Abstract
We tested for an association between variable number of tandem repeats in the canine androgen receptor (AR) gene and personality differences in Japanese Akita Inu dogs. The polymorphic trinucleotide (CAG) repeat region coding for glutamine in exon 1 of the AR gene was genotyped using genomic DNA obtained from 171 dogs. Three alleles (23, 24 and 26 repeats) were detected, and the allele frequency differed with the coat colour. We assessed the personality profiles of 100 fawn-coloured dogs (54 males and 46 females) based on a questionnaire answered by each dog's owner. The questionnaire consisted of five sub-scales (sociability, playfulness, neuroticism, aggressiveness, distractibility), and the psychometric properties were acceptable based upon internal consistency of the subscales. We found that male dogs with a short allele conferring increased AR function had higher aggressiveness scores than male dogs with longer alleles. By contrast, no evidence was found for a relationship between AR gene variants and personality in females. To our knowledge, our findings provide the first evidence of polymorphism in the AR gene being associated with canine aggression.
Keywords: aggression, androgen receptor gene, dogs, gene polymorphisms, personality
1. Introduction
Androgens such as testosterone play a key role not only in sexual differentiation of males but also in modulating human behaviour [1,2]. The human androgen receptor (AR) gene, located on the X chromosome and composed of eight exons, contains a highly polymorphic trinucleotide (CAG) repeat in exon 1, and the level of transactivation is affected by the length of the polyglutamine stretch [3]. In vitro studies have shown that a relatively short CAG repeat sequence enhances the transcriptional activity of the AR by promoting interactions between the receptor and coactivators [4]. The CAG repeat polymorphisms have been shown to affect reproductive capability and various disease risks in men [5–7]. Moreover, recent studies on human males have reported that shorter CAG repeats are associated with violent criminal behaviour and amygdala reactivity [8,9] and longer CAG repeats with cognitive ageing and male-to-female trans-sexualism [10,11]. With respect to personality, it has been suggested that there are relationships between relatively short repeat polymorphisms and extraversion, verbal aggressiveness and depression [12–14].
The aim of the present study is to explore the potential link between AR gene polymorphism and personality in domestic dogs (Canis familiaris). There are a large number of dog breeds showing behavioural variation, and consequently dogs are recognized as a model species for understanding the genetic basis of behaviour. Most of the attention has been focused on the dopamine receptor D4 gene (DRD4) which is related to novelty seeking in humans [15]. Previous studies demonstrated that polymorphism in the dog DRD4 exon 3 were associated with aggressiveness and activity/impulsivity [16,17], as well as those in intron 2 with social impulsivity [18]. The canine AR gene contains two CAG repeats referred to as the Q1 and Q2 regions of exon 1 [19]. The form and extent of influence of the AR gene on personality measures in dogs, however, is still unknown. It has been reported that AR allele frequencies differ among dog breeds [19,20]. Therefore, we focused on a specific breed, Japanese Akita Inu, which descended from bear-hunting dogs in Japan. This breed is thought to be temporarily bred for fighting and has strong guarding instincts. The genetic constitution of East Asian native breeds, including Japanese Akita Inu, has been shown to be similar to those of wolves [21]. These primitive-type dogs maintain a high genetic diversity owing to a very ancestral origin and short history of artificial selection, which enabled us to investigate the genetic control of canine behavioural variability. In our study, we tested for an association between AR gene variants in Japanese Akita Inu and personality scores obtained from a questionnaire answered by the dogs' owners.
2. Material and methods
We collected genomic DNA from buccal cells of 171 Japanese Akita Inu (83 males and 88 females; 102 fawn, 53 brindle and 16 white-coloured dogs) which were registered by the Akita Inu Introduction Foundation in Japan. Litter siblings were excluded from analysis to remove closely related dogs. All dogs were kept in their owner's house as pets. The methods for genotyping the canine AR gene have been previously reported ([19,20]; see the electronic supplementary material). Since very few polymorphisms were observed in the Q1 region in Japanese Akita Inu, only the CAG repeats in the Q2 region of the AR gene were genotyped [20]. As shown in table 1, we detected three different alleles (23, 24 and 26 repeats) in our population, and the observed allele frequencies did not differ significantly from Hardy–Weinberg equilibrium (χ2 = 0.02, p = 0.999). The distribution of allele frequencies according to coat colours showed that AR allele frequencies were different when comparing fawn and brindle dogs (χ2 = 23.52, p < 0.001).
Table 1.
Allele frequency of AR in Japanese Akita Inu (number of male dogs is shown in parentheses). (Heterozygosity (H) was calculated according to the following equation: H = 2n(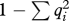 )/(2n − 1).)
)/(2n − 1).)
| n | allele frequency (qi) |
heterozygosity (H) | |||
|---|---|---|---|---|---|
| 23 | 24 | 26 | |||
| total dogs | 171 (83) | 0.58 | 0.21 | 0.22 | 0.58 |
| fawn | 102 (56) | 0.52 | 0.16 | 0.32 | 0.60 |
| brindle | 53 (24) | 0.70 | 0.26 | 0.05 | 0.45 |
| white | 16 (3) | 0.52 | 0.34 | 0.14 | 0.62 |
To eliminate the confounding effect of coat colour, our personality assessment was limited to 100 dogs with fawn-coloured coats (54 males and 46 females). All subjects were intact and sexually mature dogs with a mean age of 3.75 years old, ranging from 13 months to 7 years. Allele 23, which has the smallest number of tandem repeats, was the most common AR allele observed in Japanese Akita Inu. Hence, we categorized allele 23 as short-type (S), and the other relatively scarce alleles 24 and 26 as long-types (L). Based on this grouping, five genotype categories were set as follows: S/− (23/−, n = 30) and L/− (24/− and 26/−, n = 24) for males; and S/S (23/23, n = 13), S/L (23/24 and 23/26, n = 19) and L/L (24/24, 24/26 and 26/26, n = 14) for females. We asked the owners of the subject dogs (all men; mean age, 46.8 years old) to fill out a questionnaire assessing the dogs' personalities. The questionnaire consisted of 30 items designed to characterize the five personality traits of aggressiveness, distractibility, neuroticism, playfulness and sociability (items contained in the aggressiveness trait are shown in table 2). The owners indicated the extent to which a personality description was applicable to the subject dog for each item, using a six-point scale. To confirm the factor structure of the questionnaire, we performed a factor analysis. The scale scores for each dog were calculated as the average scores of items belonging to each of the factors. A one-way analysis of variance with a Bonferroni multiple test correction was used to assess statistical significance for association of the scale scores with the AR genotype (see the electronic supplementary material).
Table 2.
Items of the aggressiveness scale applied in the present study.
| adjective | defining statement |
|---|---|
| dominant | behaves only as he/she pleases and becomes aggressive when interrupted |
| defiant | becomes aggressive, even towards you or other family members when he/she is annoyed |
| moody | seems to be in a bad mood constantly and displays mood swings frequently |
| irritable | impatient and gets angry at people as well as other dogs that he/she dislikes |
| aggressive | threatens or initiates fights with people as well as other dogs, that he/she dislikes |
3. Results
A factor analysis with Varimax rotation on the 30-item ratings of 100 dogs confirmed that the observed factor structure was simple and equivalent to our pre-arranged five personality dimensions (electronic supplementary material, table S1). The internal consistencies of the five personality scales assessed by Cronbach's α were found to be satisfactory, ranging from 0.71 for distractibility to 0.88 for sociability. Table 3 shows the mean scores of each of the personality traits by AR genotypes. We found that the AR genotype in males was significantly associated with only the aggressiveness scale after the Bonferroni correction (p < 0.01), indicating that dogs carrying the S/− genotype displayed higher aggressiveness scores than dogs carrying the L/− genotype (F1,52 = 7.50, p = 0.008). Furthermore, there was no significant difference in any personality scores between the two genotypes included in the L/− category (the 24/− and the 26/− genotype; see the electronic supplementary material). When analysing females, no significant difference was found between genotypes on any of the personality scales in females (table 3).
Table 3.
Mean scores (s.d.) of each of the personality scales as a function of genotype categories. (The p-value in italics indicates a significant value.)
| personality scale | male |
F-value | p | female |
F-value | p | |||
|---|---|---|---|---|---|---|---|---|---|
| S/− | L/− | S/S | S/L | L/L | |||||
| n | 30 | 24 | 13 | 19 | 14 | ||||
| sociability | 3.20 (1.05) | 3.74 (1.16) | 3.09 | 0.09 | 3.71 (1.48) | 3.81 (1.64) | 3.83 (1.72) | 0.02 | 0.98 |
| playfulness | 3.32 (0.73) | 3.42 (1.03) | 0.17 | 0.68 | 3.33 (0.90) | 3.34 (0.79) | 3.30 (0.78) | 0.01 | 0.99 |
| neuroticism | 3.72 (1.07) | 3.35 (1.12) | 1.52 | 0.22 | 3.67 (1.13) | 3.60 (1.15) | 3.48 (1.01) | 0.10 | 0.90 |
| aggressiveness | 3.16 (1.07) | 2.30 (1.23) | 7.50 | <0.01 | 2.06 (1.05) | 2.01 (0.77) | 1.90 (0.81) | 0.12 | 0.88 |
| distractibility | 3.02 (0.92) | 3.10 (1.00) | 0.10 | 0.76 | 2.67 (0.68) | 2.50 (0.77) | 2.75 (0.66) | 0.53 | 0.59 |
4. Discussion
Consistent with previous reports, our genotyping demonstrated that the Q2 region of the AR gene in Japanese Akita Inu had three different alleles and maintained a high genetic diversity with Hardy–Weinberg equilibrium. To ensure the most homogeneous genetic and physiological background possible, we limited our subjects to intact and sexually mature dogs with fawn-coloured coats. We obtained the personality scores of individual dogs from a questionnaire answered by the owners. This indirect method for phenotyping has been accepted for the study of dogs if the psychometric criteria are fulfilled [22]. In this study, internal consistency of subscales was confirmed as rationales for applying the owner-assessment approach (see the electronic supplementary material for test–retest reliability). This suggests that the questionnaire used here is an appropriate tool for providing a rough assessment of personality differences among dogs.
We found that the CAG repeat polymorphism in the AR gene was associated with owner-assessed aggressiveness in male Japanese Akita Inu. It was reasonable that male dogs possessing a short CAG repeat, whose AR responsivity is assumed to be increased according to human studies, had higher aggressiveness scores than dogs with relatively long alleles. These data broaden our knowledge about the influence of the AR gene on men's behaviour [8,12,13] by providing a valid animal model. No evidence was found for a relationship between AR gene variants and personality in female dogs. This could be attributed to a gender difference in androgenic activity. Many studies have demonstrated that males are typically more responsive than females to the behavioural and neuroendocrine actions of androgens [1]. Although the molecular function of the canine AR gene variants should be confirmed, our results suggest that dog personality traits related to aggression might be partially mediated through the androgen receptor. To our knowledge, the present study is the first demonstration of an association between AR gene polymorphism and dog aggressiveness.
Acknowledgements
The present study was approved by the ethical committee at the Wildlife Research Center, Kyoto University (no. WRC-2010-EC001).
We thank Hiroshi Hirama, Manabu Kumagai, Naoki Ohba and members of the Akita Inu Introduction Foundation in Japan for data collection. This study was supported by JSPS Research Fellowships for Young Scientists (no. 213762) to A.K. and by the MEXT with a Grant-in-aid (no. 21310150) to M.I.-M.
References
- 1.Collaer M. L., Hines M. 1995. Human behavioral sex differences: a role for gonadal hormones during early development? Psychol. Bull. 118, 55–107 10.1037/0033-2909.118.1.55 (doi:10.1037/0033-2909.118.1.55) [DOI] [PubMed] [Google Scholar]
- 2.Zitzmann M., Nieschlag E. 2003. The CAG repeat polymorphism within the androgen receptor gene and maleness. Int. J. Androl. 26, 76–83 10.1046/j.1365-2605.2003.00393.x (doi:10.1046/j.1365-2605.2003.00393.x) [DOI] [PubMed] [Google Scholar]
- 3.Gelmann E. P. 2002. Molecular biology of the androgen receptor. J. Clin. Oncol. 20, 3001–3015 10.1200/JCO.2002.10.018 (doi:10.1200/JCO.2002.10.018) [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain N. L., Driver E. D., Miesfeld R. L. 1994. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucl. Acids Res. 22, 3181–3186 10.1093/nar/22.15.3181 (doi:10.1093/nar/22.15.3181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choong C. S., Wilson E. M. 1998. Trinucleotide repeats in the human androgen receptor: a molecular basis for disease. J. Mol. Endocrinol. 21, 235–257 10.1677/jme.0.0210235 (doi:10.1677/jme.0.0210235) [DOI] [PubMed] [Google Scholar]
- 6.Tut T. L., Ghadessy F. J., Trifiro M. A., Pinsky L., Yong E. L. 1997. Long polyglutamine tracts in the androgen receptor are associated with reduced trans-activation, impaired sperm production, and male infertility. J. Clin. Endocrinol. Metab. 82, 3777–3782 10.1210/jc.82.11.3777 (doi:10.1210/jc.82.11.3777) [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E., Stampfer M. J., Krithivas K., Brown M., Brufsky A., Talcott J., Hennekens C. H., Kantoff P. W. 1997. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc. Natl Acad. Sci. USA 94, 3320–3323 10.1073/pnas.94.7.3320 (doi:10.1073/pnas.94.7.3320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajender S., Pandu G., Sharma J. D., Gandhi K. P. C., Singh L., Thangaraj K. 2008. Reduced CAG repeats length in androgen receptor gene is associated with violent criminal behavior. Int. J. Legal Med. 122, 367–372 10.1007/s00414-008-0225-7 (doi:10.1007/s00414-008-0225-7) [DOI] [PubMed] [Google Scholar]
- 9.Manuck S. B., Marsland A. L., Flory J. D., Gorka A., Ferrell R. E., Hariri A. R. 2010. Salivary testosterone and a trinucleotide (CAG) length polymorphism in the androgen receptor gene predict amygdala reactivity in men. Psychoneuroendocrinology 35, 94–104 10.1016/j.psyneuen.2009.04.013 (doi:10.1016/j.psyneuen.2009.04.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaffe K., Edwards E. R., Lui L. Y., Zmuda J. M., Ferrell R. E., Cauley J. A. 2003. Androgen receptor CAG repeat polymorphism is associated with cognitive function in older men. Biol. Psychiatry 54, 943–946 10.1016/S0006-3223(03)00115-X (doi:10.1016/S0006-3223(03)00115-X) [DOI] [PubMed] [Google Scholar]
- 11.Hare L., Bernard P., Sanchez F. J., Baird P. N., Vilain E., Kennedy T., Harley V. R. 2009. Androgen receptor repeat length polymorphism associated with male-to-female transsexualism. Biol. Psychiatry 65, 93–96 10.1016/j.biopsych.2008.08.033 (doi:10.1016/j.biopsych.2008.08.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westberg L., et al. 2009. Influence of androgen receptor repeat polymorphisms on personality traits in men. J. Psychiatry Neurosci. 34, 205–213 [PMC free article] [PubMed] [Google Scholar]
- 13.Jonsson E. G., et al. 2001. Androgen receptor trinucleotide repeat polymorphism and personality traits. Psychiatr. Genet. 11, 19–23 10.1097/00041444-200103000-00004 (doi:10.1097/00041444-200103000-00004) [DOI] [PubMed] [Google Scholar]
- 14.Seidman S. N., Araujo A. B., Roose S. P., McKinlay J. B. 2001. Testosterone level, androgen receptor polymorphism, and depressive symptoms in middle-aged men. Biol. Psychiatry 50, 371–376 10.1016/S0006-3223(01)01148-9 (doi:10.1016/S0006-3223(01)01148-9) [DOI] [PubMed] [Google Scholar]
- 15.Ebstein R. P., et al. 1996. Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of novelty seeking. Nat. Genet. 12, 78–80 10.1038/ng0196-78 (doi:10.1038/ng0196-78) [DOI] [PubMed] [Google Scholar]
- 16.Ito H., et al. 2004. Allele frequency distribution of the canine dopamine receptor D4 gene exon III and I in 23 breeds. J. Vet. Med. Sci. 66, 815–820 10.1292/jvms.66.815 (doi:10.1292/jvms.66.815) [DOI] [PubMed] [Google Scholar]
- 17.Hejjas K., et al. 2007. Association of polymorphisms in the dopamine D4 receptor gene and the activity/impulsivity endophenotype in dogs. Anim. Genet. 38, 629–633 10.1111/j.1365-2052.2007.01657.x (doi:10.1111/j.1365-2052.2007.01657.x) [DOI] [PubMed] [Google Scholar]
- 18.Hejjas K., Kubinyi E., Ronai Z., Szekely A., Vas J., Miklosi A., Sasvari-Szekely M., Kereszturi E. 2009. Molecular and behavioral analysis of the intron 2 repeat polymorphism in the canine dopamine D4 receptor gene. Genes Brain Behav. 8, 330–336 10.1111/j.1601-183X.2008.00475.x (doi:10.1111/j.1601-183X.2008.00475.x) [DOI] [PubMed] [Google Scholar]
- 19.Maejima M., et al. 2005. Allelic variation of two poly-glutamine repeats in the canine androgen receptor gene. J. Anim. Genet. 32, 3–11 [Google Scholar]
- 20.Koshimura A., et al. 2006. Genetic diversity in dog breeds of Asian origin and Asian native dogs. Rep. Soc. Res. Native Livestock 23, 189–207 (In Japanese, with English abstract) [Google Scholar]
- 21.Savolainen P., Zhang Y., Luo J., Lundeberg J., Leitner T. 2002. Genetic evidence for an east Asian origin of domestic dogs. Science 298, 1610–1613 10.1126/science.1073906 (doi:10.1126/science.1073906) [DOI] [PubMed] [Google Scholar]
- 22.Jones A. C., Gosling S. D. 2005. Temperament and personality in dogs (Canis familiaris): a review and evaluation of past research. Appl. Anim. Behav. Sci. 95, 1–53 10.1016/j.applanim.2005.04.008 (doi:10.1016/j.applanim.2005.04.008) [DOI] [Google Scholar]


