Abstract
Consensus is growing among ecologists that energy and the factors influencing its utilization can play overarching roles in regulating large-scale patterns of biodiversity. The deep sea—the world's largest ecosystem—has simplified energetic inputs and thus provides an excellent opportunity to study how these processes structure spatial diversity patterns. Two factors influencing energy availability and use are chemical (productive) and thermal energy, here represented as seafloor particulate organic carbon (POC) flux and temperature. We related regional patterns of benthic molluscan diversity in the North Atlantic to these factors, to conduct an explicit test of species–energy relationships in the modern day fauna of the deep ocean. Spatial regression analyses in a model-averaging framework indicated that POC flux had a substantially higher relative importance than temperature for both gastropods and protobranch bivalves, although high correlations between variables prevented definitive interpretation. This contrasts with recent research on temporal variation in fossil diversity from deep-sea cores, where temperature is generally a more significant predictor. These differences may reflect the scales of time and space at which productivity and temperature operate, or differences in body size; but both lines of evidence implicate processes influencing energy utilization as major determinants of deep-sea species diversity.
Keywords: species–energy, productivity, temperature, mollusc, diversity
1. Introduction
Energy and the factors influencing its utilization have emerged as potential unifying causes of spatial and temporal gradients in biodiversity. Three distinct types of energy have been postulated to play a role in structuring ecological communities; radiation (light), thermal (heat) and chemical (Gibbs free energy in reduced carbon compounds in tissues; [1]). In the vast deep-sea soft-sediment environment, photosynthetically active radiation is entirely absent. This simplified system of energetic inputs, together with the extremes of both thermal and chemical energy experienced by these organisms, mean that it represents an excellent testbed for species–energy hypotheses.
Particulate organic carbon (POC) flux represents the ‘rain’ of chemical energy available to deep-sea organisms from the euphotic zone. Flux into the ocean's interior decreases with depth as the material is remineralized and with distance seaward from productive coastal waters. This results in an exponential decline in benthic biomass and abundance with increasing ocean depth [2]. Temperature per se is not a form of energy, but often interpreted as an expression of thermal energy [1]. It directly influences the rate processes of population dynamics and biotic interactions, and regulates the biochemical kinetics of metabolism [3,4].
Recently, intriguing evidence has emerged that temporal variation in deep-sea meiofaunal diversity can be related to temperature. Hunt et al. [5] compared foraminiferan diversity during the last 130 000 years to proxy variables for temperature and productivity. Meta-analysis showed a significant and positive overall temporal relationship with temperature, and no relationship with productivity. Deep-sea ostracod diversity in individual cores has also shown a positive relationship to temporal variation in temperature and a negative relationship to productivity [6,7]. Furthermore, temperature is the most consistent predictor of modern broad-scale species diversity for a wide array of taxa in the shallow ocean [8].
Bathymetric gradients of benthic molluscan diversity are often unimodal, peaking at mid-bathyal depths [9]. Diversity appears to be depressed at abyssal depths because extremely low densities make populations vulnerable to chronic local extinction. Causes of lower diversity at upper bathyal depths are much less clear, but may result from accelerated competitive exclusion owing to greater population growth driven by proximity to high coastal production, or guild interactions resulting from bioturbation by large mobile megafauna [10].
Here, we use spatially explicit regression models to determine the effects of POC flux, temperature and depth (a potential confounding factor) on diversity in modern deep-sea bivalves [11] and gastropods [12], collected from four basins (North American, West European, Guiana and Gambia) in the North Atlantic (figure 1). Although previous studies have related diversity to the environment in the deep sea, this is, to our knowledge, the first study to explicitly test for species–energy relationships in the modern day fauna of the deep ocean. Earlier efforts to infer the relationship between diversity and productivity relied on three proxy variables for productivity: depth, animal density and the application of POC flux-depth algorithms to surface production [9]. We use a newer model developed by Lutz et al. [13] which integrates global data on satellite imagery with POC flux measured at deep-moored sediment traps to provide a more direct estimate of food supply at depth.
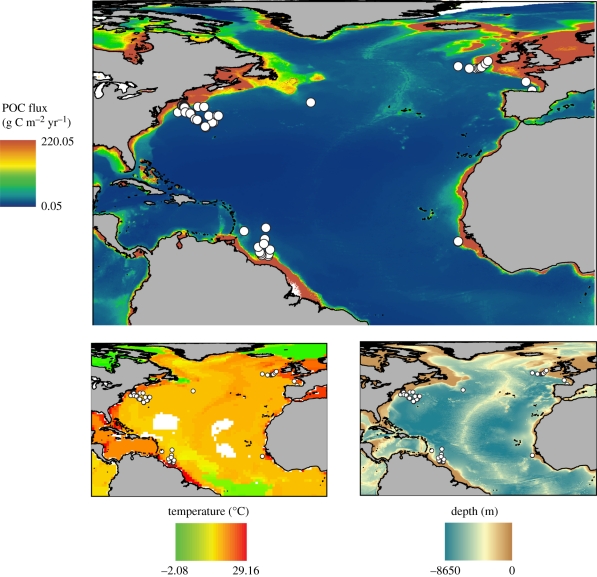
Figure 1.
Locations of study samples (white circles) superimposed on particulate organic carbon (POC) flux, temperature and bathymetry.
2. Material and methods
All samples were collected by epibenthic sleds, which sample seabed-surface sediments over an area of 10–100 m2 per tow, and washed on a 420 µm sieve. Locality data (figure 1) are provided in Allen & Sanders [11] and Stuart & Rex [12]. Sampled species diversity was standardized using the Sanders–Hurlbert expected number of species [14] normalized to sample sizes of 100 for protobranch bivalves and 50 for gastropods (protobranch bivalves being more abundant). POC flux to the seafloor was estimated by the Lutz et al. [13] model. Bottom temperature data were derived from the World Ocean Atlas 2005 [15], and interpolated to sample depths.
We used spatially explicit models to account for the potential effects of spatial autocorrelation on inference, using a spatial eigenvector model (SEVM) approach [16]. Environmental data were natural log-transformed and centred. Spatial models were run in an information-theoretic model-averaging framework [17] following the approach of O'Hara & Tittensor [18], with linear and quadratic terms for temperature, POC flux and depth, and using the small sample size-corrected version of Akaike's Information Criterion to assess goodness of fit. All possible combinations of parameters (except quadratic terms without main effects) were used (26 models). Moran's I tests indicated no significant spatial autocorrelation remained in spatial model residuals (p > 0.05 for all models). Analyses used R, with the package ‘Spdep’ for spatial analyses [19,20].
3. Results
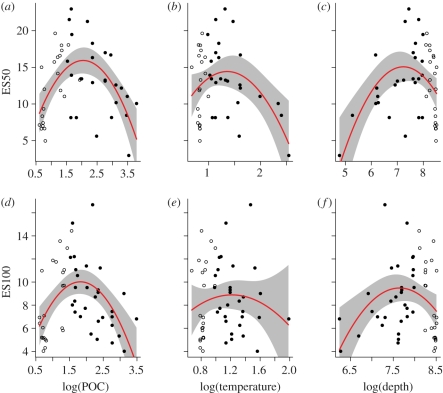
Figure 2 shows the non-spatial relationships of gastropod and bivalve diversity to POC flux, temperature and depth. All three factors showed unimodal trends, peaking at intermediate environmental values. It is noteworthy that a single high-temperature outlier is responsible for the downward slope at the upper end of the bivalve relationship, suggesting that the general trend is not yet robust.
Figure 2.
(a–c) Trends of deep-sea benthic gastropod and (d–f) protobranch bivalve species diversity E(Sn) against particulate organic carbon flux (POC), bottom temperature and depth in the North Atlantic. Closed circles represent samples from less than 3000 m, and open circles greater than 3000 m. Red lines are quadratic linear (non-spatial) model fits, grey-shaded regions represent 95% confidence intervals.
Spatial model-averaged results indicated that POC flux had the highest relative importance (summed Akaike weights) for both gastropods and bivalves by a substantial margin (table 1). More traditional statistical modelling frameworks (minimal adequate and single factor quadratic spatial regression models) also supported this result, with the minimal adequate model for both taxa being composed solely of POC flux and its quadratic term. All three variables were highly correlated (absolute value of r between 0.7 and 0.9), limiting inference, but our results imply that POC flux has the greatest potential effect on deep-sea molluscan diversity.
Table 1.
Model-averaged results. (Spatial eigenvector models were fitted for all possible combinations of parameters and then model-averaged; quadratic terms were not allowed to enter the model without main effects. ‘Relative importance’ is the summed Akaike weights across all models in which a variable occurs; a relative importance of less than 0.3 in a balanced design such as this one is suggestive of minimal or no importance for a predictor variable [17].)
| variable | relative importance | parameter estimate | unconditional standard error |
|---|---|---|---|
| gastropods | |||
| intercept | 1 | 15.54 | 1.17 |
| log(POC) | 0.92 | 1.31 | 1.49 |
| [log(POC)]2 | 0.89 | −3.01 | 1.43 |
| log(temperature) | 0.33 | −0.87 | 4.35 |
| [log(temperature)]2 | 0.09 | 0.42 | 2.56 |
| log(depth) | 0.39 | −0.34 | 2.57 |
| [log(depth)]2 | 0.16 | −0.29 | 0.91 |
| bivalves | |||
| intercept | 1 | 9.95 | 0.41 |
| log(POC) | 1 | 0.71 | 1.03 |
| [log(POC)]2 | 1 | −2.79 | 0.73 |
| log(temperature) | 0.32 | −0.77 | 2.23 |
| [log(temperature)]2 | 0.11 | 0.65 | 2.34 |
| log(depth) | 0.30 | 0.32 | 1.48 |
| [log(depth)]2 | 0.14 | 0.30 | 0.92 |
A striking feature of the dataset is the rapid decline in diversity below 3000 m (figure 2c,f). This difference in diversity between the bathyal zone and the abyss is currently a major focus of deep-sea ecology [9,21].
4. Discussion
The strong and consistent relationship between POC flux and diversity and its substantially greater relative importance than other factors tested (table 1) suggest that chemical energy may be a general driver of molluscan diversity across the full bathymetry between the continental shelf and the abyss. Our results contrast with recent temporal analyses of fossil foraminiferans and ostracods, in which diversities were linear negative functions of or unrelated to productivity [5,22]. This difference may be partly related to body size. Foraminiferans and ostracods are minute meiofauna, whereas molluscs are larger macrofaunal animals. Meiofaunal populations respond to phytodetrital deposition more rapidly than macrofauna, and remain more abundant and diverse at abyssal depths (reviewed in [9]). Even a modest increase in food supply may trigger local competitive displacement and depressed diversity in the same way that macrofauna may respond to heavy nutrient loading at upper bathyal depths.
Another explanation for differences between the temporal meiofaunal studies and our spatial study may be related to the dynamic range of productivity. POC flux varied by around twentyfold (figure 2), whereas productivity indices in the temporal studies appeared to vary by fivefold or less [5,22]. Thus, our study is more likely to elucidate the full range of potential effects of productivity on deep-sea diversity.
Although we were necessarily limited to using rarefied data (ES50/ES100) owing to the semi-quantitative epibenthic sled sampling, such an approach does mean that we can rule out several species–energy mechanisms as being responsible for the observed patterns. Mechanisms that operate through density-related (number of individuals per unit area) processes, namely the ‘sampling effect’ and ‘more individuals’ hypotheses [23] cannot be responsible for our results, which are standardized to identical numbers of individuals at each site. Thus, the species–energy relationship we have described must be driven by density-independent mechanisms (i.e. not dependent on changes in the number of individuals per unit area). While density-dependent mechanisms may also be in operation, these would strengthen observed relationships: for deep-sea consumers such mechanisms would operate through chemical energy processes (increased POC flux energy available to sustain larger population sizes). We do note that if this is the case, the shape of the relationship could change at the upper end (from unimodal to linear). A comparison of rarefied and unrarefied data, when feasible, may help to separate density-dependent and density-independent mechanisms through which energy can influence species richness.
We have provided evidence for a unimodal relationship between energy and deep-sea molluscan diversity (figure 2). Although we cannot conclusively determine whether this species–energy relationship is moderated directly through the supply of chemical energy (POC flux), or indirectly through thermal effects on rate processes (temperature), once accounting for spatial autocorrelation we find greater support for the former. The predictive strength of both chemical and thermal variables points to some form of energy, or factors influencing energy utilization, as major determinants of deep-sea species diversity at large scales of space and time.
Acknowledgements
This research was supported by The Census of the Diversity of Abyssal Marine Life (CeDAMar), a programme of the Census of Marine Life (CoML). D.P.T. acknowledges support from the FMAP programme of the CoML. C.R.M. is supported by the National Evolutionary Synthesis Center (NESCent), NSF no. EF-0905606. We thank the article reviewers for their constructive criticism.
References
- 1.Clarke A., Gaston K. J. 2006. Climate, energy and diversity. Proc. R. Soc. B 273, 2257–2266 10.1098/rspb.2006.3545 (doi:10.1098/rspb.2006.3545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rex M. A., et al. 2006. Global bathymetric patterns of standing stock and body size in the deep-sea benthos. Mar. Ecol. Prog. Ser. 317, 1–8 10.3354/meps317001 (doi:10.3354/meps317001) [DOI] [Google Scholar]
- 3.Allen A. P., Brown J. H., Gillooly J. F. 2002. Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science 297, 1545–1548 10.1126/science.1072380 (doi:10.1126/science.1072380) [DOI] [PubMed] [Google Scholar]
- 4.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B. 2004. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 10.1890/03-9000 (doi:10.1890/03-9000) [DOI] [Google Scholar]
- 5.Hunt G., Cronin T. M., Roy K. 2005. Species–energy relationships in the deep sea: a test using the quaternary fossil record. Ecol. Lett. 8, 739–747 10.1111/j.1461-0248.2005.00778.x (doi:10.1111/j.1461-0248.2005.00778.x) [DOI] [Google Scholar]
- 6.Cronin T. M., Raymo M. E. 1997. Orbital forcing of deep-sea benthic species diversity. Nature 385, 624–627 10.1038/385624a0 (doi:10.1038/385624a0) [DOI] [Google Scholar]
- 7.Yasuhara M., Cronin T. M. 2008. Climatic influences on deep-sea ostracode (Crustacea) diversity for the last three million years. Ecology 89(Suppl.), S53–S65 10.1890/07-1021.1 (doi:10.1890/07-1021.1) [DOI] [PubMed] [Google Scholar]
- 8.Tittensor D. P., Mora C., Jetz W., Lotze H. K., Ricard D., Vanden Berghe E., Worm B. 2010. Global patterns and predictors of marine biodiversity across taxa. Nature 466, 1098–1101 10.1038/nature09329 (doi:10.1038/nature09329) [DOI] [PubMed] [Google Scholar]
- 9.Rex M. A., Etter R. J. 2010. Deep-sea biodiversity: pattern and scale. Cambridge, MA: Harvard University Press [Google Scholar]
- 10.McClain C. R., Barry J. P. 2010. Habitat heterogeneity, disturbance, and productivity work in concert to regulate biodiversity in deep submarine canyons. Ecology 91, 964–976 10.1890/09-0087.1 (doi:10.1890/09-0087.1) [DOI] [PubMed] [Google Scholar]
- 11.Allen J. A., Sanders H. L. 1996. The zoogeography, diversity and origin of the deep-sea protobranch bivalves of the Atlantic: the epilogue. Prog. Oceanogr. 38, 95–153 10.1016/S0079-6611(96)00011-0 (doi:10.1016/S0079-6611(96)00011-0) [DOI] [Google Scholar]
- 12.Stuart C. T., Rex M. A. 2009. Bathymetric patterns of deep-sea gastropod species diversity in 10 basins of the Atlantic Ocean and Norwegian Sea. Mar. Ecol. 30, 164–180 10.1111/j.1439-0485.2008.00269.x (doi:10.1111/j.1439-0485.2008.00269.x) [DOI] [Google Scholar]
- 13.Lutz M. J., Caldeira K., Dunbar R. B., Behrenfeld M. J. 2007. Seasonal rhythms of net primary production and particulate organic carbon flux describe biological pump efficiency in the global ocean. J. Geophys. Res. 112, C10011. 10.1029/2006JC003706 (doi:10.1029/2006JC003706) [DOI] [Google Scholar]
- 14.Hurlbert S. H. 1971. The nonconcept of species diversity: a critique and alternative parameters. Ecology 52, 577–586 10.2307/1934145 (doi:10.2307/1934145) [DOI] [PubMed] [Google Scholar]
- 15.Locarnini R. A., Mishonov A. V., Antonov J. I., Boyer T. P., Garcia H. E. 2006. World Ocean Atlas 2005, vol. 1: temperature. (ed. Levitus S.). Washington, DC: US Government Printing Office [Google Scholar]
- 16.Dormann C. F., et al. 2007. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30, 609–628 10.1111/j.2007.0906-7590.05171.x (doi:10.1111/j.2007.0906-7590.05171.x) [DOI] [Google Scholar]
- 17.Burnham K. P., Anderson D. R. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer [Google Scholar]
- 18.O'Hara T. D., Tittensor D. P. 2010. Environmental drivers of ophiuroid species richness on seamounts. Mar. Ecol. 31(Suppl. 1), 26–38 10.1111/j.1439-0485.2010.00373.x (doi:10.1111/j.1439-0485.2010.00373.x) [DOI] [Google Scholar]
- 19.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org (ISBN 3-900051-07-0). [Google Scholar]
- 20.Bivand R. & with contributions by 24 others 2010. Spdep: spatial dependence: weighting schemes, statistics and models. R package, v. 0.5-21. See http://www.CRAN.R-project.org/package=spdep
- 21.Smith C. R., De Leo F. C., Bernardino A. F., Sweetman A. K., Martinez Arbizu P. 2008. Abyssal food limitation, ecosystem structure and climate change. Trends Ecol. Evol. 23, 518–528 10.1016/j.tree.2008.05.002 (doi:10.1016/j.tree.2008.05.002) [DOI] [PubMed] [Google Scholar]
- 22.Yasuhara M., Hunt G., Cronin T. M., Okahashi H. 2009. Temporal latitudinal-gradient dynamics and tropical instability of deep-sea species diversity. Proc. Natl Acad. Sci. USA 106, 21 717–21 720 10.1073/pnas.0910935106 (doi:10.1073/pnas.0910935106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans K. L., Warren P. H., Gaston K. J. 2005. Speceis–energy relationships at the macroecological scale: a review of the mechanisms. Biol. Rev. 80, 1–25 10.1017/S1464793104006517 (doi:10.1017/S1464793104006517) [DOI] [PubMed] [Google Scholar]