Both C., te Marvelde L. 2007. Climate change and timing of avian breeding and migration throughout Europe. Climate Res. 35, 93–105 10.3354/cr00716 (doi:10.3354/cr00716) [DOI] [Google Scholar]
- 11.Nielsen J. T., Møller A. P. 2006. Effects of food abundance, density and climate change on reproduction in the sparrowhawk Accipiter nisus. Oecologia 149, 505–518 10.1007/s00442-006-0451-y (doi:10.1007/s00442-006-0451-y) [DOI] [PubMed] [Google Scholar]
- 12.Weidinger K., Kral M. 2007. Climatic effects on arrival and laying dates in a long-distance migrant, the Collared Flycatcher Ficedula albicollis. Ibis 149, 836–847 10.1111/j.1474-919X.2007.00719.x (doi:10.1111/j.1474-919X.2007.00719.x) [DOI] [Google Scholar]
- 13.Charmantier A., McCleery R. H., Cole L. R., Perrins C., Kruuk L. E. B., Sheldon B. C. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803 10.1126/science.1157174 (doi:10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 14.Nussey D. H., Postma E., Gienapp P., Visser M. E. 2005. Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306 10.1126/science.1117004 (doi:10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- 15.Gienapp P., Leimu R., Merila J. 2007. Responses to climate change in avian migration time—microevolution versus phenotypic plasticity. Climate Res. 35, 25–35 10.3354/cr00712 (doi:10.3354/cr00712) [DOI] [Google Scholar]
- 16.Both C., Bouwhuis S., Lessells C. M., Visser M. E. 2006. Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83 10.1038/nature04539 (doi:10.1038/nature04539) [DOI] [PubMed] [Google Scholar]
- 17.Visser M. E., et al. 2003. Variable responses to large-scale climate change in European Parus populations. Proc. R. Soc. Lond. B 270, 367–372 10.1098/rspb.2002.2244 (doi:10.1098/rspb.2002.2244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant P. R., Grant B. R. 1996. Finch communities in a climatically fluctuating environment. In Long-term studies of vertebrate communities (eds Cody M. L., Smallwood J. A.), ch. 12, pp. 343–390 Amsterdam, The Netherlands: Elsevier Inc [Google Scholar]
- 19.Burgess M. D., Nicoll M. A. C., Jones C. G., Norris K. 2008. Restricted dispersal reduces the strength of spatial density dependence in a tropical bird population. Proc. R. Soc. B 275, 1209–1216 10.1098/rspb.2007.1751 (doi:10.1098/rspb.2007.1751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones C. G., Heck W., Lewis R. E., Mungroo Y., Slade G., Cade T. J. 1995. The restoration of the Mauritius kestrel (Falco punctatus) population. Ibis 137, S173–S180 10.1111/j.1474-919X.1995.tb08439.x (doi:10.1111/j.1474-919X.1995.tb08439.x) [DOI] [Google Scholar]
- 21.Nicoll M. A. C., Jones C. G., Norris K. 2003. Declining survival rates in a reintroduced population of the Mauritius kestrel: evidence for non-linear density dependence and environmental stochasticity. J. Anim. Ecol. 72, 917–926 10.1046/j.1365-2656.2003.00768.x (doi:10.1046/j.1365-2656.2003.00768.x) [DOI] [Google Scholar]
- 22.Burgess M. D. 2008. Spatial patterns and population dynamics of a reintroduced Mauritius kestrel (Falco punctatus) population. PhD thesis, University of Reading, Reading, UK [Google Scholar]
- 23.Nicoll M. A. C., Jones C. G., Norris K. 2006. The impact of harvesting on a formerly endangered tropical bird: insights from life-history theory. J. Appl. Ecol. 43, 567–575 10.1111/j.1365-2664.2006.01165.x (doi:10.1111/j.1365-2664.2006.01165.x) [DOI] [Google Scholar]
- 24.Ewing S. R. 2005. The occurrence and consequences of inbreeding in a reintroduced population of the Mauritius Kestrel (Falco punctatus). PhD thesis, University of Glasgow, Glasgow, UK [Google Scholar]
- 25.Senapathi D., Underwood F. M., Black E. C., Nicoll M. A. C., Norris K. 2009. Evidence for long-term regional changes in precipitation on the East Coast Mountains in Mauritius. Int. J. Climatol. 30, 1164–1177 10.1002/joc.1953 (doi:10.1002/joc.1953) [DOI] [Google Scholar]
- 26.Nicoll M. A. C. 2004. The ecology and management of a re-introduced population of the Mauritius kestrel (Falco punctatus). PhD thesis, University of Reading, Reading, UK [Google Scholar]
- 27.van de Pol M., Wright J. 2009. A simple method for distinguishing within- versus between-subject effects using mixed models. An. Behav. 77, 753–758 10.1016/j.anbehav.2008.11.006 (doi:10.1016/j.anbehav.2008.11.006) [DOI] [Google Scholar]
- 28.van de Pol M., Verhulst S. 2006. Age-dependent traits: A new statistical method to separate within- and between-individual effects. Am. Nat. 167, 766–773 10.1086/503331 (doi:10.1086/503331) [DOI] [PubMed] [Google Scholar]
- 29.Boyle W. A., Norris D. R., Guglielmo C. G. 2010. Storms drive altitudinal migration in a tropical bird. Proc. R. Soc. B 277, 2511–2519 10.1098/rspb.2010.0344 (doi:10.1098/rspb.2010.0344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visser M. E., van Noordwijk A. J., Tinbergen J. M., Lessells C. M. 1998. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. Lond. B 265, 1867–1870 10.1098/rspb.1998.0514 (doi:10.1098/rspb.1998.0514) [DOI] [Google Scholar]
- 31.Lehikoinen A., Byholm P., Ranta E., Saurola P., Valkama J., Korpimäki E., Pietiäinen H., Henttonen H. 2009. Reproduction of the common buzzard at its northern range margin under climatic change. Oikos 118, 829–836 10.1111/j.1600-0706.2008.17440.x (doi:10.1111/j.1600-0706.2008.17440.x) [DOI] [Google Scholar]
- 32.Mearns R., Newton I. 1988. Factors affecting breeding success of peregrines in south Scotland. J. Anim. Ecol. 57, 903–916 10.2307/5100 (doi:10.2307/5100) [DOI] [Google Scholar]
- 33.Kostrzewa A., Kostrzewa R. 1990. The relationship of spring and summer weather with density and breeding performance of the Buzzard Buteo buteo, Goshawk Accipiter gentilis and Kestrel Falco tinnunculus. Ibis 132, 550–559 10.1111/j.1474-919X.1990.tb00278.x (doi:10.1111/j.1474-919X.1990.tb00278.x) [DOI] [Google Scholar]
- 34.Krüger O. 2004. The importance of competition, food, habitat, weather and phenotype for the reproduction of Buzzard Buteo buteo. Bird Study 51, 125–132 10.1080/00063650409461344 (doi:10.1080/00063650409461344) [DOI] [Google Scholar]
- 35.Rodriguez C., Bustamante J. 2003. The effect of weather on lesser kestrel breeding success: can climate change explain historical population declines? J. Anim. Ecol. 72, 793–810 10.1046/j.1365-2656.2003.00757.x (doi:10.1046/j.1365-2656.2003.00757.x) [DOI] [Google Scholar]
- 36.Selas V. 2001. Breeding density and brood size of Common Buzzard Buteo buteo in relation to snow cover in spring. Ardea 89, 471–479 [Google Scholar]
- 37.Costantini D., Dell'Omo G. 2006. Environmental and genetic components of oxidative stress in wild kestrel nestlings (Falco tinnunculus). J. Comp. Physiol. B 176, 575–579 10.1007/s00360-006-0080-0 (doi:10.1007/s00360-006-0080-0) [DOI] [PubMed] [Google Scholar]
- 38.Wilson S., Arcese P. 2003. El Nino drives timing of breeding but not population growth in the song sparrow (Melospiza melodia). Proc. Natl Acad. Sci. USA 100, 11 139–11 142 10.1073/pnas.1931407100 (doi:10.1073/pnas.1931407100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehikoinen A., Kilpi M., Ost M. 2006. Winter climate affects subsequent breeding success of common eiders. Global Change Biol. 12, 1355–1365 10.1111/j.1365-2486.2006.01162.x (doi:10.1111/j.1365-2486.2006.01162.x) [DOI] [Google Scholar]
- 40.Dunn P. O., Winkler D. W. 2010. Effects of climate change on timing of breeding and reproductive success in birds. In Effects of climate change on birds (eds Moller A. P., Fiedler W., Berthold P.), pp. 113–128 Oxford, UK: Oxford University Press [Google Scholar]
- 41.Elsner J. B., Kossin J. P., Jagger T. H. 2008. The increasing intensity of the strongest tropical cyclones. Nature 455, 92–95 10.1038/nature07234 (doi:10.1038/nature07234) [DOI] [PubMed] [Google Scholar]
- 42.Emanuel K. 2005. Increasing destructiveness of tropical cyclones over the past 30 years. Nature 436, 686–688 10.1038/nature03906 (doi:10.1038/nature03906) [DOI] [PubMed] [Google Scholar]
- 43.Zhang X., Zwiers F. W., Hegerl G. C., Lambert F. H., Gillett N. P., Solomon S., Stott P. A., Nozawa T. 2007. Detection of human influence on twentieth-century precipitation trends. Nature 448, 461–465 10.1038/nature06025 (doi:10.1038/nature06025) [DOI] [PubMed] [Google Scholar]
- 44.Myers N., Mittermeier R. A., Mittermeier C. G., da Fonseca G. A. B., Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 10.1038/35002501 (doi:10.1038/35002501) [DOI] [PubMed] [Google Scholar]