Abstract
Differences between sexes in cognitive processes are widespread in humans and permeate many, if not most, cognitive domains. In animal cognition research, however, possible sex differences are still often neglected. Here, we provide striking evidence for a sex-specific response in an object permanence task in domestic dogs (Canis familiaris). Female dogs responded with significantly increased looking times to a violation of expectancy—a ball ‘magically’ changing size while temporarily occluded. By contrast, male dogs, irrespective of their neuter status, did not respond to the size constancy violation. These results indicate that sex differences in basic cognitive processes may extend to mammals in general, and call for increased consideration of possible sex effects when analysing and interpreting data in animal cognition.
Keywords: cognition, dog, sex difference, object permanence
1. Introduction
Sex differences in cognitive abilities are widespread in humans, for example in perceptual, visual–spatial, verbal and quantitative-mathematical abilities [1,2], although the extent and basis of these differences remain controversial (e.g. [3]). In non-human mammals as well, some sex differences are well documented, such as differences in spatial abilities between males and females in some polygynous rodents (reviewed in [4]), or differences in learning performances in conditioning tasks (reviewed in [5]). However, in many other domains, animal cognition research still widely ignores possible sex differences, despite earlier calls to the contrary (e.g. [6,7]).
Since sex differences in cognitive abilities are probably explained by effects of hormonal differences (either selected for directly or as a by-product of other traits [1,2]), we expect them to be widespread in all mammals and extend to most, if not all, cognitive domains, including physical cognition. Here we tested male and female domestic dogs (Canis familiaris) in an object permanence task. Unlike previous studies of object permanence in dogs [8–11], which focused on visible and invisible displacements, our experiment aimed to determine their understanding that objects do not change their size while temporarily occluded (size constancy), an ability that develops in children in the first year of life [12,13]. We used the expectancy-violation paradigm, which has been successfully transferred from children to dogs previously (e.g. for a numerosity task [14]). With this approach, a subject's sensitivity to perceptual changes can be tested. However, it does not necessarily imply complex cognitive processing (e.g. [15]). To rule out possible novelty confounds associated with shaping trials [16], we conducted single-test trials only in a between-subjects design (following [17]).
2. Material and methods
(a). Subjects and apparatus
Fifty privately owned dogs aged 1–10 years and of various breeds participated in this study—25 males and 25 females (see the electronic supplementary material, table S1, for details). They were shown either an expected or an unexpected event in a between-subjects design. The dogs were assigned randomly to one of the two conditions with the stipulation of a balanced sex ratio in each condition.
The unexpected event consisted of a ball disappearing behind a screen and a ball of different size (but otherwise identical) appearing on the other side. For the expected event, the appearing ball had the same size as the disappearing ball (note that also for this event, two balls were used). The start positions of the two balls were 40 cm to the left of the screen (disappearing ball) and behind the screen (appearing ball, see also electronic supplementary material, figure S1). The end position was behind the screen (disappearing ball) and 40 cm to the right of the screen (appearing ball). The balls used were blue tennis balls with a diameter of 6.5 cm (small) or 15 cm (large). Each dog was shown one of the following four sequences: (i) small ball disappears, another small ball appears (expected event), (ii) small ball disappears, large ball appears (unexpected event), (iii) large ball disappears, another large ball appears (expected event), and (iv) large ball disappears, small ball appears (unexpected event).
The experimental apparatus (see the electronic supplementary material, figure S1) consisted of a 2 m wooden plank fitted with rails along which the balls were pulled by means of attached transparent nylon strings. The centre part of the plank was occluded by a 93 cm long screen. The strings were operated by the experimenter while hidden behind a barrier placed at the extension of the rail plank. A camera placed at the edge of the barrier on the ground allowed the experimenter to see when the dog was sitting calmly and oriented towards the apparatus. The experiment was recorded with four cameras, one of which was aimed directly at the dog while seated, the remaining ones were placed in three corners of the room.
(b). Procedure
All tests were conducted in the same 5 × 6.4 m large room at the Clever Dog Lab in Vienna. At the beginning of the test, the dog entered the room together with its owner and was allowed to explore the set-up with no balls present for 1 min. Thereafter, the owner was asked to play with the dog for 30 s each with the large and the small ball. The owner and the dog then briefly left the room whereupon the experimenter (Ch.M.) placed the two balls in their respective positions. After the experimenter had taken her position behind the barrier, the dog and the owner re-entered the room, the owner took a seat 2 m in front of the screen, placed the dog between the legs and put on a blindfold. Once the dog was calm and facing the apparatus, the experimenter started the sequence: by means of attached transparent strings, a ball was pulled behind the barrier, whereupon a ball of the same or different size (previously hidden behind the barrier) was pulled out at the other end (see the electronic supplementary material, video clip, for an example).
(c). Analysis
The time that the dog spent looking at the appearing ball was determined from video recordings using frame-by-frame analysis in Solomon Coder v. 10.09 (András Péter, Eötvös Loránd University, Budapest, Hungary). Looking time was defined as the time spent motionless with the head oriented towards the ball, starting from the time when the ball appeared from behind the screen and ending with the first head movement (upwards, downwards or sideways). We used head direction as a proxy of gaze direction (as in e.g. [14,18]), since pupil direction could not be reliably determined with our set-up. This is because the dogs' heads had to remain mobile to look first at the disappearing ball and then at the appearing ball and thus we could not use close-up recording of the subjects' eyes. All the videos were coded by S.D. and Ch.M. (consensus coding). A random sample (20 of the 50 videos) was additionally coded by C.A.M. and inter-observer reliability for looking times was high (Cronbach's α = 0.99, maximum deviation between coders = 2.2 s).
Looking time data were log-transformed and analysed in R v. 2.10.0 using a general linear model (GLM) with the following variables included as predictors: experimental condition (expected versus unexpected), subject sex, size of the appearing ball (large or small) and play motivation (proportion of time oriented to the ball in the play session as determined from the video recordings).
3. Results
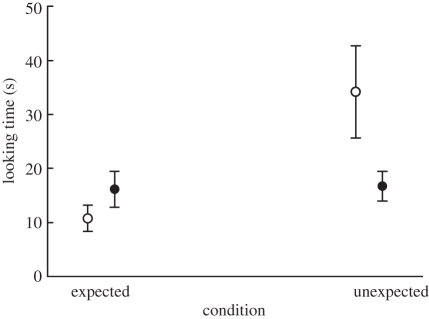
Overall, looking times were longer when an unexpected event was shown than when an expected event was shown (GLM, F1,45 = 10.3, p = 0.002). However, this effect was restricted to females and absent for males (sex × condition interaction: F1,44 = 5.67, p = 0.022, figure 1). The missing effect for males was not due to neuter status, as neutered and intact males looked equally long at the two conditions (neuter status × condition interaction: F1,21 = 0.28, p = 0.60; the electronic supplementary material, table S2). Likewise, no influence of neuter status on looking time was found for the females (neuter status × condition interaction: F1,21 = 0.17, p = 0.68; the electronic supplementary material, table S2). Neither play motivation nor the size of the appearing ball had an effect on the dogs' looking time (table 1).
Figure 1.
Looking time at the appearing ball in the expected and unexpected conditions for males and females separately. Shown are mean ± s.e.m. Filled circles, male; open circles, female.
Table 1.
Factors influencing looking time at the appearing ball. Statistics are given for each factor entered last in a sequential model.
| explanatory term | F-statistic | d.f. | p |
|---|---|---|---|
| play motivationa | 0.39 | 1,45 | 0.53 |
| size of appearing ball (large, small) | 2.35 | 1,45 | 0.13 |
| sex (male, female) | 0.01 | 1,45 | 0.94 |
| condition (expected, unexpected) | 10.4 | 1,45 | 0.002 |
| sex × condition interaction | 5.67 | 1,44 | 0.022 |
aPer cent of time playing with the balls in the familiarization phase.
4. Discussion
Our results show a striking sex difference in a basic cognitive task: female dogs, but not male dogs, responded to a size constancy violation with a significant increase in looking time. While sex differences in spatial cognition tasks have been demonstrated in various rodent and primate species (reviewed in [4]), where males generally outperform females, our study to our knowledge provides the first demonstration of such a sex-specific performance in a physical cognition task in a non-human mammal. This result is supported by our reanalysis of the data presented in Rooijakkers et al. [11], which revealed a similar female advantage in another object permanence task in dogs, although not significant, possibly owing to small sample size (p = 0.079; see the electronic supplementary material, table S3 for details). Together, these results suggest that a sex-biased performance in object permanence tasks might be a robust phenomenon in dogs.
Three main mechanisms are typically cited as basis for sex differences in the performance in cognitive tasks: (i) sex-specific selection pressures in the past, (ii) sex-specific environmental influences during ontogeny, and (iii) cognitive differences between sexes as a by-product of other sex differences. We believe that the latter explanation is the most likely basis for our results for the following reasons.
Sex differences in performance in spatial cognition is typically explained by sex-specific selection pressures reflecting home range size and lifestyle [4]. In addition, some of the numerous sex differences found in humans may be explained by selection pressures that operated in the past on hunter–gatherer societies [1,2]. In dogs and their pack-living ancestors, however, life styles were unlikely to have differed systematically between the sexes and any division of labour probably did not extend beyond the task of rearing offspring [19]. Thus, there is no basis for a selection pressure on dog's ancestors that could account for the sex-specific response found here.
Particularly in humans, sex-specific child-rearing practices may contribute to some cognitive sex differences [1,2]. This mechanism is unlikely to explain our results since, unlike in some primates (e.g. [20]), there is little evidence for such sex-specific experiences in dogs.
Finally, sex differences in cognitive skills may also occur as an unselected by-product of other sex differences, as the brain of young mammals responds to different levels of sex hormones with sex-specific differentiation (resulting in an ‘androgenized brain’ in male mammals [1,2]). Furthermore, even current oestrogen and androgen levels in adults have been shown to influence performance in a variety of cognitive tasks in humans [1]. Since sex hormones have such far-reaching effects on mammalian brains, we suggest that they may cause a variety of basic cognitive differences between male and female mammals, such as the differential response in an object permanence task presented above. In our specific case, the results are most probably explained by sex-specific effects on brain differentiation in early life, rather than current hormone levels, as later neutering did not change the performance. Furthermore, the detected sex difference is unlikely to be mediated by a simple difference in attention, since overall males did not look less long at the stimuli than females, but may reflect different information-processing strategies between sexes as has been hypothesized for humans [21].
Our study suggests that sex differences in basic cognitive processes may occur widely in mammals, possibly as a consequence of sex-specific differentiation of the brain. Such results will be useful to judge hypotheses put forward to explain differences in cognitive skills between species. In dogs for example, selection pressures associated with domestication are often put forward as an explanation for presence or absence of particular cognitive skills [22,23]. This hypothesis loses support when the ability in question is found only in one of the two sexes. We thus call upon animal cognition researchers to explore possible sex differences in their datasets, rather than being content to report the sex distribution of the sample of subjects studied, and to interpret data on animal cognition with caution when only subjects of one sex were tested or when their sex distribution is strongly biased.
Acknowledgements
This study was funded by the Austrian Science Fund (P21418 to L.H.). The Clever Dog Lab is supported by Royal Canin, the University of Vienna and a private sponsor. We thank András Péter for the behaviour coding software Solomon.
References
- 1.Kimura D. 1999. Sex and cognition. Cambridge, MA: MIT Press [Google Scholar]
- 2.Halpern D. F. 2000. Sex differences in cognitive abilities, 3rd edn. London, UK: Lawrence Erlbaum Associates [Google Scholar]
- 3.Spelke E. S. 2005. Sex differences in intrinsic aptitude for mathematics and science? A critical review. Am. Psychol. 60, 950–958 10.1037/0003-066X.60.9.950 (doi:10.1037/0003-066X.60.9.950) [DOI] [PubMed] [Google Scholar]
- 4.Healy S. D., Bacon I. E., Haggis O., Harris A. P., Kelley L. A. 2009. Explanations for variation in cognitive ability: behavioural ecology meets comparative cognition. Behav. Process. 80, 288–294 10.1016/j.beproc.2008.10.002 (doi:10.1016/j.beproc.2008.10.002) [DOI] [PubMed] [Google Scholar]
- 5.Dalla C., Shors T. J. 2009. Sex differences in learning processes of classical and operant conditioning. Physiol. Behav. 97, 229–238 10.1016/j.physbeh.2009.02.035 (doi:10.1016/j.physbeh.2009.02.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laland K. N., Reader S. M. 1999. Foraging innovation in the guppy. Anim. Behav. 57, 331–340 10.1006/anbe.1998.0967 (doi:10.1006/anbe.1998.0967) [DOI] [PubMed] [Google Scholar]
- 7.Choleris E., Kavaliers M. 1999. Social learning in animals: sex differences and neurobiological analysis. Pharmacol. Biochem. Behav. 64, 767–776 10.1016/S0091-3057(99)00141-0 (doi:10.1016/S0091-3057(99)00141-0) [DOI] [PubMed] [Google Scholar]
- 8.Gagnon S., Dore F. Y. 1994. Cross-sectional study of object permanence in domestic puppies (Canis familiaris). J. Comp. Psychol. 108, 220–232 10.1037/0735-7036.108.3.220 (doi:10.1037/0735-7036.108.3.220) [DOI] [PubMed] [Google Scholar]
- 9.Fiset S., LeBlanc V. 2007. Invisible displacement understanding in domestic dogs (Canis familiaris): the role of visual cues in search behavior. Anim. Cogn. 10, 211–224 10.1007/s10071-006-0060-5 (doi:10.1007/s10071-006-0060-5) [DOI] [PubMed] [Google Scholar]
- 10.Collier-Baker E., Davis J. M., Suddendorf T. 2004. Do dogs (Canis familiaris) understand invisible displacement? J. Comp. Psychol. 118, 421–433 10.1037/0735-7036.118.4.421 (doi:10.1037/0735-7036.118.4.421) [DOI] [PubMed] [Google Scholar]
- 11.Rooijakkers E. F., Kaminski J., Call J. 2009. Comparing dogs and great apes in their ability to visually track object transpositions. Anim. Cogn. 12, 789–796 10.1007/s10071-009-0238-8 (doi:10.1007/s10071-009-0238-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenzie B. E., Tootell H. E., Day R. H. 1980. Development of visual size constancy during the 1st year of human infancy. Dev. Psychol. 16, 163–174 10.1037/0012-1649.16.3.163 (doi:10.1037/0012-1649.16.3.163) [DOI] [Google Scholar]
- 13.Baillargeon R., Devos J. 1991. Object permanence in young infants - further evidence. Child Dev. 62, 1227–1246 10.2307/1130803 (doi:10.2307/1130803) [DOI] [PubMed] [Google Scholar]
- 14.West R. E., Young R. J. 2002. Do domestic dogs show any evidence of being able to count? Anim. Cogn. 5, 183–186 10.1007/s10071-002-0140-0 (doi:10.1007/s10071-002-0140-0) [DOI] [PubMed] [Google Scholar]
- 15.Schöner G., Thelen E. 2006. Using dynamic field theory to rethink infant habituation. Psychol. Rev. 113, 273–299 10.1037/0033-295X.113.2.273 (doi:10.1037/0033-295X.113.2.273) [DOI] [PubMed] [Google Scholar]
- 16.Bogartz R. S., Shinskey J. L., Speaker C. J. 1997. Interpreting infant looking: the event set × event set design. Dev. Psychol. 33, 408–422 10.1037/0012-1649.33.3.408 (doi:10.1037/0012-1649.33.3.408) [DOI] [PubMed] [Google Scholar]
- 17.Wang S. H., Baillargeon R., Brueckner L. 2004. Young infants' reasoning about hidden objects: evidence from violation-of-expectation tasks with test trials only. Cognition 93, 167–198 10.1016/j.cognition.2003.09.012 (doi:10.1016/j.cognition.2003.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farago T., Pongracz P., Miklosi A., Huber L., Viranyi Z., Range F. 2010. Dogs' expectation about signalers' body size by virtue of their growls. PLoS ONE 5, e15175. 10.1371/journal.pone.0015175 (doi:10.1371/journal.pone.0015175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mech L. D., Boitani L. 2003. Wolves: behavior, ecology and conservation. Chicago, IL: University of Chicago Press [Google Scholar]
- 20.Kahlenberg S. M., Wrangham R. W. 2010. Sex differences in chimpanzee's use of sticks as play objects resemble those of children. Curr. Biol. 20, R1067–R1068 10.1016/j.cub.2010.11.024 (doi:10.1016/j.cub.2010.11.024) [DOI] [PubMed] [Google Scholar]
- 21.Meyers-Levy J. 1989. Gender differences in information processing: a selectivity interpretation. In Cognitive and affective responses to advertising (eds Cafferata P. C., Tybout A. M.), pp. 219–260 Lexington, MA: Lexington Books [Google Scholar]
- 22.Hare B., Brown M., Williamson C., Tomasello M. 2002. The domestication of social cognition in dogs. Science 298, 1634–1636 10.1126/science.1072702 (doi:10.1126/science.1072702) [DOI] [PubMed] [Google Scholar]
- 23.Hare B., Tomasello M. 2005. Human-like social skills in dogs? Trends Cogn. Sci. 9, 439–444 10.1016/j.tics.2005.07.003 (doi:10.1016/j.tics.2005.07.003) [DOI] [PubMed] [Google Scholar]