Abstract
Fisheries bycatch is a recognized threat to marine megafauna. Addressing bycatch of pelagic species however is challenging owing to the dynamic nature of marine environments and vagility of these organisms. In order to assess the potential for species to overlap with fisheries, we propose applying dynamic habitat models to determine relative probabilities of species occurrence for specific oceanographic conditions. We demonstrate this approach by modelling habitats for Laysan (Phoebastria immutabilis) and black-footed albatrosses (Phoebastria nigripes) using telemetry data and relating their occurrence probabilities to observations of Hawaii-based longline fisheries in 1997–2000. We found that modelled habitat preference probabilities of black-footed albatrosses were high within some areas of the fishing range of the Hawaiian fleet and such preferences were important in explaining bycatch occurrence. Conversely, modelled habitats of Laysan albatrosses overlapped little with Hawaii-based longline fisheries and did little to explain the bycatch of this species. Estimated patterns of albatross habitat overlap with the Hawaiian fleet corresponded to bycatch observations: black-footed albatrosses were more frequently caught in this fishery despite being 10 times less abundant than Laysan albatrosses. This case study demonstrates that dynamic habitat models based on telemetry data may help to project interactions with pelagic animals relative to environmental features and that such an approach can serve as a tool to guide conservation and management decisions.
Keywords: bycatch, dynamic habitat, marine conservation, modelling, telemetry
1. Introduction
Fisheries bycatch is a major threat to marine megafauna, including seabirds, marine mammals, sea turtles and sharks [1]. With ocean-wide distributions and movement patterns, these animals interact with a wide range of fishing gear and fisheries across all oceans and seas. Unintentional capture of these and other non-target species, termed bycatch, during fisheries operations is an issue of conservation concern both because of the waste of discarded individuals and the threat to populations of long-lived species. Bycatch research and management actions have led to progress in mitigating bycatch for some species in several regions [2–4], but bycatch remains a challenge to fishery sustainability.
For bycatch to occur there must be spatial and temporal overlap of fishing operations with the distribution of species that are susceptible to particular fishing gear. Although it is possible to track the location of fishing activity for some fisheries (e.g. via logbooks, observer programmes, vessel-monitoring systems), understanding distributions and movements of marine megafauna continues to be a challenge. The development and use of a variety of electronic tags for animal tracking have revolutionized our knowledge of the spatio-temporal ecology of marine animals over the last decade (e.g. [5,6]), and conservation applications of these data are emerging (e.g. [7–9]). Previous efforts have used tracking information to make general inferences about the overlap of marine megafauna with fisheries. One of the best-known examples is the ‘Tracking Ocean Wanderers’ initiative by BirdLife International, a compilation of tracking data of Procellariiform seabirds (i.e. albatrosses and petrels) and overlays of species distributions with longline fishing effort, exclusive economic zones and jurisdictional areas of Regional Fisheries Management Organizations [10]. Similar studies exist for sea turtles and marine mammals. For example, high-use habitats of leatherbacks (Dermochelys coriacea) have been identified in the northwest Atlantic, representing areas of turtle concentration and possible fishery threats [11]. Likewise, tracking harbour porpoises (Phocoena phocoena) in the Bay of Fundy and Gulf of Maine has resulted in insights into the periods when the animals visit areas of high gillnet fishing activity [12].
The majority of published bycatch studies that use animal telemetry data overlay static layers of animal distributions and fishing effort data to identify areas of potential interaction (e.g. [8,10,13,14]). This approach has proved to be a useful means to meet conservation and fishery management objectives for some applications. However, static overlays are, by definition, incapable of accurately representing the dynamic nature of the ocean environment, which includes intra- (seasonal) and inter-annual (between year) variation that occurs at both the basin and meso-scales. As such, they cannot provide a mechanistic understanding of animal–fishery interactions in the context of highly variable oceanographic conditions, e.g. storms or other anomalies or El Niño/La Niña-Southern Oscillation. Because static overlays integrate over large spatial and temporal scales, they miss meso-scale oceanographic features that are targeted by both human fishers [13,15] and animals (e.g. [16–20]). In response to fluctuating physical and biological features in the ocean, marine animals cue on meso-scale oceanographic features when moving and selecting habitats, and changes in these small-scale oceanographic features result in shifts in animal distribution (e.g. [16–24]). Because fishing vessels also track environmental features [25], variation in oceanographic conditions also influences the likelihood of fisheries encounters. To mitigate fisheries bycatch effectively and to minimize overlap with fishing operations, we need a clear understanding of the underlying mechanisms that influence bycatch interactions [26]. Such information can allow managers to develop strategies to avoid fisheries interactions at a finer scale than is possible with static overlays, while accounting for dynamic ocean conditions. Based on the large body of literature characterizing the dynamic nature of marine vertebrate distribution, oceanographic conditions and fishing activities, we a priori suggest that dynamic models which accurately relate animal and fishery distributions to oceanographic characteristics should perform as well as static models in spatially predictable environments, and are likely to generate more accurate projections of interactions between bycatch-impacted species and fishing vessels over time and space in the less predictable ocean environment.
Here, we integrate animal tracking data with fisheries information to determine the relative probability of encounter between two albatross species (Hawaii-breeding Laysan (Phoebastria immutabilis) and black-footed albatrosses (Phoebastria nigripes)) and United States (US) pelagic longline fishing vessels based in Hawaii. We present a dynamic habitat preference model based on albatross tracking and concurrent oceanographic data, and then relate modelled predictions of bird occurrence through time to known locations of the fishing fleet and its associated bycatch. Before bycatch mitigation measures were enacted in 2000, the Hawaii-based longline fleet took an estimated 1000–2000 individuals of each albatross species annually [27–29]. Although bycatch mitigation measures have substantially reduced albatross bycatch in the Hawaiian longline fleet since 2000, incidental bycatch remains a significant threat to albatross populations that encounter other fleets in the North Pacific Ocean and to albatrosses in other ocean regions [10,30]. The quantitative approach presented here captures the dynamic nature of albatross distributions in the North Pacific and provides a method for integrating fisheries and telemetry data to evaluate the overall level of risk from interactions and to dynamically identify likely bycatch ‘hotspots’ from an oceanographic perspective.
2. Material and methods
(a). Datasets
We used three types of data in this study: (i) telemetry data of post-breeding Laysan and black-footed albatrosses using archival tags; (ii) remotely sensed oceanographic data associated with albatross distribution and longline fisheries; and (iii) seabird bycatch in the US Hawaii-based longline fisheries recorded by onboard observers.
Albatross tracking data were collected during the post-breeding seasons (March–November) of 2004–2006 by the Tagging of Pacific Predators research group [6]. Adult Laysan and black-footed albatrosses were fitted with Lotek LTD 2400 light level and temperature-based geolocator archival tags (for further description see [31]). Tagging was conducted at their nesting colony on Tern Island in the atoll of French Frigate Shoals, Northwestern Hawaiian Islands (23°52′ N, 166°17′ W) during the breeding season. Tags were recovered when birds returned to the colony for the subsequent breeding season in November and recorded data were delimited to encompass the post-breeding period only. Tracking data from 28 Laysan and 24 black-footed albatrosses were used in this study (see the electronic supplementary material, S1). Archival tag data were processed following methods described in Shaffer et al. [31] and two daily positions (at universal time noon and midnight) were obtained for each bird after data were filtered and interpolated [32]. On average, Laysan albatross tracks lasted for 172 days (range 58–268 days) and black-footed albatross tracks lasted for 165 days (range 139–259 days).
Because static maps of bird telemetry locations shift considerably between years (see the electronic supplementary material, S1), we used tracking information of post-breeding albatrosses to develop preferred habitat models of albatrosses in the Northern Pacific. When breeding, albatrosses are central place foragers, so they must return to their nests within a few days. This restricts their foraging to the vicinity of the nesting colony thus significantly reducing the available habitat they can exploit during this time [18]. By contrast, during the post-breeding period, albatrosses are not constrained by the need to return to their chick at the colony and therefore, have more time to travel farther to locate more favourable habitats. Using tracking data from post-breeding albatrosses, who are no longer constrained by colony location, provides insight into bycatch likelihood as albatrosses exploit a broader ocean environment. These data also more accurately characterize the distribution of non-breeding birds, a substantial proportion of the adult population.
Because albatrosses were tagged and tags were recovered at the breeding grounds, we assumed that the first and last several locations of a trajectory did not reflect foraging habitats but rather the birds' seasonal migration corridors from and to breeding sites; we therefore removed the first 5 and the last 5 days of tracking of each bird in our analyses. Further, we assumed that albatrosses cue on similar ocean habitat characteristics year-round and that they are most likely to be caught by fishing gear deployed in their preferred foraging habitats. In this analysis, we relate albatross bycatch observed in Hawaii fisheries year-round to concurrently modelled albatross habitats, which were developed using tracking information of non-breeding birds.
Two types of environmental parameters were used as candidate predictors in habitat and bycatch models: static variables describing topographic features and dynamic variables obtained from publicly available remotely sensed physical and biological oceanography datasets (table 1). We sampled depth from the S2004 bathymetric dataset [33]. Ocean bottom slope (measured in degrees) was calculated from the S2004 bathymetric data using the slope function in ArcGIS v. 9.2 [34]. We identified the shelf break by selecting pixels with bathymetric slope values greater than or equal to 1° and falling within a depth range between 100 and 200 m. We then calculated the distance to the continental shelf by generating a raster using the path distance function available in ArcGIS v. 9.2 [34]. Sea surface temperature (SST) was sampled from the NOAA Pathfinder Advanced Very High Resolution Radiometer (AVHRR) v. 5 dataset. We used monthly SST composite images to avoid data loss owing to cloud cover. SST front probabilities were calculated as monthly composites, assessing frequency of SST front occurrence relative to the number of times a particular image pixel was visible during a month using two daily SST images (i.e. not covered by clouds). SST fronts were detected for water masses with SSTs differing greater than or equal to 0.5°C applying an algorithm available in Marine Geospatial Ecology Tool (MGET) package [35]. Sea surface height (SSH) data, collected by the Poseidon-2 altimeter on the Jason-1 spacecraft, were downloaded from Aviso website (http://www.aviso.oceanobs.com/). We used the Delayed-Time MSLA updated dataset. Ocean productivity data were obtained from Oregon State University; these values were calculated using a conventional Vertically Generalized Production Model (VGPM) [36]. For the VGPM, net primary production is a function of chlorophyll, available light, and the photosynthetic efficiency, and is measured as milligrams of carbon fixed per square metre per day.
Table 1.
List of remotely sensed oceanographic variables used in the analysis.
| no | variable | source | spatial resolution | temporal resolution | description |
|---|---|---|---|---|---|
| 1 | depth | S2004 | 0.017° | static | |
| 2 | ocean bottom slope | S2004 | 0.017° | static | slope of the ocean bottom in degrees calculated using bathymetry data |
| 3 | distance to shelf break | S2004 | 0.017° | static | custom-generated raster of distance to the continental shelf |
| 4 | sea surface temperature (SST) | Pathfinder AVHRR v5 | 0.17° | monthly | monthly mean temperature |
| 5 | SST front probability | Pathfinder AVHRR v5 | 0.17° | monthly | custom-generated images, identifying daily SST fronts (0.5°C), calculating monthly front probabilities and then smoothing probabilities over 10 × 10 pixel window |
| 6 | sea surface height (SSH) | Aviso-merged-delayed time updated MSLA | 0.33° | 7 days | SSH deviation from 7 year mean SSH above the geoid |
| 7 | ocean productivity (VGPM) | Oregon State University | 0.17° | 8 days | derived images obtained from Oregon State University (http://www.science.oregonstate.edu/ocean.productivity/) |
Seabird bycatch was recorded by the Hawaii Longline Observer Programme, which is part of the US National Marine Fisheries Service Observer Programme (described in Cousins [27] and Kinan [29]). We used seabird bycatch data from September 1997 to August 2000. The start date was determined by the availability of Sea-viewing Wide Field-of-view Sensor (SeaWiFS) remotely sensed ocean colour information and the end date was set to predate the implementation of seabird bycatch mitigation measures that effectively reduced seabird bycatch by the Hawaii-based fleet [29]. We excluded records of fishing operations that took place to the south of 15° N parallel from our analyses, as neither bycatch nor the typical distributional range of Laysan and black-footed albatrosses extends further south [28,37].
(b). Modelling albatross oceanographic habitats
To model albatross oceanographic habitats during the post-breeding period we used generalized additive models (GAMs), which are commonly used to model and predict habitat distributions [38]. Because the albatross tracking data consisted of presence-only locations, we simulated pseudo-absence positions so that a logistic GAM using a binomial response variable could be applied [38,39]. This analysis identified habitat characteristics where birds were present relative to habitats they could have selected during the same time frame.
(i). Simulation of pseudo-absence locations
We used correlated random walks (CRWs) to simulate pseudo-absence positions that represented locations where birds could have occurred [40]. Movement parameters to generate CRWs for individual birds were estimated based on observed movement characteristics of each tracked albatross. Parameters included the number of steps corresponding to a number of recorded albatross locations, mean, standard deviation, minimum and maximum step length values (km), and mean and standard deviation of turning angles. Each simulated location had an identification (ID) corresponding to the bird ID and a date of a paired actual track, and each simulated walk started at Tern Island. We restricted simulated walks to not occur over land, and to occur within the area defined by the minimum convex polygon (MCP) that encompassed all observed albatross track locations of both species. The MCP was increased by a 202 km buffer to account for the average error of temperature-based geolocator archival tags [31].
We tested the effects of different numbers of CRWs per individual bird on performance of habitat models aiming to determine an optimal number of simulated pseudo-absence locations. We ran a set of models of the same structure, consisting of all actual albatross tracks (separately for each species) and varying numbers of simulated trajectories. For each sample size (number of simulated tracks per bird), we measured the change in χ2-value of each parameter and model performance measured as area under the receiver operator curve (AUC). χ2-values and AUC stabilized with sample sizes of about 30–45 simulated tracks for each observed albatross track (see the electronic supplementary material, S2). On this basis, we set our sample size at 50 simulated tracks for each observed track.
We sampled oceanographic variables for each location of the real and simulated albatross tracks for the corresponding dates. To account for estimated error distribution of archival tags [31], we used a spatial kernel sampling technique to calculate a spatially weighted average of the oceanographic data for each location using a bivariate Gaussian distribution with s.e. 202 km (detailed description of this sampling technique provided in Teo et al. [41]).
(ii). Habitat modelling
Before running the analyses, all records with incomplete environmental information, e.g. where remotely sensed data were not available owing to cloud cover, were removed from the datasets. This resulted in the elimination of about 10 per cent of all records. Then, the predictor variables (table 1) were checked for intercorrelation using Spearman's rank correlation matrix. Variables for which the correlation coefficient exceeded 0.75 were not included in the same group of candidate predictors. For example, the correlation between distance to shelf break and SST variables was 0.85; therefore, these variables were not considered in the same candidate model. To account for possible nonlinear relationships between response and predictor variables, we used a smoothed spline fit with all predictors in GAMs. We used an information-theoretic approach for model selection, applying Akaike's Information Criteria (AIC) to rank models according to their degree of parsimony [42].
(iii). Evaluation of habitat models
The receiver operating characteristic (ROC) curves were used to evaluate the performance of the albatross habitat models [43]. We used a cross-validation technique by randomly splitting our datasets into two parts: ‘training’, consisting of two-third of all records and ‘testing’, consisting of one-third of all records. We used the training dataset to develop the model, which was then evaluated using the testing dataset. The AUC was used to discriminate between true and false positive rates of model predictions. Generally, AUC values of 0.5–0.7 indicate low model accuracy, values of 0.7–0.9 indicate reasonable model performance and values greater than 0.9 indicate very good accuracy [43]. We used the cut-off point along the ROC curve to discriminate the prediction values into either ‘suitable habitat’ or ‘unsuitable habitat’. We used automatic selection of the cut-off point by the software (ROCR package in R; [44]), which identifies the point on the ROC curve that is closest to the point of ideal classification, offering the highest possible sensitivity with the lowest proportion of false positives.
A time series of locations along the track of the same individual can be serially correlated with respect to their associated habitat characteristics, and therefore may affect results of habitat modelling [45]. To test whether serial correlation was an issue in our analysis, we ran models using every fifth location in the observed and simulated albatross tracks. Then, we compared parameter importance and AUCs of these models with our standard models based on all bird locations. Albatross habitat modelling and model evaluation were conducted in software R v. 2.8.0 [46] and using fit GAM, Plot ROC of the binary classification model, and predict GAM from rasters tools available in the MGET package for ArcGIS [35].
(c). Modelling albatross bycatch
In albatross bycatch models, the response variable was the number of albatrosses caught per longline set, with separate models for each species. We applied GAMs with a log link and Tweedie distribution, which have been proved to outperform other distributions when modelling zero-inflated data in similar fisheries catch and effort datasets [47,48]. To characterize the oceanic environment where the longline sets occurred, we used SST measured at the beginning of longline sets and sampled other oceanographic variables (table 1) from 20 randomly generated points along the length of each deployed line (between start and end gear setting positions). We did not consider the haul locations and the area fished during the longline soak time [49], as fatal seabird interactions typically occur while longlines are being set [50]. We used the function Sample Rasters Listed in Fields of the MGET package for ArcGIS [35] to sample spatially and temporally corresponding oceanographic data (table 1). We averaged oceanographic parameter values of the 20 points generated along each longline set. Further, because oceanographic features were correlated among longline sets within a fishing trip, we averaged environmental values and summed albatross bycatch across all longline sets within each trip and ran bycatch models using trip as a sampling unit and number of albatrosses caught as a response variable. Fishing vessels usually deploy several longline sets (average 12, range 1–28) during each trip and therefore characteristics of longline sets correlate within a trip owing to spatial and temporal proximity [28].
Hawaii-based longline fisheries can be categorized by target fish species, generally either swordfish or tunas. But such a categorical variable would essentially be a proxy for the different environmental characteristics associated with targeting these different groups, which are fished for in largely different areas using different gear configurations [25,27]. We did not include target species as a variable since we were already evaluating environmental variables directly, and because albatrosses are generally caught while deploying gear, we assumed that technical characteristics of different longline gear for different target species do not affect albatross bycatch. This assumption was supported by McCracken [28], who detected no effect of target fish catches on albatross bycatch in the Hawaii-based longline fisheries.
In addition to environmental data, we included as a predictor variable the estimated albatross habitat occurrence probability, which we calculated for each longline set using sampled oceanographic variables and developed a dynamic albatross habitat model. Before running the bycatch models, the predictor variables were checked for intercorrelation, and variables with Spearman's rank correlation exceeding 0.75 were not included in the same group of candidate predictors. We used an information-theoretic approach for model selection and ranking [42].
3. Results
(a). Albatross oceanographic habitats
(i). Laysan albatrosses
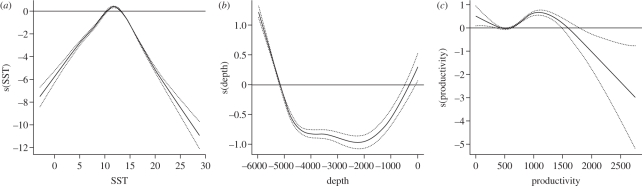
The best-fitting model-predicting Laysan albatross oceanographic habitat included depth, bottom slope, productivity, SST, SST front and SSH. Based on comparison of χ2-values, habitat occurrence probability varied most strongly with changes in SST, followed by variation in ocean depth and productivity (figure 1 and electronic supplementary material, S3). According to model response curves, Laysan albatross occurrence probabilities increased at SSTs between 10°C and 13°C, either shallow or great water depths, medium ocean productivity, steeper bottom slopes and negative SSH values (figure 1 and the electronic supplementary material, S3). SST fronts had the lowest explanatory power among predictors and model response curve did not show a clear relationship with the response variable (see the electronic supplementary material, S3). Distance to continental shelf break, which correlated highly with SST and therefore was considered in a competing candidate variable group, was a substantially less important predictor of bird habitat than SST (ΔAIC = 6885 between models of analogous structure with distance to continental shelf break versus SST). Evaluation of the model performance using an ROC curve indicated very good model accuracy with AUC = 0.93 (see the electronic supplementary material, S4). The predictive habitat maps of post-breeding Laysan albatrosses also showed very good fit with telemetry locations of this species (figure 2).
Figure 1.
Response curves of three most influential variables in Laysan albatross oceanographic habitat model (GAM).
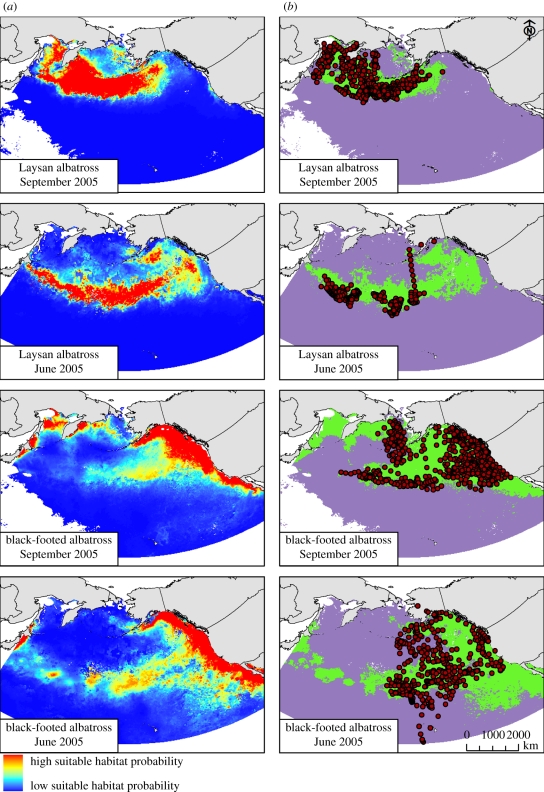
Figure 2.
Examples of dynamic habitat model performance: (a) predictive habitats maps and (b) observed tracking locations overlaid over binary habitat suitability maps of Laysan and black-footed albatrosses during June and September of the year 2005. Binary habitat suitability maps differentiate model predictions using the cut-off point of ROC curve. Purple regions, unsuitable habitat; green regions, suitable habitat; red circles, bird tracking fixes.
(ii). Black-footed albatrosses
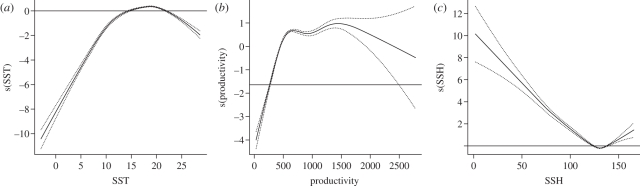
The model predicting black-footed albatross oceanographic habitats with the best fit included depth, bottom slope, productivity, SST, SST front probability and SSH (see the electronic supplementary material, S3). Model response curves indicated that black-footed albatrosses prefer habitats with SST between 14°C and 23°C, high productivity, either negative or positive SSH values, gentle slopes, water depths of less than 4000 m, and low SST frontal activity zones (figure 3, electronic supplementary material, S3). Model performance according to ROC curve was good with AUC = 0.85 (see the electronic supplementary material, S4) and predictive habitat maps created using the optimal model matched distribution of black-footed albatross telemetry locations reasonably well throughout most of the Northern Pacific Ocean (figure 3). However, predicted suitable habitat probability maps also suggested that highly suitable habitats occurred in areas where black-footed albatrosses tagged during this study were not observed (e.g. the Okhotsk Sea; figure 2) and where the species has not been reported as regularly occurring in the recent past [37,51].
Figure 3.
Response curves of three most influential variables in black-footed albatross oceanographic habitat model (GAM).
Owing to high correlation between distance to continental shelf break and SST, these terms were both tested in the model separately. As with Laysan albatrosses, SST performed substantially better and the distance to continental shelf break variable was excluded from the best-fitting model (ΔAIC = 3933 between models of analogous structure with distance to continental shelf break versus SST).
To test whether albatross habitat models were influenced by serial correlation, we developed models on sub-sampled datasets consisting of every fifth location along observed and simulated albatross tracks. The most plausible models of these sub-sampled datasets appeared to be very similar to those developed from full datasets for both albatross species in terms of which variables were included in the best models and their importance, model performance according to AUC, and shape of response curves (electronic supplementary material, S3). We therefore conclude that results of our analyses were not affected by serial correlation.
(b). Modelling albatross bycatch
Only 10 of the 1471 longline sets (0.7%) occurred in areas identified by our models as probable Laysan albatross habitat, i.e. areas with habitat suitability likelihood in excess of the cut-off point of the ROC curve (electronic supplementary material, S4). Longline fishing overlapped more with areas identified as black-footed albatross habitat (173 sets or 12%). Modelled probabilities of black-footed albatrosses occurrence correlated highly with SST and ocean productivity (r = −0.75 and r = 0.82, respectively). Therefore, we ran competing bycatch models with separate variable groups including either SST, ocean productivity or modelled black-footed albatross occurrence. Correlation between modelled Laysan albatross habitat occurrence and other predictors did not exceed 0.75, therefore all predictors were considered together.
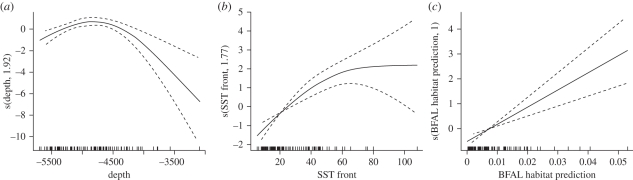
The most plausible bycatch model for Laysan albatross included ocean productivity, bottom slope, SST fronts and SSH. The Laysan albatross habitat probability variable was not significant in bycatch models of Laysan albatrosses, which is not surprising given that our models did not predict high use probability by this species in the area fished by the Hawaii-based longline fleet (electronic supplementary material, S5). By contrast, modelled black-footed albatross habitat probability was one of the predictors (together with depth and SST fronts) in the best bycatch model for black-footed albatross (figure 4 and electronic supplementary material, S5). The relationship between the response variable and black-footed albatross habitat probability was positive, indicating that black-footed albatross bycatch was more likely to occur in areas where environmental conditions suggested higher probability of preferred habitat occurrence (figure 4). Competing model sets with SST and ocean productivity among predictor variables performed less well than the model set with the black-footed albatross habitat probability (ΔAIC = 8.6 and ΔAIC = 4.7, respectively).
Figure 4.
Response curves of black-footed albatross bycatch model indicate that this species is more likely to be caught in areas of higher probability of its modelled habitat and frequent SST fronts.
4. Discussion
The dynamic nature of marine environments creates selective pressure favouring vagility as an adaptive mechanism of large pelagic vertebrates. Characterizing habitat associations between wide-ranging marine vertebrates and the vast dynamic seascape over which they forage requires an appropriate analytical framework. Using tracking data, we developed dynamic habitat models for two highly mobile pelagic species—Laysan and black-footed albatrosses. These birds roam extensively over the North Pacific and encounter a wide range of habitat conditions ranging from oligotrophic sub-tropical waters to highly productive sub-polar and continental shelf regions. Our habitat models showed distinct spatial segregation between these two species, a finding supported by previous research [8,18,21,52]. Dynamic habitat models take species distribution beyond static range maps and provide information on probabilities of habitat use given environmental conditions at a particular place and time. Our modelling approach demonstrates that preferred habitats of both albatross species are distributed at high latitudes and far away from the main breeding colonies located in the Hawaii Islands during the post-breeding period of these birds (July–November). Conversely, during the early breeding season (December–February), albatrosses find similar oceanographic conditions much closer to their breeding grounds. The results of our habitat-modelling exercise further support earlier work which suggests that seabirds adapt their distribution during the annual cycle to ocean resources [16,18,20]. Performance of habitat models differed between the two albatross species despite input data being of the same quality. Most likely, there are important factors determining black-footed albatross distribution that has not been covered by covariates used in our models, whereas the same variables predicted ocean habitats of Laysan albatrosses with higher precision.
For species occupying their full range in spatially–temporally predictable environments, the emergent projections of a dynamic process may look similar to those of static models. In less predictable situations, however, dynamic models can provide novel insights. Our models predicted suitable habitats for Laysan albatrosses along the California Current despite the fact that the birds tracked in our study did not travel there. It is known, however, that Laysan albatrosses occur in that region: a small population breeds on Guadalupe Island, Mexico, and these birds forage in rich waters of the California Current (W. R. Henry 2003, unpublished data). This exemplifies the usefulness of dynamic models in cases where a sample of tracking data does not fully represent the population. A notable discrepancy between our black-footed albatross habitat model and the known distribution of this species was in the northwest Pacific Ocean. Our model predicted suitable oceanic habitats for black-footed albatrosses in the Sea of Okhotsk during the post-breeding period (July–October; figure 2). While literature sources and tracking data do not suggest the current distribution range of this species extending into that area [37,51,53], populations of albatrosses in the Northern Pacific suffered severe exploitation (deliberate killing for feathers) during the nineteenth and early twentieth centuries [54] and therefore their current range may be smaller than historic or possible range of this species. There is ample archaeological evidence indicating that albatrosses were widespread in the Northern Pacific, including along the coasts of the Sea of Okhotsk and the Sea of Japan in the pre-modern times [54,55]. Moreover, there is a small (ca 2500 pairs [56]) population of black-footed albatrosses that breeds in the Western Pacific (Torishima, Senkaku and Bonin Islands, Japan) but limited data exist on the at-sea distribution and movements of this population [53]. Whether our model has correctly identified potential or recent black-footed albatross range is unknown; nevertheless, our model predictions underscore the potential for dynamic models to provide new information on animal distributions.
Although marine animal-tracking studies are numerous, we believe that tracking data often remain underused particularly in resource management. Whereas the majority of authors restrict their analyses to a description of habitat use and spatial patterns of tracked animals, relatively few make predictions about the extent of habitat suitability, particularly in dynamic environments [45,57] and there are even fewer examples of dynamic habitat models that are then used to answer an applied environmental management question (but see [57,58]). For seabirds, tracking data have primarily been used to indicate general and qualitative spatial overlap between birds and fisheries when analysing seabird bycatch (e.g. [8,10,13]). A key concern here is that telemetry studies are typically restricted to tracking relatively few individuals over short time periods. For wide-ranging species, these sample individuals are unlikely to accurately characterize the species' distribution or its temporal variation, especially in response to periodic or anomalous events (e.g. El Niño cycles, prey regime changes [59]). We also attempted to analyse our albatross telemetry data using static plots of area use. However, these static maps yielded patchy and varying distribution maps that could not be linked to fisheries data (see the electronic supplementary material, S1). Dynamic habitat modelling addresses this problem by quantifying environmental relationships to allow predictions of animal and fisheries distributions (and their probable overlap) beyond recorded observations. This issue was evident in our own dataset; dynamic models described Laysan albatross habitat in the California Current where they are known to occur but where none of our tracked albatrosses travelled. Thus, dynamic models can provide a more realistic and mechanistic interpretation of system dynamics than static approaches.
Fisheries bycatch is a major threat affecting albatross populations [30,60] and one management approach to limit albatross-fishing vessel interactions is to minimize their overlap. In our study, modelled oceanic habitat occurrence of Laysan albatrosses was not a significant covariate in bycatch models of this species owing to limited overlap between Hawaii-based longline fisheries and predicted habitats of Laysan albatrosses. This finding is consistent with earlier studies reporting that Laysan albatrosses frequently travel far from breeding colonies in the Hawaiian Islands and well beyond the operating range of Hawaii-based longline fisheries during the breeding season [18,21]. By contrast, explanatory bycatch models clearly indicated that oceanic habitat occurrence of black-footed albatrosses was among the most significant covariates predicting bycatch of this species. Our model predicted a higher probability of encounter between Hawaii-based longline fleet with black-footed albatrosses than with Laysan albatrosses. This pattern is borne out in NOAA observer data, which indicate that Laysan albatrosses, despite being 10 times as abundant as black-footed albatrosses [37], were less frequently caught by Hawaii-based longline fisheries [27,29].
With respect to data availability, the Hawaii longline fishery served as an ideal case study in that necessary datasets (observer, logbook, telemetry) are robust. However, this fishery has worked for years to reduce seabird (and other protected species) bycatch through a range of management strategies [61]. As such, the number of bycatch encounters and the area of overlap are far lower than would be observed in fleets that have not implemented mitigation measures. This fleet also has a far smaller range relative to both bird species. A full application of this model for these species would require similar datasets from other fishing fleets across the Northern and Central Pacific [8,62]. With information about temporal and spatial extent of other fisheries and along with fisheries observer data, it would be possible to consider the full susceptibility of Laysan and black-footed albatrosses to bycatch across their entire ranges.
We showed that the probability of bird occurrence can be inferred from remotely sensed data in a dynamic way. We also demonstrated that modelled spatial distribution of birds can be a major factor explaining the risk of fisheries bycatch. This approach can therefore lead to adaptive fisheries management practices, thus serving the cause of both sustainable fisheries and conservation of highly mobile species of marine megafauna.
Acknowledgements
All protocols were approved by the UCSC Institutional Animal Care and Use Committee.
This study was funded by the Gordon and Betty Moore Foundation as a part of Project GloBAL (global bycatch assessment of long-lived species). We also thank the Gordon and Betty Moore, David and Lucile Packard, Alfred P. Sloan Foundations, and the National Ocean Partnership Programme (N00014–02–1–1012), and the Office of Naval Research (N00014–00–1–0880 and N00014–03–1–0651) for financial assistance to the Tagging of Pacific Pelagics (TOPP) programme. The Hawaiian Islands National Wildlife Refuge, US Fish and Wildlife Service, Department of the Interior granted a permission to conduct research on Tern Island.
References
- 1.Lewison R. L., Crowder L. B., Read A. J., Freeman S. A. 2004. Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol. Evol. 19, 598–604 10.1016/j.tree.2004.09.004 (doi:10.1016/j.tree.2004.09.004) [DOI] [Google Scholar]
- 2.Hall M. A. 1998. An ecological view of the tuna–dolphin problem: impacts and trade-offs. Rev. Fish Biol. Fish. 8, 1–34 10.1023/A:1008854816580 (doi:10.1023/A:1008854816580) [DOI] [Google Scholar]
- 3.Bull L. S. 2007. Reducing seabird bycatch in longline, trawl and gillnet fisheries. Fish Fish. 8, 31–56 10.1111/j.1467-2979.2007.00234.x (doi:10.1111/j.1467-2979.2007.00234.x) [DOI] [Google Scholar]
- 4.Cox T. M., Lewison R. L., Žydelis R., Crowder L. B., Safina C., Read A. J. 2007. Comparing effectiveness of experimental and implemented bycatch reduction measures: the ideal and the real. Conserv. Biol. 21, 1155–1164 10.1111/j.1523-1739.2007.00772.x (doi:10.1111/j.1523-1739.2007.00772.x) [DOI] [PubMed] [Google Scholar]
- 5.Burger A. E., Shaffer S. A. 2008. Perspectives in ornithology: application of tracking and data-logging technology in research and conservation of seabirds. Auk 125, 253–264 10.1525/auk.2008.1408 (doi:10.1525/auk.2008.1408) [DOI] [Google Scholar]
- 6.Block B. A., Costa D. P., Bograd S. J. 2010. A view of the ocean from Pacific predators. In Life in the world's oceans (ed. Alasdair D.), pp. 291–311 McIntyre, GA: Blackwell Publishing Ltd; 10.1002/9781444325508.ch15 (doi:10.1002/9781444325508.ch15) [DOI] [Google Scholar]
- 7.Peckham S. H., Diaz D. M., Walli A., Ruiz G., Crowder L. B., Nichols W. J. 2007. Small-scale fisheries bycatch jeopardizes endangered Pacific loggerhead turtles. PLoS ONE 2, e1041. 10.1371/journal.pone.0001041 (doi:10.1371/journal.pone.0001041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer K. N., Suryan R. M., Roby D. D., Balogh G. R. 2009. Post-breeding season distribution of black-footed and Laysan albatrosses satellite-tagged in Alaska: interspecific differences in spatial overlap with North Pacific fisheries. Biol. Conserv. 142, 751–760 10.1016/j.biocon.2008.12.007 (doi:10.1016/j.biocon.2008.12.007) [DOI] [Google Scholar]
- 9.Tremblay Y., Bertrand S., Henry R. W., Kappes M. A., Costa D. P., Shaffer S. A. 2009. Analytical approaches to investigating seabird–environment interactions: a review. Mar. Ecol. Prog. Ser. 391, 153–163 10.3354/meps08146 (doi:10.3354/meps08146) [DOI] [Google Scholar]
- 10.BirdLife International 2004. Tracking ocean wanderers: the global distribution of albatrosses and petrels. Results from the Global Procellariiform Tracking Workshop, 1–5 September 2003, Gordon's Bay, South Africa Cambridge, UK: BirdLife International [Google Scholar]
- 11.James M. C., Ottensmeyer C. A., Myers R. A. 2005. Identification of high-use habitat and threats to leatherback sea turtles in northern waters: new directions for conservation. Ecol. Lett. 8, 195–201 10.1111/j.1461-0248.2004.00710.x (doi:10.1111/j.1461-0248.2004.00710.x) [DOI] [Google Scholar]
- 12.Read A. J., Westgate A. J. 1997. Monitoring the movements of harbour porpoises (Phocoena phocoena) with satellite telemetry. Mar. Biol. 130, 315–322 10.1007/s002270050251 (doi:10.1007/s002270050251) [DOI] [Google Scholar]
- 13.Hyrenbach K. D., Dotson R. C. 2003. Assessing the susceptibility of female black-footed albatross (Phoebastria nigripes) to longline fisheries during their post-breeding dispersal: an integrated approach. Biol. Conserv. 112, 391–404 10.1016/S0006-3207(02)00337-3 (doi:10.1016/S0006-3207(02)00337-3) [DOI] [Google Scholar]
- 14.Phillips R. A., Silk J. R. D., Croxall J. P., Afanasyev V. 2006. Year-round distribution of white-chinned petrels from South Georgia: relationships with oceanography and fisheries. Biol. Conserv. 129, 336–347 10.1016/j.biocon.2005.10.046 (doi:10.1016/j.biocon.2005.10.046) [DOI] [Google Scholar]
- 15.Tuck G. N., Polacheck T., Bulman C. M. 2003. Spatio-temporal trends of longline fishing effort in the Southern Ocean and implication for seabird bycatch. Biol. Conserv. 114, 1–27 10.1016/S0006-3207(02)00378-6 (doi:10.1016/S0006-3207(02)00378-6) [DOI] [Google Scholar]
- 16.Shaffer S. A., et al. 2006. Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer. Proc. Natl Acad. Sci. USA 103, 12 799–12 802 10.1073/pnas.0603715103 (doi:10.1073/pnas.0603715103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa D. P., Huckstadt L. A., Crocker D. E., McDonald B. I., Goebel M. E., Fedak M. A. 2010. Approaches to studying climatic change and its role on the habitat selection of Antarctic pinnipeds. Integr. Comp. Biol. 50, 1018–1030 10.1093/icb/icq054 (doi:10.1093/icb/icq054) [DOI] [PubMed] [Google Scholar]
- 18.Kappes M. A., Shaffer S. A., Tremblay Y., Foley D. G., Palacios D. M., Robinson P. W., Bograd S. J., Costa D. P. 2010. Hawaiian albatrosses track interannual variability of marine habitats in the North Pacific. Prog. Oceanogr. 8, 246–260 10.1016/j.pocean.2010.04.012 (doi:10.1016/j.pocean.2010.04.012) [DOI] [Google Scholar]
- 19.Péron C., Authier M., Barbraud C., Delord K., Besson D., Weimerskirch H. 2010. Interdecadal changes in at-sea distribution and abundance of subantarctic seabirds along a latitudinal gradient in the Southern Indian Ocean. Glob. Change Biol. 16, 1895–1909 10.1111/j.1365-2486.2010.02169.x (doi:10.1111/j.1365-2486.2010.02169.x) [DOI] [Google Scholar]
- 20.Dias M. P., Granadeiro J. P., Phillips R. A., Alonso H., Catry P. In press Breaking the routine: individual Cory's shearwaters shift winter destinations between hemispheres and across ocean basins. Proc. R. Soc. B 278, 1786–1793 10.1098/rspb.2010.2114 (doi:10.1098/rspb.2010.2114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyrenbach K. D., Fernandez P., Anderson D. J. 2002. Oceanographic habitats of two sympatric North Pacific albatrosses during the breeding season. Mar. Ecol. Prog. Ser. 233, 283–301 10.3354/meps233283 (doi:10.3354/meps233283) [DOI] [Google Scholar]
- 22.Weimerskirch H. 2007. Are seabirds foraging for unpredictable resources? Deep Sea Res. Part II 54, 211–223 10.1016/j.dsr2.2006.11.013 (doi:10.1016/j.dsr2.2006.11.013) [DOI] [Google Scholar]
- 23.Eckert S. A., Moore J. E., Dunn D. C., Van Buiten R. S., Eckert K. L., Halpin P. Modeling loggerhead turtle movement in the Mediterranean: importance of body size and oceanography. Ecol. Appl. 18, 290–308 10.1890/06-2107.1 (doi:10.1890/06-2107.1) [DOI] [PubMed] [Google Scholar]
- 24.Tew Kai E., Rossi V., Sudre J., Weimerskirch H., Lopez C., Hernandez-Garcia E., Marsac F., Garçon V. 2009. Top marine predators track Lagrangian coherent structures. Proc. Natl Acad. Sci. USA 106, 8245–8250 10.1073/pnas.0811034106 (doi:10.1073/pnas.0811034106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bigelow K. A., Boggs C. H., He X. 1999. Environmental effects on swordfish and blue shark catch rates in the US North Pacific longline fishery. Fish Oceanogr. 8, 178–198 10.1046/j.1365-2419.1999.00105.x (doi:10.1046/j.1365-2419.1999.00105.x) [DOI] [Google Scholar]
- 26.Polovina J. J., Kobayashi D. R., Parker D. M., Seki M. P., Balazs G. H. 2000. Turtles on the edge: movement of loggerhead turtles (Caretta caretta) along oceanic fronts, spanning longline fishing grounds in the central North Pacific, 1997–1998. Fish Oceanogr. 9, 71–82 10.1046/j.1365-2419.2000.00123.x (doi:10.1046/j.1365-2419.2000.00123.x) [DOI] [Google Scholar]
- 27.Cousins K. L. 2001. The black-footed albatross population biology workshop: a step to understanding the impacts of longline fishing on the seabird populations. In Seabird bycatch: trends, roadblocks, and solutions (eds Melvin E. F., Parrish J. K.), pp. 95–114 Fairbanks, AK: University of Alaska Sea Grant; (AK-SG-01-01). [Google Scholar]
- 28.McCracken M. L. 2001. Estimation of albatross take in the Hawaiian longline fisheries. Southwest Fisheries Science Center Administrative Report no. H-01-03 National Marine Fisheries Service, NOAA, Honolulu, HI, USA [Google Scholar]
- 29.Kinan I. 2003. Annual report on seabird interactions and mitigation efforts in the Hawaii-based longline fishery for calendar years 2000 and 2001. Report no. AR-PIR-03-02. National Marine Fisheries Service, Pacific Island Regional Office, Honolulu, HI, USA [Google Scholar]
- 30.Baker G. B., Gales R., Hamilton S., Wilkinson V. 2002. Albatrosses and petrels in Australia: a review of their conservation and management. Emu 102, 71–97 10.1071/MU01036 (doi:10.1071/MU01036) [DOI] [Google Scholar]
- 31.Shaffer S. A., Tremblay Y., Awkerman J. A., Henry R. W., Teo S. L. H., Anderson D. J., Croll D. A., Block B. A., Costa D. P. 2005. Comparison of light- and SST-based geolocation with satellite telemetry in free-ranging albatrosses. Mar. Biol. 147, 833–843 10.1007/s00227-005-1631-8 (doi:10.1007/s00227-005-1631-8) [DOI] [Google Scholar]
- 32.Tremblay Y., et al. 2006. Interpolation of animal tracking data in a fluid environment. J. Exp. Biol. 209, 128–140 10.1242/jeb.01970 (doi:10.1242/jeb.01970) [DOI] [PubMed] [Google Scholar]
- 33.Marks K., Smith W. H. F. 2006. An evaluation of publicly available global bathymetry grids. Mar. Geophys. Res. 27, 19–34 10.1007/s11001-005-2095-4 (doi:10.1007/s11001-005-2095-4) [DOI] [Google Scholar]
- 34.ESRI 2006. ARCGIS. Redlands, CA: Environmental Systems Research Institute [Google Scholar]
- 35.Roberts J. J., Best B. D., Dunn D. C., Treml E. A., Halpin P. N. 2010. Marine geospatial ecology tools: an integrated framework for ecological geoprocessing with ArcGIS, Python, R, MATLAB, and C++. Environ. Model. Softw. 25, 1197–1207 10.1016/j.envsoft.2010.03.029 (doi:10.1016/j.envsoft.2010.03.029) [DOI] [Google Scholar]
- 36.Behrenfeld M. J., Falkowski P. G. 1997. Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol. Oceanogr. 42, 1–20 10.4319/lo.1997.42.1.0001 (doi:10.4319/lo.1997.42.1.0001) [DOI] [Google Scholar]
- 37.BirdLife International 2009. Data zone: species factsheets. See http://www.birdlife.org on 11/8/2009
- 38.Guisan A., Edwards T. C., Jr, Hastie T. 2002. Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol. Model. 157, 89–100 10.1016/S0304-3800(02)00204-1 (doi:10.1016/S0304-3800(02)00204-1) [DOI] [Google Scholar]
- 39.Brotons L., Thuiller W., Araújo M. B., Hirzel A. H. 2004. Presence–absence versus presence-only modelling methods for predicting bird habitat suitability. Ecography 27, 437–448 10.1111/j.0906-7590.2004.03764.x (doi:10.1111/j.0906-7590.2004.03764.x) [DOI] [Google Scholar]
- 40.Kareiva P. M., Shigesada N. 1983. Analyzing insect movement as a correlated random walk. Oecologia 56, 234–238 10.1007/BF00379695 (doi:10.1007/BF00379695) [DOI] [PubMed] [Google Scholar]
- 41.Teo S. H., Boustany A. M., Block B. A. 2007. Oceanographic preferences of Atlantic bluefin tuna, Thunnus thynnus, on their Gulf of Mexico breeding grounds. Mar. Biol. 152, 1105–1119 10.1007/s00227-007-0758-1 (doi:10.1007/s00227-007-0758-1) [DOI] [Google Scholar]
- 42.Burnham K. P., Anderson D. R. 2002. Model selection and multimodel inference: a practical information-theoretical approach, 2nd edn. New York, NY: Springer-Verlag; 10.1016/j.ecolmodel.2003.11.004 (doi:10.1016/j.ecolmodel.2003.11.004) [DOI] [Google Scholar]
- 43.Pearce J., Ferrier S. 2000. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Model. 133, 225–245 10.1016/S0304-3800(00)00322-7 (doi:10.1016/S0304-3800(00)00322-7) [DOI] [Google Scholar]
- 44.Sing T., Sander O., Beerenwinkel N., Lengauer T. 2007. Package ‘ROCR’: visualizing the performance of scoring classifiers. See: http://rocr.bioinf.mpi-sb.mpg.de/ [DOI] [PubMed]
- 45.Aarts G., MacKenzie M., McConnell B., Fedak M., Matthioloulos J. 2008. Estimating space-use and habitat preference from wildlife telemetry data. Ecography 31, 140–160 10.1111/j.2007.0906-7590.05236.x (doi:10.1111/j.2007.0906-7590.05236.x) [DOI] [Google Scholar]
- 46.R Development Core Team 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See: http://www.R-project.org [Google Scholar]
- 47.Candy S. 2004. Modelling catch and effort data using generalised linear models, the Tweedie distribution, random vessel effects and random stratum-by-year effects. CCAMLR Sci. 11, 59–80 [Google Scholar]
- 48.Shono H. 2008. Application of the Tweedie distribution to zero-catch data in CPUE analysis. Fish. Res. 93, 154–162 10.1016/j.fishres.2008.03.006 (doi:10.1016/j.fishres.2008.03.006) [DOI] [Google Scholar]
- 49.Dunn D. C., Kot K. Y., Halpin P. N. 2008. A comparison of methods to spatially represent pelagic longline fishing effort in catch and bycatch studies. Fish. Res. 92, 268–276 10.1016/j.fishres.2008.01.006 (doi:10.1016/j.fishres.2008.01.006) [DOI] [Google Scholar]
- 50.Gilman E., Freifeld H. 2003. Seabird mortality in North Pacific longline fisheries. Endangered Species Update 20, 35–46 [Google Scholar]
- 51.Shuntov V. P. 1972. Morskie ptitsy i biologicheskaya struktura okeana (Sea birds and biological structure of the ocean). Vladivostok, USSR: Far East Publishing House [Google Scholar]
- 52.Suryan R. M., Fischer K. N. 2010. Stable isotope analysis and satellite tracking reveal interspecific resource partitioning of non-breeding albatrosses off Alaska. Can. J. Zool. 88, 299–305 10.1139/Z10-002 (doi:10.1139/Z10-002) [DOI] [Google Scholar]
- 53.Kawakami K., Suzuki H., Horikoshi K., Chiba H., Fukuda A., Higuchi H. 2006. The foraging ranges of black-footed albatross Diomedea nigripes breeding in the Bonin Islands, southern Japan, as determined by GPS tracking. Ornithol. Sci. 5, 187–191 10.2326/1347-0558(2006)5[187:TFROBA]2.0.CO;2 (doi:10.2326/1347-0558(2006)5[187:TFROBA]2.0.CO;2) [DOI] [Google Scholar]
- 54.Yesner D. R. 1976. Aleutian Island albatrosses: a population history. Auk 93, 263–280 [Google Scholar]
- 55.Eda M., Higuchi H. 2004. Distribution of albatross remains in the Far East regions during the Holocene, based on zooarchaeological remains. Zool. Sci. 21, 771–783 10.2108/zsj.21.771 (doi:10.2108/zsj.21.771) [DOI] [PubMed] [Google Scholar]
- 56.Naughton M. B., Romano M. D., Zimmerman T. S. 2007. A conservation action plan for black-footed albatross (Phoebastria nigripes) and Laysan albatross (P. immutabilis), v. 1.0. USFWS, Migratory Birds and Habitat Programs, Pacific Region. See http://www.fws.gov/pacific/migratorybirds/conservation.htm
- 57.Laran S., Gannier A. 2008. Spatial and temporal prediction of fin whale distribution in the northwestern Mediterranean Sea. ICES J. Mar. Sci. 65, 1260–1269 10.1093/icesjms/fsn086 (doi:10.1093/icesjms/fsn086) [DOI] [Google Scholar]
- 58.Panigada S., Zanardelli M., MacKenzie M., Donovan C., Mélin F., Hammond P. S. 2008. Modelling habitat preferences for fin whales and striped dolphins in the Pelagos Sanctuary (western Mediterranean Sea) with physiographic and remote sensing variables. Remote Sens. Environ. 112, 3400–3412 10.1016/j.rse.2007.11.017 (doi:10.1016/j.rse.2007.11.017) [DOI] [Google Scholar]
- 59.Weise M. J., Costa D. P., Kudela R. M. 2006. Movement and diving behavior of male California sea lion (Zalophus californianus) during anomalous oceanographic conditions of 2005 compared to those of 2004. Geophys. Res. Lett. 33, L22S10. 10.1029/2006GL027113 (doi:10.1029/2006GL027113) [DOI] [Google Scholar]
- 60.Croxall J. P. 1998. Research and conservation: a future for albatrosses? In Albatross biology and conservation (eds Robertson G., Gales R.), pp. 269–290 Chipping Norton, Australia: Surrey Beatty and Sons [Google Scholar]
- 61.Gilman E., Brothers N., Kobayashi D. 2005. Principles and approaches to abate seabird by-catch in longline fisheries. Fish Fish. 6, 35–49 10.1111/j.1467-2679.2005.00175.x (doi:10.1111/j.1467-2679.2005.00175.x) [DOI] [Google Scholar]
- 62.Lewison R. L., Crowder L. B. 2003. Estimating fishery bycatch and effects on a vulnerable seabird population. Ecol. Appl. 13, 743–753 10.1890/1051-0761(2003)013[0743:EFBAEO]2.0.CO;2] (doi:10.1890/1051-0761(2003)013[0743:EFBAEO]2.0.CO;2]) [DOI] [Google Scholar]