Abstract
Fishes use multiple flexible fins in order to move and maintain stability in a complex fluid environment. We used a new approach, a volumetric velocimetry imaging system, to provide the first instantaneous three-dimensional views of wake structures as they are produced by freely swimming fishes. This new technology allowed us to demonstrate conclusively the linked ring vortex wake pattern that is produced by the symmetrical (homocercal) tail of fishes, and to visualize for the first time the three-dimensional vortex wake interaction between the dorsal and anal fins and the tail. We found that the dorsal and anal fin wakes were rapidly (within one tail beat) assimilated into the caudal fin vortex wake. These results show that volumetric imaging of biologically generated flow patterns can reveal new features of locomotor dynamics, and provides an avenue for future investigations of the diversity of fish swimming patterns and their hydrodynamic consequences.
Keywords: fish, swimming, locomotion, volumetric imaging, vortex
1. Introduction
Fluid environments challenge organisms with constantly changing hydrodynamic perturbations, and fishes with flexible fins must continuously react to the surrounding fluid to maintain stability and forward propulsion. In order to understand how fishes achieve normal steady locomotion, researchers have studied the kinematics of fish fins with increasingly accurate techniques, moving from planar to three-dimensional methods [1–7]. However, understanding the motion of the fins alone does not explain how fishes move; the viscous fluid in which they swim must also be considered.
Until now, analysis of hydrodynamic wake structures has been limited to two-dimensional particle image velocimetry (PIV) techniques and stereo-PIV, which gives three-dimensional velocity vector components for vectors within a plane [3,6,8–16]. While these techniques have given us a great deal of insight into the fluid mechanics of fish locomotion, the current method of inferring three-dimensional wake structure from repeated two-dimensional PIV slices produces considerable room for error. A mechanism that instantaneously captures a three-dimensional wake structure is needed in order to understand fully the fluid interactions between fishes and their environment, and among the different fins used for propulsion [16,17].
The goal of this paper is to use a novel volumetric velocimetry system to test for the vortex wake structures hypothesized, using two-dimensional techniques in fishes swimming with an externally symmetrical (homocercal) caudal fin [11,12], and to examine the interaction between the dorsal and anal fin wake and the tail fin, which has been technically difficult to do with traditional imaging approaches [16].
2. Material and methods
Four bluegill sunfish (Lepomis macrochirus; mean total length (TL) = 15.5 cm) and one cichlid fish (Pseudotropheus greshakei; TL = 12 cm) were kept in individual 40 l aquaria with 12 L : 12 D cycles and fed three times weekly. For data capture, fish were placed into a 160 l recirculating flow tank with an 80 × 20 × 20 cm working area, and trained to swim in the centre of flow, away from the walls.
Fish swam in a flow tank seeded with 50 µm plastic particles suspended in flow. The fluid downstream of the swimming bluegill was illuminated by a 120 mJ dual-head pulse laser pulsed at a frequency of 7.25 Hz (electronic supplementary material, figure S1), synchronized with a volumetric 3-component velocimetry (V3V) volumetric flow imaging system (TSI Incorporated, Shoreview, Minnesota, USA). Particle position and displacements were calculated between laser pulses, using V3V software as detailed in Troolin & Longmire [18] and Pereira et al. [19].
Vector data were visualized in Tecplot 360, and Reynolds (Re) and Strouhal (St) numbers, jet angle and mean vorticity maximum were quantified. Jet angle was defined as the angle relative to the downstream flow (as in Lauder & Drucker [12]) and mean vorticity maxima were determined from the centre of vorticity of most recently formed, complete (detached from tail) vortex ring.
3. Results
Fishes swam steadily at 1.1 body lengths per second (L s−1; Re = 28 600 ± 316, St = 0.50 ± 0.04) and 1.5 L s−1 (Re = 38 300 ± 1350, St = 0.39 ± 0.02) with the tail and dorsal and anal fins inside the laser volume, as well as upstream, for a minimum of 2 s, during which time 15 volumes were captured in any one sequence and 41 sequences captured in total. The homocercal tail of steadily swimming teleost fishes produced a series of linked ring vortices (figure 1). Vortices were also shed from the dorsal and ventral trailing edges of the caudal fin (figure 2). The caudal fin of bluegill and the cichlid produced a jet with a mean angle of 65.91 ± 1.38° relative to the downstream flow, with no significant difference between the two swim speeds (p < 0.0001). The mean maximum vorticity of rings produced by fishes swimming at 1.1 L s−1 was 9.38 ± 0.84 s−1 and 16.25 ± 1.26 s−1 by fishes swimming at 1.5 L s−1.
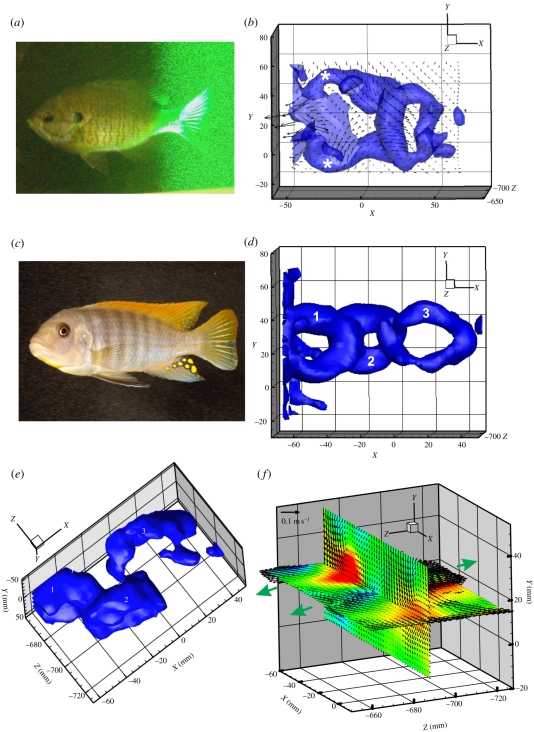
Figure 1.
Instantaneous volumetric wake visualization of vortices produced by the symmetrical tail of freely swimming fishes. (a) Bluegill sunfish (Lepomis macrochirus) swimming with the tail, dorsal and anal fins within the laser light volume and the vorticity isosurface (at 5.0 s−1), to show the linked vortex rings produced by the tail. (b) White asterisks denote trailing edge vortices separating from the caudal fin of the bluegill. (c,d) Cichlid fish Pseudotropheus greshakei swimming just upstream of the laser volume and the linked chain vortex wake produced by the tail. (e) Angled view from below to show the vorticity isosurface and the three tail vortices (numbers correspond to rings shown in panel (d)). (f) Two orthogonal slices through the tail vortex wake to show the three-dimensional structure of the wake and the alternating jet flows (green arrows).
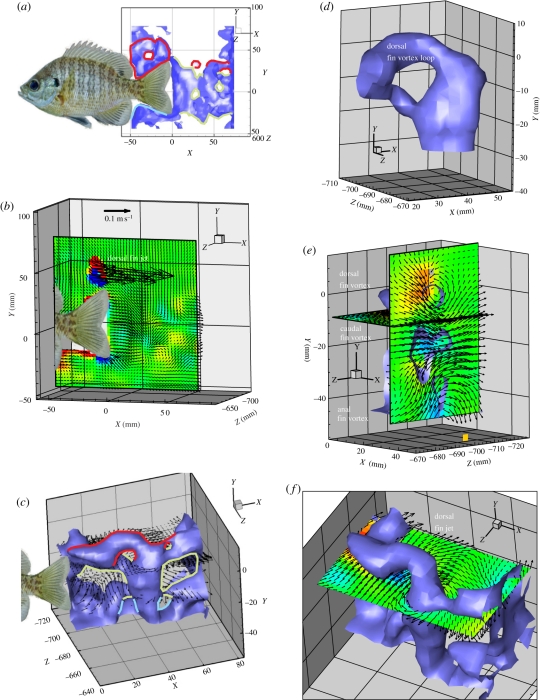
Figure 2.
(a) Vortical wake produced by dorsal (red), anal (blue) and caudal (green) fins of freely swimming bluegill sunfish (at 1.5 L s−1) isosurfaced by absolute vorticity (5.0 s−1). Alternating dorsal and anal fin vortices are visible due to location of these fins within the capture volume. (b) Slice of isosurface in (a), contoured by Z vorticity. (c) Three-quarter view of wake interaction of vortices produced by dorsal (red), anal (blue) and caudal (green) fins of live swimming bluegill (1.5 L s−1) isosurfaced by absolute vorticity (5.0 s−1). Velocity vectors are drawn from XZ planes bisecting the dorsal and caudal fin vortices, with every third velocity vector shown for clarity. (d) Close view of the dorsal fin vortex loop in (c) joining the caudal vortex ring. (e) Sections through the vortex wake to show the dorsal, caudal and anal fin vortices in the YZ plane and the resulting side jet (directed to the right in this figure). (f) Horizontal slice through the vortex wake to show the side momentum produced by the dorsal fin. Fish image has been inserted into panels a, b and c to indicate the location of the fins.
Dorsal fin wake properties were highly variable; jet angle ranged from 17.8 to 92.6° (mean = 65.32 ± 13.7°) relative to downstream flow with lower jet angles being correlated with higher swim speeds, and mean maximum vorticity ranged from 5.6 to 22.3 s−1 (mean = 12.9 ± 3.02 s−1). Conversely, anal fins produced a more consistent jet angle of 90.32 ± 0.32° with a mean maximum vorticity of 6.33 ± 1.53 s−1. In some cases, the wake produced by the dorsal or anal fin could be seen clearly anterior to the location of the caudal fin (figure 2c,e). Downstream of the caudal fin, vortices produced by the dorsal or anal fin assimilated with the most recent fully formed caudal vortex; once a second vortex ring had been produced by the caudal fin, the dorsal or anal fin vortex was nearly completely entrained by its corresponding caudal fin vortex (figure 2c,d). The vorticity magnitude produced by the dorsal and anal fins was not significantly different from the vorticity magnitude produced by the caudal fin during associated tail beats (p < 0.01).
4. Discussion
The results presented here represent the first application of instantaneous volumetric PIV, or V3V, to be used in the study of swimming hydrodynamics of teleost fishes. Previous attempts to understand wake hydrodynamics produced by a homocercal tail employed repeated two-dimensional PIV techniques or stereo-PIV [15], to attempt to infer the three-dimensional wake structure produced by swimming fishes, or computational fluid dynamics (CFD) modelling techniques [20]. The ability to capture experimentally a three-dimensional snapshot of the wake structure created by a single individual removes the problem of aligning two-dimensional slices collected at different times. In addition, the three-dimensional structure of the wake shed by the dorsal and anal fins, and how this vorticity interacts with tail vortices, has not been directly visualized previously.
Two teleost fishes, cichlid and bluegill sunfish, both possessing homocercal (externally symmetrical) tails, produced single vortex ring structures with each lateral pass of the tail, and volumetric imaging produces wake flow patterns that closely match current models based on two-dimensional analyses in most respects (figure 1a–d; [11,12]). As expected, continuous tail beats resulted in the formation of a linked chain of vortex rings. This same structure was also hypothesized for the mackerel, which possesses a more forked homocercal tail, using both planar PIV and three-dimensional CFD techniques [14,20].
Using multiple two-dimensional PIV slices in the transverse plane, Tytell [16] inferred that the dorsal and anal fin wakes would interact with the caudal fin and the wake it produced, but the duration and extent of median fin wake interaction was not known due to the inability to image the area of interaction above and below the tail in three dimensions. It is now evident that the dorsal and anal fin wakes are rapidly entrained by the caudal fin wake, approximately within the timeframe of a subsequent tail beat (figure 2). Volumetric images revealed a great deal more variability of the dorsal fin jet angle and mean maximum vorticity when compared with the relatively consistent anal fin wake. While the mean angle of the jet produced by the dorsal fin here (65.32 ± 13.7°) was similar to the 62.4 ± 1.8° jet angle discovered by Drucker & Lauder [9], the extent to which dorsal fin jet angle varied was not found in previous research. The dorsal fin produced a jet at lower angles to the direction of motion when the fish swam at higher speeds, suggesting that a greater portion of the jet contributed thrust and added momentum to the caudal fin vortex than compared with swimming at lower speeds.
Volumetric imaging of flows produced by moving animals is a long-sought goal in comparative biomechanics and allows previously hypothesized fluid motion resulting from fin and body movements to be quantified in three dimensions, without the assumptions inherent in reconstructing flow patterns from two-dimensional slices. These results show that volumetric imaging of biologically generated flow patterns can reveal new features of locomotor dynamics, and provides an avenue for future investigations of the diversity of fish swimming patterns and their hydrodynamic consequences.
Acknowledgements
We thank members of the Lauder Laboratory for assistance with animal care, and NSF EFRI-0938043 for research support.
References
- 1.Alben S., Madden P. G. A., Lauder G. V. 2007. The mechanics of active fin-shape control in ray-finned fishes. J. R. Soc. Interface 4, 243–256 10.1098/rsif.2006.0181 (doi:10.1098/rsif.2006.0181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bainbridge R. 1963. Caudal fin and body movement in the propulsion of some fish. J. Exp. Biol. 40, 23–56 [Google Scholar]
- 3.Drucker E. G., Lauder G. V. 2005. Locomotor function of the dorsal fin in rainbow trout: kinematic patterns and hydrodynamic forces. J. Exp. Biol. 208, 4479–4494 10.1242/jeb.01922 (doi:10.1242/jeb.01922) [DOI] [PubMed] [Google Scholar]
- 4.Flammang B. E., Lauder G. V. 2008. Speed-dependent intrinsic caudal fin muscle recruitment during steady swimming in bluegill sunfish, Lepomis macrochirus. J. Exp. Biol. 211, 587–598 10.1242/jeb.012096 (doi:10.1242/jeb.012096) [DOI] [PubMed] [Google Scholar]
- 5.Jayne B. C., Lozada A. F., Lauder G. V. 1996. Function of the dorsal fin in bluegill sunfish: motor patterns during four distinct locomotor behaviors. J. Morphol. 228, 307–326 (doi:10.1002/(SICI)1097-4687(199606)228:3<307::AID-JMOR3>3.0.CO;2-Z) [DOI] [PubMed] [Google Scholar]
- 6.Muller U. K., van den Huevel B. L. E., Stamhuis E. J., Videler J. J. 1997. Fish foot prints: morphology and energetics of the wake behind a continuously swimming mullet (Chelon labrosus). J. Exp. Biol. 200, 2893–2906 [DOI] [PubMed] [Google Scholar]
- 7.Standen E. M., Lauder G. V. 2005. Dorsal and anal fin function in bluegill sunfish Lepomis macrochirus: three-dimensional kinematics during propulsion and maneuvering. J. Exp. Biol. 208, 2753–2763 10.1242/jeb.01706 (doi:10.1242/jeb.01706) [DOI] [PubMed] [Google Scholar]
- 8.Drucker E. G., Lauder G. V. 1999. Locomotor forces on a swimming fish: three-dimensional vortex wake dynamics quantified using digital particle image velocimetry. J. Exp. Biol. 202, 2393–2412 [DOI] [PubMed] [Google Scholar]
- 9.Drucker E. G., Lauder G. V. 2001. Locomotor function of the dorsal fin in teleost fishes: experimental analysis of wake forces in sunfish. J. Exp. Biol. 204, 2943–2958 [DOI] [PubMed] [Google Scholar]
- 10.Drucker E. G., Lauder G. V. 2001. Wake dynamics and fluid forces of turning maneuvers in sunfish. J. Exp. Biol. 204, 431–442 [DOI] [PubMed] [Google Scholar]
- 11.Lauder G. V. 2000. Function of the caudal fin during locomotion in fishes: kinematics, flow visualization, and evolutionary patterns. Am. Zool. 40, 101–122 10.1668/0003-1569(2000)040[0101:FOTCFD]2.0.CO;2 (doi:10.1668/0003-1569(2000)040[0101:FOTCFD]2.0.CO;2) [DOI] [Google Scholar]
- 12.Lauder G. V., Drucker E. G. 2002. Forces, fishes, and fluids: hydrodynamic mechanisms of aquatic locomotion. News Physiol. Sci. 17, 235–240 [DOI] [PubMed] [Google Scholar]
- 13.Lauder G. V., Madden P. G. A. 2007. Fish locomotion: kinematics and hydrodynamics of flexible foil-like fins. Exp. Fluids 43, 641–653 10.1007/s00348-007-0357-4 (doi:10.1007/s00348-007-0357-4) [DOI] [Google Scholar]
- 14.Lauder G. V., Nauen J. C., Drucker E. G. 2002. Experimental hydrodynamics and evolution: function of median fins in ray-finned fishes. Integr. Comp. Biol. 42, 1009–1017 10.1093/icb/42.5.1009 (doi:10.1093/icb/42.5.1009) [DOI] [PubMed] [Google Scholar]
- 15.Nauen J. C., Lauder G. V. 2002. Quantification of the wake of rainbow trout (Oncorhynchus mykiss) using three-dimensional stereoscopic digital particle image velocimetry. J. Exp. Biol. 205, 3271–3279 [DOI] [PubMed] [Google Scholar]
- 16.Tytell E. D. 2006. Median fin function in bluegill sunfish Lepomis macrochirus: streamwise vortex structure during steady swimming. J. Exp. Biol. 209, 1516–1534 10.1242/jeb.02154 (doi:10.1242/jeb.02154) [DOI] [PubMed] [Google Scholar]
- 17.Tytell E. D., Standen E. M., Lauder G. V. 2008. Escaping flatland: three-dimensional kinematics and hydrodynamics of median fins in fishes. J. Exp. Biol. 211, 187–195 10.1242/jeb.008128 (doi:10.1242/jeb.008128) [DOI] [PubMed] [Google Scholar]
- 18.Troolin D. R., Longmire E. K. 2010. Volumetric velocity measurements of vortex rings from inclined exits. Exp. Fluids 48, 409–420 10.1007/s00348-009-0745-z (doi:10.1007/s00348-009-0745-z) [DOI] [Google Scholar]
- 19.Pereira F., Gharib M., Dabiri D., Modarress D. 2000. Defocusing digital particle image velocimetry: a 3-component 3-dimensional DPIV measurement technique. Application to bubbly flows. Exp. Fluids 29, 78–84 10.1007/s003480070010 (doi:10.1007/s003480070010) [DOI] [Google Scholar]
- 20.Borazjani I., Sotiropoulos F. 2008. Numerical investigation of the hydrodynamics of carangiform swimming in the transitional and inertial flow regimes. J. Exp. Biol. 211, 1541–1558 10.1242/jeb.015644 (doi:10.1242/jeb.015644) [DOI] [PubMed] [Google Scholar]