Abstract
In social insects, resource allocation is a key factor that influences colony survival and growth. Optimal allocation to queens and brood is essential for maximum colony productivity, requiring colony members to have information on the total reproductive power in colonies. However, the mechanisms regulating egg production relative to the current labour force for brood care remain poorly known. Recently, a volatile chemical was identified as a termite queen pheromone that inhibits the differentiation of new neotenic reproductives (secondary reproductives developed from nymphs or workers) in Reticulitermes speratus. The same volatile chemical is also emitted by eggs. This queen pheromone would therefore be expected to act as an honest message of the reproductive power about queens. In this study, we examined how the queen pheromone influences the reproductive rate of queens in R. speratus. We compared the number of eggs produced by each queen between groups with and without exposure to artificial queen pheromone. Exposure to the pheromone resulted in a significant decrease in egg production in both single-queen and multiple-queen groups. This is the first report supporting the role of queen pheromones as a signal regulating colony-level egg production, using synthetically derived compounds in a termite.
Keywords: queen pheromone, egg production, termites, resource allocation
1. Introduction
The question of resource allocation has long been a core issue in the study of social insects. When and how resources are allocated to growth, maintenance and reproduction determines colony survival and growth [1]. In social insects, the rate of egg laying by queens is related to the capacity of the colony to rear additional members, whereas production of too few or too many eggs lowers colony growth below the maximum sustainable capacity [2,3]. To accomplish optimal resource allocation to queens and brood, colony members need to adjust investment to queens in accordance with the current reproductive power of the queens. Vargo [4] demonstrated that queen-produced pheromones were involved in inhabitation of the reproductive output of coexisting queens in the ant Solenopsis invicta, although the active compounds remain to be identified. In many eusocial Hymenoptera, cuticular hydrocarbons provide information about the egg-laying ability of queens [5–9]. However, it is difficult to determine the role of queen pheromones in the regulation of colony-level egg production, because it generally involves conflicts over reproductive output among coexisting queens.
A subterranean termite Reticulitermes speratus provides good material for investigating the role of queen pheromone in the regulation of colony-level egg production, because their asexual queen succession (AQS) system leads to secondary polygyny without conflict [10]. In this termite, colonies are typically founded by a monogamous pair, one king and one queen. Secondary queens, female neotenic reproductives taking over reproduction in the colony, are produced from within the colony during colony development. Mature field colonies of R. speratus usually have a single primary king and an average of 55.4 female neotenic reproductives, which are exclusively produced parthenogenetically by the original primary queen [10]. The AQS system enables founding queens to increase their reproductive output through reproduction of their parthenogens while retaining the transmission rate of their genes to descendants even after they themselves are replaced. In addition, egg cannibalism or production of trophic eggs has never been observed in this termite.
Queen-produced pheromones have long been believed to influence the behaviour and physiology of colony members [11–13]. Recently, a queen-produced volatile chemical consisting of n-butyl-n-butyrate and 2-methyl 1-butanol was identified as a termite queen pheromone that inhibits the differentiation of new neotenic reproductives, which remain in the natal nest and inherit reproduction, in R. speratus [14]. In addition, the same volatile chemical is emitted by eggs themselves and plays a role both as a worker attractant and inhibitor of reproductive differentiation [14]. One may reasonably predict that queen pheromones would act as an honest signal of the reproductive power of queens and thus influence the colony egg production rate.
To determine whether the queen pheromone influences the reproductive rate of queens in R. speratus, we compared the number of eggs produced by queens with and without exposure to an artificial queen pheromone. We also compared the number of eggs produced between monogynous (single-queen) and polygynous (multiple-queen) groups. In addition, we investigated changes in queen body weight and the number of eggs produced by queens to determine whether workers adjust their food provisioning of queens based on information about the current egg production rate.
2. Material and methods
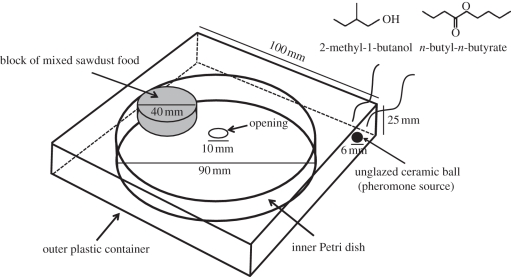
Two colonies (A and B) of R. speratus were collected from pine forests in Okayama, western Japan, in May and July 2010. We placed either one or three mature female neotenic reproductives (secondary queens) with 200 workers on a block of mixed sawdust food in a 90 mm Petri dish, which had an opening (10 mm diameter) in the lid. An unglazed ceramic ball (6 mm diameter) was located at the corner of an outer 100 mm square plastic container as a pheromone source (figure 1). We added 3 µl of artificial queen pheromone consisting of 2 µl n-butyl-n-butyrate (Sigma-Aldrich, MO, USA) and 1 µl 2-metyl-1-butanol (Wako, Osaka, Japan) on the unglazed ceramic ball every 24 h. In the control treatment, we added 3 µl of distilled water to the ceramic ball. Once absorbed by the unglazed ceramic ball, the compounds volatilized in the outer plastic container and entered the inner Petri dish through the opening in the lid. These devices were kept at 25°C in the dark for 30 days, after which we counted the number of eggs produced in each group. The period until hatching is approximately 35 days [15], and thus the number of eggs found at 30 days indicates the total number of eggs laid by the queens during the experiment. The female reproductives were weighed to the nearest 0.01 mg at the beginning and the end of the experiment. Six replicates from each treatment were conducted, and the experiment was repeated using the same two colonies.
Figure 1.
Experimental set-up for the inhibitory bioassay of synthetic volatile chemicals. We added either 3 µl of the artificial queen pheromone (2 : 1 mixture of n-butyl-n-butyrate and 2-metyl-1-butanol) or 3 µl of distilled water (negative control) to the unglazed ceramic ball every 24 h. The pheromone compounds, once absorbed by the unglazed ceramic ball, volatilize in the outer plastic container and entered the inner Petri dish through the opening in the lid.
All statistical analyses were performed using STATISTICA 06J (StatSoft, Inc., OK, USA). The data of the colony A and B were used as independent replicates. The data for the colonies in which female reproductives died during the experiment were excluded from the statistical analysis. We tested the effects of the artificial queen pheromone and queen number on the average number of eggs produced by individual queens and the total number of eggs with a nested-ANOVA to determine whether the queen pheromone and the existence of multiple queens suppress egg production by each queen. The effects of the treatment on the change in queen body weight were also analysed with a nested-ANOVA.
3. Results
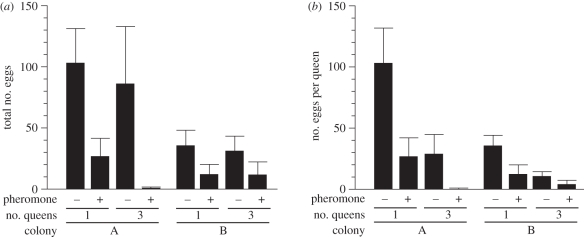
Exposure to the artificial queen pheromone significantly reduced the total number of eggs produced by the queens in each group (colony: F1,34 = 4.86, p = 0.034; pheromone: F2,34 = 8.23, p = 0.001; nested-ANOVA), whereas no significant difference was observed in the total number of eggs produced between single-queen and three-queen groups (F2,34 = 0.50, p = 0.612; figure 2a). The average number of eggs produced per queen in groups with multiple queens was significantly smaller than in groups with a single queen (colony: F1,34 = 4.00, p = 0.053; single versus multiple queens: F2,34 = 5.46, p = 0.009; figure 2b). The number of eggs laid per queen was also reduced by exposure to the artificial queen pheromone (F2,34 = 7.04, p = 0.003). No significant difference was detected in changes in queen body weight between the pheromone treatments (colony: F1,34 = 38.61, p < 0.0001; pheromone: F2,34 = 0.75, p = 0.479) or between single- and multiple-queen groups (F2,34 = 1.82, p = 0.178).
Figure 2.
Comparison of egg production between single-queen and multiple-queen groups with or without exposure to the artificial queen pheromone. (a) The total number of eggs produced by the queens in each group. The artificial pheromone significantly suppressed egg production (p < 0.01), although no significant difference was observed between single-queen and multiple-queen groups (p > 0.05). (b) The average number of eggs produced by each queen. Each queen produced significantly fewer eggs in multiple-queen groups than in single-queen groups (p < 0.01). Exposure to the pheromone significantly reduced each queen's egg production (p < 0.01). Values denote the mean ± s.e.m. of six replicates.
4. Discussion
In eusocial insects, the young brood (eggs and larvae) needs tending and nourishment by workers. Therefore, egg production must be adjusted in proportion to the capacity of the colony to rear the brood to maximize productivity [2,16]. In the presence of multiple queens, the regulation of colony-level egg production involves interactions among the queens. Our results show that total number of eggs produced in single-queen and multiple-queen groups was not significantly different when these colonies had an equal number of workers. This means that the egg production of one queen negatively affects the egg production of the other queens in a colony. Reduced egg production under exposure to the artificial queen pheromone suggests that this mutual inhibition can be caused by the volatile chemical emitted by the queens and eggs.
In this study, we found that the queen pheromone reduced egg production by queens, suggesting that this pheromone plays a role in the regulation of colony-level egg production. We propose three possible mechanisms for the process. (i) Queens make a decision, whereby nourishment by workers may be adjusted based on queen behaviour such as begging frequency. (ii) Workers make a decision, whereby they control the amount of food supplied to each queen based on pheromonal information. (iii) The queen-produced pheromone acts directly on the queen's neuroendocrine system to affect egg production. Future studies including inactivation of the pheromone receptor of queens or workers would be able to distinguish between these possibilities.
Recent identification of the termite queen pheromone has opened up new avenues for elucidating how reproductive and non-reproductive activities are regulated in colonies. In the present study, we demonstrated a novel function of the pheromone as an inhibitor of egg production in addition to the known functions as a worker attractant and an inhibitor of reproductive differentiation. This multi-functional queen pheromone, together with the multi-functional egg recognition pheromone [17,18], represents a parsimonious evolution of chemical signals involved in within-colony social communications in termites and provides important insights into how social insects accomplished reproductive harmony in the evolution of eusociality.
References
- 1.Oster G. F., Wilson E. O. 1978. Caste and ecology in the social insects. New Jersey, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 2.Tschinkel W. R. 1988. Social control of egg-laying rate in queens of the fire ant Solenopsis invicta. Physiol. Entomol. 13, 327–350 10.1111/j.1365-3032.1988.tb00484.x (doi:10.1111/j.1365-3032.1988.tb00484.x) [DOI] [Google Scholar]
- 3.Hölldobler B., Wilson E. O. 1990. The Ants. Cambridge, MA: Harvard University Press [Google Scholar]
- 4.Vargo E. L. 1992. Mutual pheromonal inhibition among queens in polygyne colonies of the fire ant Solenopsis invicta. Behav. Ecol. Sociobiol. 31, 205–210 10.1007/BF00168648 (doi:10.1007/BF00168648) [DOI] [Google Scholar]
- 5.Peeters C., Monnin T., Malosse C. 1999. Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc. R. Soc. Lond. B 266, 1323–1327 10.1098/rspb.1999.0782 (doi:10.1098/rspb.1999.0782) [DOI] [Google Scholar]
- 6.Liebig J., Peeters C., Oldham N. J., Markstadter C., Hölldobler B. 2000. Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc. Natl Acad. Sci. USA 97, 4124–4131 10.1073/pnas.97.8.4124 (doi:10.1073/pnas.97.8.4124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannonen M., Sledge M. F., Turillazzi S., Sundström L. 2002. Queen reproduction, chemical signalling and worker behaviour in polygyne colonies of the ant Formica fusca. Anim. Behav. 64, 477–485 10.1006/anbe.2002.4001 (doi:10.1006/anbe.2002.4001) [DOI] [Google Scholar]
- 8.Heinze J., Stengl B., Sledge M. F. 2002. Worker rank, reproductive status and cuticular hydrocarbon signature in the ant, Pachycondyla cf. inversa. Behav. Ecol. Sociobiol. 52, 59–65 10.1007/s00265-002-0491-1 (doi:10.1007/s00265-002-0491-1) [DOI] [Google Scholar]
- 9.Holman L., Jørgensen C. G., Nielsen J., d'Ettorre P. 2010. Identification of an ant queen pheromone regulating worker sterility. Proc. R. Soc. Lond. B 277, 3793–3800 10.1098/rspb.2010.0984 (doi:10.1098/rspb.2010.0984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuura K., Vargo E. L., Kawatsu K., Labadie P. E., Nakano H., Yashiro T., Tsuji K. 2009. Queen succession through asexual reproduction in termites. Science 323, 1687. 10.1126/science.1169702 (doi:10.1126/science.1169702) [DOI] [PubMed] [Google Scholar]
- 11.Butler C. G., Callow R. K., Johnston N. C. 1959. Extraction and purification of ‘queen substance’ from queen bees. Nature 184, 1871. 10.1038/1841871a0 (doi:10.1038/1841871a0) [DOI] [Google Scholar]
- 12.Karlson P., Lüscher M. 1959. Pheromones: a new term for a class of biologically active substances. Nature 183, 55–56 10.1038/183055a0 (doi:10.1038/183055a0) [DOI] [PubMed] [Google Scholar]
- 13.Keller L., Nonacs P. 1993. The role of queen pheromones in social insects: queen control or queen signal? Anim. Behav. 45, 787–794 10.1006/anbe.1993.1092 (doi:10.1006/anbe.1993.1092) [DOI] [Google Scholar]
- 14.Matsuura K., Himuro C., Yokoi T., Yamamoto Y., Vargo E. L., Keller L. 2010. Identification of a pheromone regulating caste differentiation in termites. Proc. Natl Acad. Sci. USA 107, 12 963–12 968 10.1073/pnas.1004675107 (doi:10.1073/pnas.1004675107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuura K., Kobayashi N. 2007. Size, hatching rate, and hatching period of sexually and asexually produced eggs in the facultatively parthenogenetic termite Reticulitermes speratus (Isoptera: Rhinotermitidae). Appl. Entomol. Zool. 42, 241–246 10.1303/aez.2007.241 (doi:10.1303/aez.2007.241) [DOI] [Google Scholar]
- 16.Matsuura K., Kobayashi N. 2010. Termite queens adjust egg size according to colony development. Behav. Ecol. 21, 1018–1023 10.1093/beheco/arq101 (doi:10.1093/beheco/arq101) [DOI] [Google Scholar]
- 17.Matsuura K., Tamura T., Kobayashi N., Yashiro T., Tatsumi S. 2007. The antibacterial protein lysozyme identified as the termite egg recognition pheromone. PLoS ONE 2, e813. 10.1371/journal.pone.0000813 (doi:10.1371/journal.pone.0000813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuura K., Yashiro T., Shimizu K., Tatsumi S., Tamura T. 2009. Cuckoo fungus mimics termite eggs by producing the cellulose-digesting enzyme beta-glucosidase. Curr. Biol. 19, 30–36 10.1016/j.cub.2008.11.030 (doi:10.1016/j.cub.2008.11.030) [DOI] [PubMed] [Google Scholar]