Abstract
BACKGROUND
Dengue is a mosquito-borne viral disease with an increasing incidence worldwide. Thrombocytopenia is a common finding in dengue virus (DV) infection; however, the underlying mechanisms remain unknown.
CASE REPORT
Here we provide the first evidence of a case of antibody formation against ADAMTS13 (ADAMTS13 inhibitor) in the course of a severe acute DV infection resulting in thrombotic microangiopathy (TMA). The patient presented with classical dengue symptoms (positive epidemiology, high fever, myalgia, predominantly in the lower limbs and lumbar region for 1 week) and, after 11 days of initial symptoms, developed TMA. Clinical and laboratorial investigation of dengue and TMA was performed.
RESULTS
The patient presented with ADAMTS13 inhibitor (IgG) during the acute phase of the disease, without anti-platelet antibodies detectable. Dengue infection had laboratorial confirmation. There were excellent clinical and laboratory responses to 11 serial plasma exchanges. Anti-ADAMTS13 inhibitor disappeared after remission of TMA and dengue resolution. No recurrence of TMA symptoms was observed after 2-year follow-up.
CONCLUSIONS
Although the real incidence of dengue-related TMA is unknown, this case provides the basis for future epidemiologic studies on acquired ADAMTS13 deficiency in DV infection. The prompt clinical recognition of this complication and early installment of specific therapy with plasma exchange are likely to improve the outcome of severe cases of dengue.
Dengue is a common viral-borne disease, caused by dengue virus (DV).1 It is the most important arthropod-borne viral disease in terms of morbidity and mortality2 with worldwide distribution.1 There are four closely related antigenically viral dengue serotypes3 but lifelong immunity is serotype-specific.4
DV infection is asymptomatic or mild in most cases, but may manifest as dengue fever or more severe forms: dengue hemorrhagic fever or dengue shock syndrome.3 Increased “unusual complications” have been observed, which may include hepatic damage, cardiomyopathy, encephalopathy, and severe hemorrhagic manifestations.2
Moderate thrombocytopenia may be present in any of the clinical manifestations, but the underlying mechanism remains unclear. Transient marrow suppression, platelet (PLT) aggregation to endothelial cells targeted by DV, hemophagocytosis, and PLT immune destruction with dengue antibody complexes are all associated with DV infection.2,5,6
Thrombotic microangiopathy (TMA) is a severe occlusive microvascular thrombotic syndrome characterized by profound thrombocytopenia, microangiopathic hemolytic anemia, and symptoms of organ ischemia. TMA includes primarily two syndromes, thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). HUS occurs more frequently in children, and most cases are associated with Shiga toxin–producing bacterial infection (D+HUS). Five to 10% of patients may have D-HUS (no history of diarrhea or gastrointestinal infection with Shiga toxin–producing organism) and seem to be related to defect in complement regulation.7
TTP is the result of an inherited or acquired deficiency in the ADAMTS13 metalloprotease activity, an enzyme that cleaves newly released large multimers of von Willebrand factor (VWF) from endothelial cells and PLTs.6,8
Some infections have been associated with TMA.7 Among these, there are reports of hepatitis C virus and parvovirus B–infected patients as well as patients with dental foci or streptococcal infection presenting the microangiopathy.9–12 Some of these infections are associated with the development of ADAMTS13 inhibitor and others have not a clear mechanism to explain the TMA. Moreover, human immunodeficiency virus (HIV) patients have been reported to develop TMA in advanced stages of the disease and many of these patients have no ADAMTS13 inhibitor detected. One possible explanation for this finding is that HIV-infected and -damaged endothelial cells release VWF, leading to localized thrombin generation and consumption of ADAMTS13. These same areas of endothelial damage may, in turn, lead to areas of turbulent flow with thrombus formation and resultant fragmentation of red blood cells (RBCs).13,14 It is possible that other infections affecting endothelial cell may present these mechanisms, leading to TMA. Here, we describe the first case of acquired ADAMTS13 deficiency due to autoantibodies against the metalloprotease after dengue viral infection.
CASE REPORT
A 45-year-old male patient was admitted to a local hospital with a history of high fever and myalgia, predominantly in the lower limbs and lumbar region for 1 week. After 11 days of the initial symptoms, the patient presented with petechiae and melena, followed by headache, reduced consciousness, and increasing jaundice within the past 48 hours. On admission, the patient presented with fever (axillary temperature, 38.2°C), tachycardia (heart rate, 106 beats/min), a blood pressure of 160 mmHg systolic and 90 mmHg diastolic, and a respiratory rate of 20/min. Laboratory data on admission are summarized in Table 1. In a 24-hour interval, clinical conditions deteriorated and the patient was placed on artificial ventilation. At this point a presumptive diagnosis of TMA was established based on the abrupt onset of thrombocytopenia, microangiopathic hemolytic anemia, elevated lactate dehydrogenase (LDH), neurologic abnormalities, and renal failure. Infusion of fresh-frozen plasma was introduced (10 mL/kg/day), until transference to a reference hospital was possible. Two days later, the patient was transferred to the intensive care unit of a university hospital where plasma exchange was available. On this admission the laboratory data (Table 1) showed an elevated level of LDH (2662 IU/L; normal range, <480 IU/L), low PLT count (11.0 × 109/L), a hemoglobin (Hb) level of 7.1 g/dL, a negative direct antiglobulin test, and negative RBC antibodies. Serum bilirubin (total and direct) was slightly elevated (1.83 and 0.77 g/dL; normal ranges, <1.0 and 0.7 g/dL, respectively), elevated serum creatinine (1.97 mg/dL), slightly prolonged prothrombin time (17 sec; control, 12.8 sec), and normal activated partial thromboplastin time (ratio, 1.01). Peripheral blood smear showed 5 to 10 schistocytes per high-power field and 14% erythroblasts of total RBCs. Flow cytometry assay15,16 was also performed to identify autoantibodies against PLTs, but the results were negative for both IgG and IgM. Other tests for antibodies against glycoprotein (GP) IIb-IIIa (Gi5), GPIa-IIa (Gi9), and GPIb-IX (anti-CD42) were also negative.15,16
TABLE 1.
Laboratory data of the patient presenting TTP, secondary to acute dengue infection at admission at local and reference hospitals (3 days interval)
| Laboratory measure | Admission at local hospital | Admission at reference hospital |
|---|---|---|
| Hb (g/L) | 8.9 | 7.1 |
| Hematocrit (%) | 24 | 21.6 |
| White blood cells (×109/L) | 5.60 | 9.40 |
| PLTs (×109/L) | 10 | 11 |
| LDH (IU/L)—NR: <480 IU/L | 3380 | 2662 |
| Bilirubin | ||
| Total (g/dL)—NR: <1.0 | 3.60 | 1.83 |
| Indirect (g/dL)—NR: <0.7 g/dL | 2.84 | 0.77 |
| Alanine aminotransferase—NR: <50 U/L | 36 | 505 |
| Aspartate aminotransferase—NR: <33 U/L | 57 | 149 |
| Creatinine (mg/dL)—NR: <1.2 mg/dL | 2.10 | 1.97 |
| Urea—NR: <50 mg/dL | 151 | 97 |
| aPTT ratio | 1.01 | |
| Prothrombin time (sec) | 17 (control, 12.8) | |
| Serologic tests (icterus-hemorrhagic fever syndrome) | Leptospirosis, hantavirus, Brazilian spotted fever, negative; Dengue, positive (IgM) | |
| Blood smear | – | Five to 10 schistocytes per high-power (×100) field; erythroblasts; polychromatophily; poikilocytosis |
aPTT = activated partial thromboplastin time; NR = normal range.
The patient lived in an area where a dengue fever outbreak was occurring. In addition, he had a positive exposure to leptospirosis due to his occupation. Dengue serology tests were performed, including Dengue IgM capture enzyme-linked immunosorbent assay (PanBio, Queensland, Australia) and DV IgM- and IgG-specific tests (Dengue DuoCassette, PanBio), and all confirmed-positive IgM results, suggesting the acute dengue viral infection. Serologic tests for leptospirosis, hantavirus, and Brazilian spotted fever, performed in a reference public health laboratory, were negative as the differential diagnosis imposed by the epidemic of DV infection in the icterus-hemorrhagic fever syndrome (as shown by a positive tourniquet test) plus thrombocytopenia.
We sought to determine whether TMA rather than icterus-hemorrhagic fever syndrome was the main underlying mechanism of the patient clinical evolution. The activity of ADAMTS13 and presence of autoantibodies against ADAMTS13 was then evaluated. The results obtained in blood samples collected at 2 weeks of the beginning of the fever showed that ADAMTS13 activity was below 5% of normal human plasma. Autoantibodies against ADAMTS13 were detected at a titer of 3 units/mL. Both ADAMTS13 activity and autoantibody inhibitors were determined by two different assays (FRETS-VWF73 and collagen-binding assay) as previously described.17,18 The anti-ADAMTS13 IgG bound specifically to both full-length ADAMTS13 and the variant deleted after the seventh TSP1 repeat (delCUB-CUB domain presents peptide sequences of complement subcomponents C1r/C1s; embryonic sea urchin protein EGF; and bone morphogenic protein-1),19 which were expressed in HEK293 cells. The antibody-antigen complexes were determined by immunoprecipitation, followed by Western blot with anti-V5 that recognizes the C-terminal V5-His tag (Fig. 1). The amount of ADAMTS13 or variant that could be pulled down with IgG from this DV-infected patient was similar to that with IgG from a TTP patient with acquired idiopathic TTP caused by known anti-ADAMTS13 IgG. Taken together, the clinical manifestations, response to the treatment, and the detection of anti-ADAMTS13 IgG autoantibody formation all suggest the diagnosis of TMA (probably TTP) concomitant to DV infection.
Fig. 1.
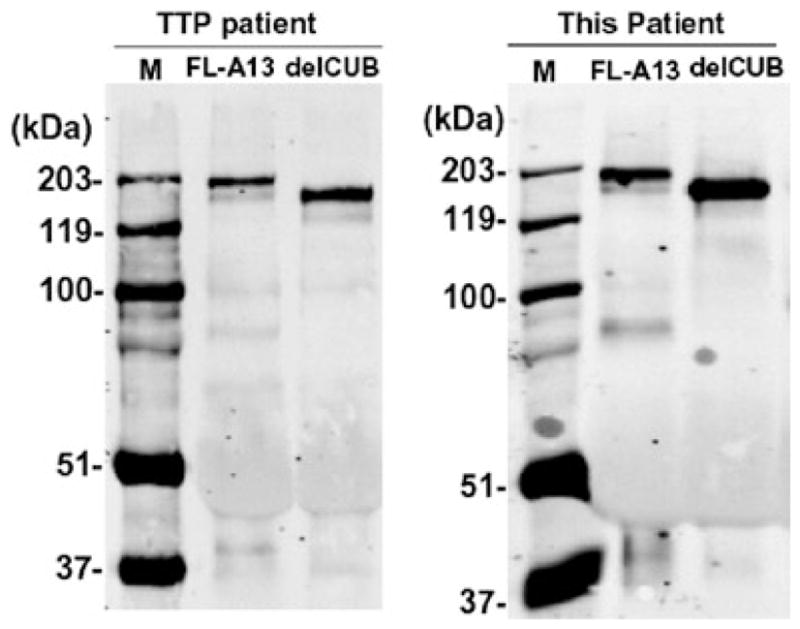
Immunoprecipitation detection of IgG antibody against ADAMTS13 in plasma. Two micrograms of purified recombinant full-length ADAMTS13 (FL-A13) and the mutant truncated after the CUB domain (delCUB) were incubated with 5 μL of plasma from a well-characterized idiopathic TTP patient and the patient with dengue (this patient) and 40 μL of protein A-Sepharose 4B for 1 hour. The bound IgG-ADAMTS13 or delCUB complex was eluted from the beads after extensive washing by heating the samples at 100°C for 5 minutes with sodium dodecyl sulfate sample buffer and detecting by Western blot with anti-V5 IgG, followed by IRDye800 fluorescent conjugated anti-mouse IgG (1:12,500) and Odyssey image system. Clearly, IgG from both patients recognized FL-A13 and delCUB proteins. The IgG from normal human plasma did not (data not shown).
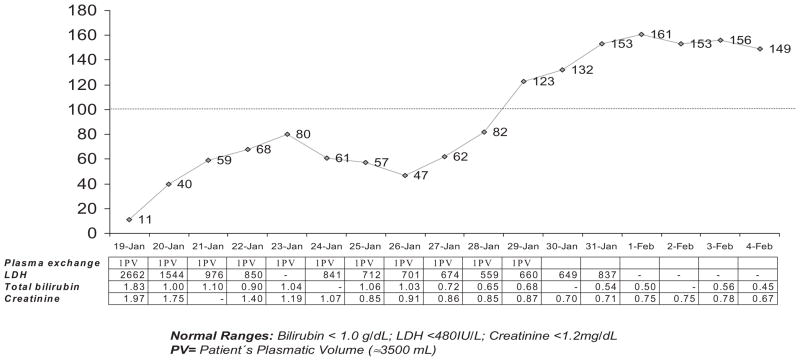
We opted for a therapy for TTP by plasma exchange therapy (1.0 plasma volume, daily) due to severity of the case, initiated before laboratorial results confirming DV infection.20 The time course of clinical and laboratorial findings showed evidence of temporal clinical and laboratorial improvements (Fig. 2). After 11 plasma exchanges, the PLT count recovered to values of 123 × 109/L and LDH decreased to 660 IU/L when plasma exchange was discontinued. At 4-month follow-up, the patient exhibited a normal PLT count and a normal level of serum LDH. Plasma ADAMTS13 activity returned to 100% of normal human plasma and no anti-ADAMTS13 inhibitor was detected at this time. No recurrence of TMA was observed after 2-year follow-up.
Fig. 2.
PLT count (×109/L;
 ), LDH (IU/L), bilirubin (g/dL), and creatinine (mg/dL) levels during case evolution with plasma exchange.
), LDH (IU/L), bilirubin (g/dL), and creatinine (mg/dL) levels during case evolution with plasma exchange.
DISCUSSION
ADAMTS13 deficiency plays a central role in the pathogenesis of hereditary or acquired idiopathic TTP.7 Here we report the first case of a neutralizing autoantibody against ADAMTS13 that developed in the course of dengue viral infection, resulting in TMA (probably TTP). The clinical evolution of DV infection and the prompted and sustained response to the TMA-specific treatment provide further evidence of the occurrence of TMA in the course of DV infection. Some clinical features such as mild coagulopathy and liver damage are not seen in TMA, but are described in DV infection.21,22
Dengue infection is often associated with rare severe complication such as dengue hemorrhagic fever and dengue shock syndrome. Here we add the development of ADAMTS13 inhibitor leading to TMA to this group as a potential mechanism underlying severe DV infection and thrombocytopenia.
We documented that antibody to ADAMTS13 developed during the course of DV and spontaneously disappeared within 4 months (after DV infection resolution). There are reports of viral-induced TTP but the direct evidence on the antibody response specific to ADAMTS13 is scarce.10,13,23 Although thrombocytopenia is a common finding in dengue hemorrhagic fever, TMA appears as a complication of DV infection and may be under recognized by physicians in many parts of the world. The prompt response to plasma exchange in TMA-related complications highlights the relevance of the clinical diagnosis of this complication in the course of DV infection.
It remains to be determined how acute DV infection induces a transient production of autoantibodies against ADAMTS13. IgM antibody against DV is generally detected when fever disappears and IgG antibody against DV generally appears at the fifth day of onset of symptoms and remains detectable for many years.24 In the case of our patient, it seems that DV infection induced the transient production of an IgG autoantibody that reacted with ADAMTS13 protease and blocked ADAMTS13 activity but not other PLT surface proteins, suggested by negative serologic tests (MAIPA and flow cytometry). The exact specificity of the antibody against ADAMTS13 epitopes was not evaluated in this study and it is possible that it may bind to ADAMTS13 at multiple sites.7 The characterization of the T-cell epitopes related to TMA and DV infection may provide insight on the disease pathogenesis and offer new potential therapeutic targets.
It is also possible that endothelial cell damage or stimulation described in DV infection,25 which is also described in HIV infection, in association with development of ADAMTS13 inhibitor described here, may contribute to the development of TMA in dengue patients.
In conclusion, special attention must be given to DV infection patients presenting signs and symptoms of TMA in future studies, to provide appropriate early investigation (presence of inhibitor autoantibodies against ADAMTS13), diagnosis, and therapeutics.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo, São Paulo State Research Support Foundation (FAPESP 2004/03605-1) to VC and from the National Institutes of Health (HL-079027) to XLZ.
We thank Dr Nicola Conran for reviewing the manuscript and suggestions.
ABBREVIATIONS
- DV
dengue virus
- GP
glycoprotein
- HUS
hemolytic uremic syndrome
- TMA
thrombotic microangiopathy
- TTP
thrombotic thrombocytopenic purpura
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
References
- 1.Guzman MG. Global voices of science. Deciphering dengue: the Cuban experience. Science. 2005;309:1495–7. doi: 10.1126/science.1115177. [DOI] [PubMed] [Google Scholar]
- 2.Rigau-Perez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV. Dengue and dengue haemorrhagic fever. Lancet. 1998;352:971–7. doi: 10.1016/s0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 3.Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis. 2002;2:33–42. doi: 10.1016/s1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 4.Wichmann O, Gascon J, Schunk M, Puente S, Siikamaki H, Gjørup I, Lopez-Velez R, Clerinx J, Peyerl-Hoffmann G, Sundøy A, Genton B, Kern P, Calleri G, de Górgolas M, Mühlberger N, Jelinek T European Network on Surveillance of Imported Infectious Diseases. Severe dengue virus infection in travelers: risk factors and laboratory indicators. J Infect Dis. 2007;195:1089–96. doi: 10.1086/512680. [DOI] [PubMed] [Google Scholar]
- 5.Lin CF, Lei HY, Liu CC, Liu HS, Yeh TM, Wang ST, Yang TI, Sheu FC, Kuo CF, Lin YS. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143–9. [PubMed] [Google Scholar]
- 6.Rigau-Perez JG. Severe dengue: the need for new case definitions. Lancet Infect Dis. 2006;6:297–302. doi: 10.1016/S1473-3099(06)70465-0. [DOI] [PubMed] [Google Scholar]
- 7.Zheng XL, Sadler JE. Pathogenesis of thrombotic microangiopathies. Annu Rev Pathol. 2008;3:249–77. doi: 10.1146/annurev.pathmechdis.3.121806.154311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO. Technical manual: dengue haemorrhagic fever: diagnosis treatment, prevention and control. Geneva: World Health Organization; 1997. [Google Scholar]
- 9.Kok RH, Wolfhagen MJ, Klosters G. A syndrome resembling thrombotic thrombocytopenic purpura associated with human parvovirus B19 infection. Clin Infect Dis. 2001;32:311–2. doi: 10.1086/318481. [DOI] [PubMed] [Google Scholar]
- 10.Yagita M, Uemura M, Nakamura T, Kunitomi A, Matsumoto M, Fujimura Y. Development of ADAMTS13 inhibitor in a patient with hepatitis C virus-related liver cirrhosis causes thrombotic thrombocytopenic purpura. J Hepatol. 2005;42:420–1. doi: 10.1016/j.jhep.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Fenner M, Frankenberger R, Pressmar K, John S, Neukam FW, Nkenke E. Life-threatening thrombotic thrombocytopenic purpura associated with dental foci. Report of two cases. J Clin Periodontol. 2004;31:1019–23. doi: 10.1111/j.1600-051X.2004.00617.x. [DOI] [PubMed] [Google Scholar]
- 12.Morrin MJ, Jones FG, McConville J, Arnold C, Mullan B, Lavery GG, McMullin MF. Thrombotic thrombocytopenic purpura secondary to Streptococcus. Transfus Apher Sci. 2006;34:153–5. doi: 10.1016/j.transci.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunther K, Garizio D, Nesara P. ADAMTS13 activity and the presence of acquired inhibitors in human immunodeficiency virus-related thrombotic thrombocytopenic purpura. Transfusion. 2007;47:1710–6. doi: 10.1111/j.1537-2995.2007.01346.x. [DOI] [PubMed] [Google Scholar]
- 14.Brecher ME, Hay SN, Park YA. Is it HIV TTP or HIV-associated thrombotic microangiopathy? J Clin Apher. 2008;23:186–90. doi: 10.1002/jca.20176. [DOI] [PubMed] [Google Scholar]
- 15.Kiefel V, Santoso S, Weisheit M, Mueller-Eckhardt C. Monoclonal antibody—specific immobilization of platelet antigens (MAIPA): a new tool for the identification of platelet-reactive antibodies. Blood. 1987;70:1722–6. [PubMed] [Google Scholar]
- 16.Castro V, Kroll H, Origa AF, Falconi MA, Marques SB, Marba ST, Passini R, Jr, Annichino-Bizzacchi JM, Costa FF, Santoso S, Arruda VR. A prospective study on the prevalence and risk factors for neonatal thrombocytopenia and platelet alloimmunization among 9332 unselected Brazilian newborns. Transfusion. 2007;47:59–66. doi: 10.1111/j.1537-2995.2007.01064.x. [DOI] [PubMed] [Google Scholar]
- 17.Gerritsen HE, Turecek PL, Schwarz HP, Lämmle B, Furlan M. Assay of von Willebrand factor (vWF)-cleaving protease based on decreased collagen binding affinity of degraded vWF: a tool for the diagnosis of thrombotic thrombocytopenic purpura (TTP) Thromb Haemost. 1999;82:1386–9. [PubMed] [Google Scholar]
- 18.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129:93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 19.Sadler JE, Moake JL, Miyata T, George JN. Recent advances in thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program. 2004:407–23. doi: 10.1182/asheducation-2004.1.407. [DOI] [PubMed] [Google Scholar]
- 20.Allford SL, Hunt BJ, Rose P, Machin SJ. Guidelines on the diagnosis and management of the thrombotic microangiopathic haemolytic anaemias. Br J Haematol. 2003;120:556–73. doi: 10.1046/j.1365-2141.2003.04049.x. [DOI] [PubMed] [Google Scholar]
- 21.Halstead SB. Dengue. Lancet. 2007;370:1644–52. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 22.Green S, Rothman A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr Opin Infect Dis. 2006;19:429–36. doi: 10.1097/01.qco.0000244047.31135.fa. [DOI] [PubMed] [Google Scholar]
- 23.Ramasubbu K, Mullick T, Koo A, Hussein M, Henderson JM, Mullen KD, Avery RK. Thrombotic microangiopathy and cytomegalovirus in liver transplant recipients: a case-based review. Transpl Infect Dis. 2003;5:98–103. doi: 10.1034/j.1399-3062.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 24.Sun DS, King CC, Huang HS, Shih YL, Lee CC, Tsai WJ, Yu CC, Chang HH. Antiplatelet autoantibodies elicited by dengue virus non-structural protein 1 cause thrombocytopenia and mortality in mice. J Thromb Haemost. 2007;5:2291–9. doi: 10.1111/j.1538-7836.2007.02754.x. [DOI] [PubMed] [Google Scholar]
- 25.Sosothikul D, Seksarn P, Pongsewalak S, Thisyakorn U, Lusher J. Activation of endothelial cells, coagulation and fibrinolysis in children with Dengue virus infection. Thromb Haemost. 2007;97:627–34. [PubMed] [Google Scholar]