Abstract
Whether the evolution of similar aposematic signals in different unpalatable species (i.e. Müllerian mimicry) is because of phenotypic convergence or advergence continues to puzzle scientists. The poison dart frog Ranitomeya imitator provides a rare example in support of the hypothesis of advergence: this species was believed to mimic numerous distinct model species because of high phenotypic variability and low genetic divergence among populations. In this study, we test the evidence in support of advergence using a population genetic framework in two localities where R. imitator is sympatric with different model species, Ranitomeya ventrimaculata and Ranitomeya variabilis. Genetic analyses revealed incomplete sorting of mitochondrial haplotypes between the two model species. These two species are also less genetically differentiated than R. imitator populations on the basis of both mitochondrial and nuclear DNA comparisons. The genetic similarity between the model species suggests that they have either diverged more recently than R. imitator populations or that they are still connected by gene flow and were misidentified as different species. An analysis of phenotypic variability indicates that the model species are as variable as R. imitator. These results do not support the hypothesis of advergence by R. imitator. Although we cannot rule out phenotypic advergence in the evolution of Müllerian mimicry, this study reopens the discussion regarding the direction of the evolution of mimicry in the R. imitator system.
Keywords: phenotypic advergence, Ranitomeya, mimicry
1. Introduction
The diversification of aposematic signals in Müllerian mimicry is a puzzling phenomenon, because mimicry is expected to promote uniformity in warning signals [1]. Yet substantial variation in aposematic signals has been shown in, for example, Neotropical butterflies [2] and poison dart frogs [3] throughout their range. Such diversification raises the question of whether both sympatric species change towards a common phenotype (i.e. convergence) or if the phenotype of one species moves towards the phenotype of another species that remains constant (i.e. advergence) as expected in Batesian mimicry [4]. Advergence is considered more likely when one species' aposematic signal is established before the arrival of a second species [3,5]. Empirical evidence, principally available indirectly for the putative case of Ranitomeya imitator, has thus far tended to support advergence [6,7].
Symula et al. [3] concluded, using a small number of individuals per population, that the phenotypically variable R. imitator mimics distinct model species in different geographical areas. This was supported by the small divergence measured between mitochondrial haplotypes of different R. imitator colour morphs compared with that observed between model species, suggesting that R. imitator had undergone a relatively recent geographical radiation during which it adopted the aposematic signals of the distinct model species it encountered [3].
However, owing to the importance of this textbook example in the field of evolutionary biology of mimicry [6,7], we re-addressed the theory of advergence in the R. imitator system using population genetics, in the localities originally used for the description of the mimicry association [3]. We compared two adjacent but phenotypically distinct populations of R. imitator and its local sympatric model species, Ranitomeya ventrimaculata and Ranitomeya variabilis. We specifically test the following predictions in support of advergence: (i) the model species display higher genetic differentiation when compared with R. imitator populations, and (ii) R. imitator is more phenotypically variable within and between regions than the model species [5].
2. Material and methods
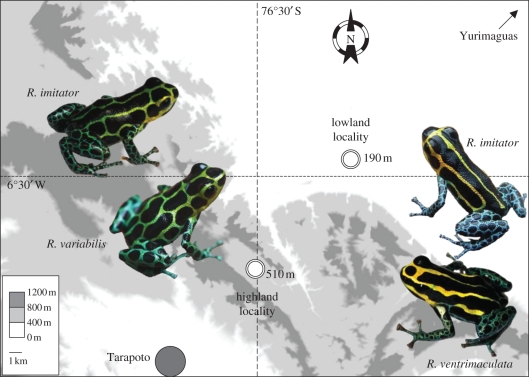
The sampling was performed in the department of San Martin (Peru) in two adjacent localities separated by ca 16 km (figure 1). A minimum of 15 individuals per species were caught in these localities (table 1). Individuals were digitally photographed under standard conditions next to a black–grey–white card for colour standardization, and a toe clipping was collected for genetic analyses.
Figure 1.
Map of the study area showing the localities under investigation and the phenotypes of the paired Ranitomeya species.
Table 1.
Genetic variability and differentiation estimated from nuclear and mitochondrial markers between populations of Ranitomeya imitator and the model species. (The associated probabilities after 999 random permutations are indicated in parenthesis. Test for relevance of molecular information is included for both the nuclear loci and mitochondrial control region.)
|
R. imitator |
model species |
|||
|---|---|---|---|---|
| R. ventrimaculata | R. variabilis | |||
| lowland | highland | (lowland) | (highland) | |
| n = 20 | n = 22 | n = 15 | n = 16 | |
| nuclear | ||||
| mean number of alleles per loci (mean ± s.d.) | 11.2 ± 2.1 | 11 ± 3.4 | 12.5 ± 3.3 | 11 ± 2.83 |
| HE (mean ± s.d.) | 0.863 ± 0.065 | 0.861 ± 0.038 | 0.879 ± 0.044 | 0.803 ± 0.123 |
| FST (95% CI) | 0.103 (0.092–0.118) (p < 0.001) | 0.023 (0–0.041) (p = 0.034) | ||
| 0.074 (0.051–0.098) (p < 0.001)a | ||||
| RST (95% CI) | 0.206 (0.123–0.266) (p < 0.001) | 0.039 (0–0.072) (p = 0.200) | ||
| 0.128 (0.098–0.157) (p < 0.001)a | ||||
| probability that RST > FST | p = 0.002 | p = 0.523 | ||
| mitochondrial | ||||
| number of haplotypes | 7 | 3 | 3 | 4 |
| HD (mean ± s.d) | 0.901 ± 0.076 | 0.432 ± 0.132 | 0.670 ± 0.081 | 0.591 ± 0.121 |
| FST | 0.343 (p < 0.001) | 0.083 (p = 0.069) | ||
| φST | 0.596 (p < 0.001) | 0.115 (p = 0.019) | ||
| probability that φST > FST | p = 0.042 | p = 0.438 | ||
aDifferentiation measured using the four loci displaying the lowest differentiation.
The control region was used to assess the genetic diversity of the mitochondrial genome. The primers 5′-AATGTATATGCCATTATC-3′ and 5′-GAAATATTATAGACCTATATC-3′ designed on Oophaga pumilio sequences [8] successfully amplified a segment of 274 bp for R. imitator and 273 bp for both model species (GenBank accession nos. JF340126–JF340141). Haplotype frequencies were assessed using single strand conformation polymorphism and the distinct haplotypes were sequenced. Sequence alignment was performed using ClustalW. Genetic differentiation between populations was evaluated with both haplotype frequency (FST) and sequences (ϕST) using Arlequin v. 3.1 [9]. Arlequin was also used to infer a haplotype minimum spanning network and to assess the mean number of mutations between populations.
Genetic diversity of the nuclear genome was assessed with nine highly variable microsatellite loci: RimiA06, RimiA07, RimiB01, RimiB02b, RimiB07b, RimiB11, RimiC05b, Rimi E02b, RimiF06 for Ranitomeya imitator and four loci: RvarA09, RvarB01, RvarF08 and RvarG12 for both R. variabilis and R. ventrimaculata according to Brown et al. [10]. Exact tests for Hardy–Weinberg equilibrium, differentiation in allele frequencies and estimation of FST and RST were computed using Arlequin v. 3.1 [9]. The relevance of these metrics (nuclear DNA (nDNA) FST versus RST and mitochondrial DNA (mtDNA) FST versus ϕST) was tested using SPAGeDI [11]. Structure v. 2.3 [12] was used without prior population information to confirm population organization. Runs were performed with a burn-in length and Markov chain Monte Carlo of 100 000 generations and 10 runs were performed for K = 2–5. The best number of clusters K was a posteriori determined according to Evano et al. [13].
The individual photographs were corrected for ambient light colour and the dominant hue of the Hue, Saturation and Brightness colour domain was used to quantify the conspicuous dorsal coloration of individuals [14]. Each individual was then assigned to one of the three following qualitative bins based on their hue: light orange (30°–60°), yellow (61°–90°) and green (91°–120°). The dorsal pattern in the localities studied consisted of three well-separated longitudinal stripes with different degrees of reticulation between them. Each individual was assigned to a qualitative bin based on the amount of reticulation: no reticulation (0), slightly reticulated (1–2), reticulated (3–4) and highly reticulated (5+). Comparison of variance between sites was assessed using Levene's test (W).
3. Results
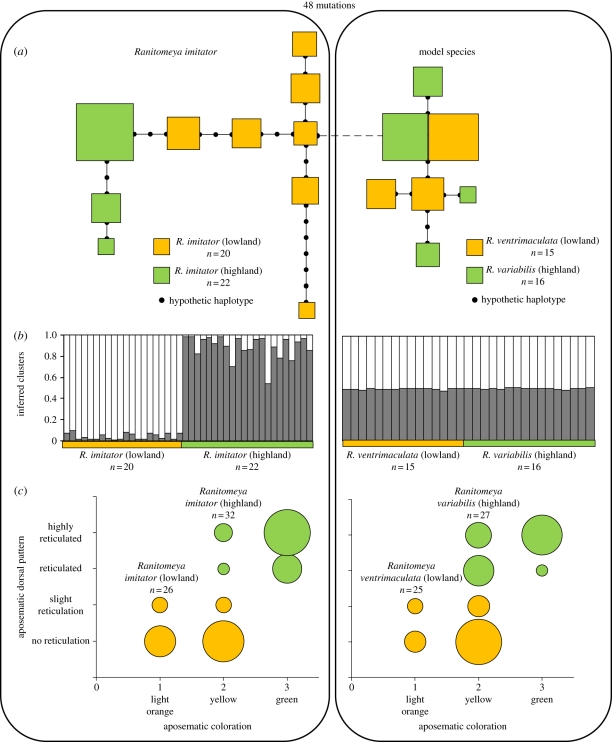
Both the mitochondrial and nuclear markers displayed high diversity within populations (table 1). The mitochondrial minimum spanning network (figure 2a) revealed that populations of R. imitator are characterized by distinct haplotypes, with a mean number of 1.84 mutations between populations. On the other hand, the haplotypes of R. ventrimaculata/variabilis are intermingled and both species had one common haplotype in high frequency, resulting in a number of mutations (0.14) one order of magnitude lower than that of R. imitator. All populations were in Hardy–Weinberg equilibrium for nuclear markers and exact tests of differentiation are significant (p < 0.001). The estimates of differentiation assessed with mtDNA and nDNA (table 1) are higher between populations of R. imitator than between the model species, even when the four loci displaying the lowest differentiation are used to compensate for the different number of loci available between species. SPAGeDI results confirm that the use of the mutational information (FST versus RST) is relevant for R. imitator but not between model species (table 1). This is concordant with the Structure results, where R. imitator individuals are clearly separated into two populations according to their geographical location, while individuals of R. ventrimaculata/variabilis failed to be properly assigned to their species of origin for all K-values tested (figure 2b).
Figure 2.
Genetic and phenotypic differences between the Ranitomeya imitator populations and between model species. (a) Minimum spanning network inferred from the mitochondrial DNA haplotypes. Each box represents a distinct haplotype and its size refers to the relative abundance within populations. (b) Individual assignment among K = 2 clusters (black and white) using Structure and (c) frequency distribution of the different aposematic phenotypes (as colours and dorsal patterns) found within the different populations. The size of the circles represents the relative abundance of the phenotypes.
The comparison of phenotypic variability between R. ventrimaculata and R. imitator living in the lowland localities (W1,6 = 0.194, p = 0.675) and between R. variabilis and R. imitator in the highland (W1,6 = 0.286, p = 0.612) show that both species display similar levels of variation and that they share the same aposematic signal in each locality (figure 2c). When the results for both localities are pooled, R. imitator and R. ventrimaculata/variabilis exhibit the same level of phenotypic variability (W1,14 = 0.007, p = 0.936).
4. Discussion
The predictions of the advergence hypothesis in R. imitator, regarding genetic differentiation and phenotypic variability [3,5], were not supported by the results of the present study.
Results of this study revealed that, in spite of a clearly distinct aposematic signal [15], R. ventrimaculata and R. variabilis display very low genetic differentiation. Incomplete sorting of mtDNA haplotypes, FST values not significantly different from zero and the inability to assign individuals to its species of origin (Structure) clearly indicate a recent separation of these groups. While it is not possible in the absence of data on reproductive isolation to determine whether R. ventrimaculata and R. variabilis are populations of the same species or distinct species, we can conclude that these two groups were recently connected by a common ancestor in both of these scenarios. This is consistent with previous phylogenetic analyses which show that R. ventrimaculata is a polyphyletic taxa characterized by the R. variabilis and R. ventrimaculata from our studied localities being closely related but clearly different from other R. ventrimaculata [16].
These findings are important because the identification of the mimicking species is in part based on phenotypic variability; i.e. the mimicking species is expected to be more phenotypically variable across localities than its model species [5]. Since R. ventrimaculata and R. variabilis belong to the same lineage and have most probably diverged recently, they are in fact as variable as R. imitator in the present system.
Another striking result is that the populations of R. imitator are far more genetically differentiated than those of R. ventrimaculata/variabilis for both genomes. This result is inconsistent with the results of Symula et al. [3], whose objective was to assess the Müllerian mimicry relationship but not its direction. The difference is most probably the result of the small number of individuals analysed by Symula et al. [3], which prevented them from accurately assessing the levels of differentiation between these genetically diverse species. This casts doubt on the hypothesis that populations of R. imitator diversified after the model species and adopted their aposematic signal [3]. To assess the directionality in mimicry, the chronology of founding events in a given locality is essential. However, it is also important to note that genetic differentiation may represent an inappropriate measure of this chronology in the absence of complete phylogeographic information.
Symula et al. [3] also discussed a third species, Ranitomeya summersi (previously called Ranitomeya fantastica), which is mimicked by another colour morph of R. imitator. Because this clade is genetically very distinct from both R. imitator and R. ventrimaculata/variabilis, it could be argued that genetic differences between the model species remain higher than among the R. imitator colour morphs. However, numerous other colour variants in the R. fantasticus clade have been discovered since the initial description of the system, some of which are sympatric and involved in mimicry with both R. imitator and R. ventrimaculata/variabilis [17]. Because all species involved in this Müllerian mimicry show high phenotypic variability, conclusions from phenotypic variability or genetic distances between clades remain inconclusive in this system.
In conclusion, our results cast doubt on the evidence previously used to infer the hypothesis of mimetic advergence in the Ranitomeya species, as our results contradict two key predictions used in the initial description of the system. This study is of particular importance because the R. imitator system is commonly considered to provide the strongest empirical evidence for advergence [6,7]. This does not mean that the theory of advergence is false or that R. imitator is not the mimic, but rather that no empirical evidence exists as of yet. This study reopens the discussion concerning the direction of mimicry in the R. imitator system.
Acknowledgements
The experiments were approved by the University of Montreal's ethics committee and by the Instituto Nacional de Recursos Naturales del Peru which provided the collecting and export permits: no. 005-2008-INRENA-IFFS-DCB and CITES no. 11067.
The authors would like to thank Melanie McClure, Méloé Prud'Homme, Christelle Leung and Jason Brown for their valuable help. This research was supported by a Natural Sciences and Engineering Research Council scholarship of Canada to M.C.
References
- 1.Müller F. 1878. Über die Vortheile der Mimicry bei Schmetterlingen. Zoologischer Anzeiger 1, 54–55 [Google Scholar]
- 2.Brower A. V. Z. 1996. Parallel race formation and the evolution of mimicry in Heliconius butterflies: a phylogenetic hypothesis from mitochondrial DNA sequences. Evolution 50, 195–221 10.2307/2410794 (doi:10.2307/2410794) [DOI] [PubMed] [Google Scholar]
- 3.Symula R., Schulte R., Summers K. 2001. Molecular phylogenetic evidence for a mimetic radiation in Peruvian poison frogs supports a Müllerian mimicry hypothesis. Proc. R. Soc. Lond. B 268, 2415–2421 10.1098/rspb.2001.1812 (doi:10.1098/rspb.2001.1812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallet J. 2001. Causes and consequences of a lack of coevolution in Müllerian mimicry. Evol. Ecol. 13, 777–806 10.1023/A:1011060330515 (doi:10.1023/A:1011060330515) [DOI] [Google Scholar]
- 5.Mallet J., Joron M. 1999. Evolution of diversity in warning color and mimicry: polymorphisms, shifting balance, and speciation. Annu. Rev. Ecol. Syst. 30, 201–233 10.1146/annurev.ecolsys.30.1.201 (doi:10.1146/annurev.ecolsys.30.1.201) [DOI] [Google Scholar]
- 6.Ruxton G. D., Sherratt T. N., Speed M. P. 2004. Avoiding attack: the evolutionary ecology of crypsis, aposematism, and mimicry. Oxford, UK: Oxford University Press [Google Scholar]
- 7.Sherratt T. N. 2008. The evolution of Müllerian mimicry. Naturwissenschaften 95, 681–695 10.1007/s00114-008-0403-y (doi:10.1007/s00114-008-0403-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang I. J., Shaffer H. B. 2008. Rapid color evolution in an aposematic species: a phylogenetic analysis of color variation in the strikingly polymorphic strawberry poison-dart frog. Evolution 62, 2742–2759 10.1111/j.1558-5646.2008.00507.x (doi:10.1111/j.1558-5646.2008.00507.x) [DOI] [PubMed] [Google Scholar]
- 9.Excoffier L., Laval G., Schneider S. 2005. Arlequin v. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinform. Online 1, 47–50 [PMC free article] [PubMed] [Google Scholar]
- 10.Brown J. L., Chouteau M., Glenn T., Summers K. 2009. The development and analysis of twenty-one microsatellite loci for three species of Amazonian poison frogs. Conserv. Genet. Resour. 1, 149–151 10.1007/s12686-009-9036-5 (doi:10.1007/s12686-009-9036-5) [DOI] [Google Scholar]
- 11.Hardy O. J., Vekemans X. 2002. SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2, 618–620 10.1046/j.1471-8278.2002.00305.x (doi:10.1046/j.1471-8278.2002.00305.x) [DOI] [Google Scholar]
- 12.Pritchard J. K., Stephens M., Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155, 945–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evano G., Regnaut S., Goudet J. 2005. Detecting the number of clusters of individuals using the software Structure: a simulation study. Mol. Ecol. 14, 2611–2620 10.1111/j.1365-294X.2005.02553.x (doi:10.1111/j.1365-294X.2005.02553.x) [DOI] [PubMed] [Google Scholar]
- 14.Robertson J. M., Robertson A. D. 2008. Spatial and temporal patterns of phenotypic variation in a Neotropical frog. J. Biogeogr. 35, 830–843 10.1111/j.1365-2699.2007.01824.x (doi:10.1111/j.1365-2699.2007.01824.x) [DOI] [Google Scholar]
- 15.Zimmerman H., Zimmerman E. 1988. Etho-taxonomie und zoogeographische Artengruppenbildung bei Pfeilgiftfröschen (Anura: Dendrobatidae). Salamandra 24, 125–160 [Google Scholar]
- 16.Symula R., Schulte R., Summers K. 2003. Molecular systematic and phylogeography of Amazonian poison frogs of the genus Dendrobates. Mol. Phylogenet. Evol. 26, 452–475 10.1016/S1055-7903(02)00367-6 (doi:10.1016/S1055-7903(02)00367-6) [DOI] [PubMed] [Google Scholar]
- 17.Brown J. L., Twomey E., Pepper M., Rodriguez M. S. 2008. Revision of the Ranitomeya fantastica species complex with description of two new species from Central Peru (Anura: Dendrobatidae). Zootaxa 1832, 1–24 [Google Scholar]