Abstract
DHA (docosahexaenoic acid, C22:6,n−3) has been shown to promote neurite growth and synaptogenesis in embryonic hippocampal neurons, supporting the importance of DHA known for hippocampus-related learning and memory function. In the present study, we demonstrate that DHA metabolism to DEA (N-docosahexaenoylethanolamide) is a significant mechanism for hippocampal neuronal development, contributing to synaptic function. We found that a fatty acid amide hydrolase inhibitor URB597 potentiates DHA-induced neurite growth, synaptogenesis and synaptic protein expression. Active metabolism of DHA to DEA was observed in embryonic day 18 hippocampal neuronal cultures, which was increased further by URB597. Synthetic DEA promoted hippocampal neurite growth and synaptogenesis at substantially lower concentrations in comparison with DHA. DEA-treated neurons increased the expression of synapsins and glutamate receptor subunits and exhibited enhanced glutamatergic synaptic activity, as was the case for DHA. The DEA level in mouse fetal hippocampi was altered according to the maternal dietary supply of n−3 fatty acids, suggesting that DEA formation is a relevant in vivo process responding to the DHA status. In conclusion, DHA metabolism to DEA is a significant biochemical mechanism for neurite growth, synaptogenesis and synaptic protein expression, leading to enhanced glutamatergic synaptic function. The novel DEA-dependent mechanism offers a new molecular insight into hippocampal neurodevelopment and function.
Keywords: docosahexaenoic acid (DHA), N-docosahexaenoylethanolamide (DEA), hippocampus, neurite growth, neuron, synaptogenesis
INTRODUCTION
DHA(docosahexaenoic acid, C22:6,n−3) is an n−3 polyunsaturated fatty acid that is highly enriched in the brain [1]. Developmental accretion of DHA has been shown to improve hippocampus-related learning and memory function in humans and rodents [2–6], whereas opposite effects were observed by depleting this fatty acid in the brain [7–9]. A unique role of DHA in promoting hippocampal neuronal survival [10] as well as neurite development [11,12], synaptogenesis and glutamatergic synaptic activity has been demonstrated in embryonic hippocampal cultures [12]. In vivo significance of DHA on hippocampal neurogenesis, neurite growth and synaptogenesis has also been suggested in animal models where the DHA status was manipulated either by dietary means [11,12] or using fat-1 transgenic mice [13]. In addition, it has been reported that n−3 fatty acids promote neurite outgrowth in sensory neurons during development and aging [14]. Previous studies have indicated biotransformation of DHA to bioactive mediators, including resolvins [15] and neuroprotectin D1, a 10,17S-hydroxy docosatriene derivative of DHA [16]. Nevertheless, involvement of DHA metabolites in hippocampal development has not been demonstrated. In the present study, we hypothesized that some of the DHA effects on hippocampal development is mediated by its metabolites. We found active biosynthesis of DEA (N-docosahexaenoylethanolamide) in developing hippocampi as well as the hippocampal neuronal culture. Treatment of hippocampal neurons with DEA promoted neurite growth, synaptogenesis and expression of glutamate receptor subunits and enhanced glutamatergic synaptic activity as in the case with DHA, but at substantially lower concentrations. Our results suggest that DEA is an active component of DHA-mediated hippocampal development.
EXPERIMENTAL
Animals and diets
For in vivo alteration of fatty acid composition, mice at 2 days of pregnancy were fed with either an n−3 fatty-acid-adequate or -deficient diet throughout the pregnancy. The n−3 fatty acid content in the adequate diet was 2.5%(w/w) LNA (linolenic acid, C18:3,n−3) plus 0.9% DHA, whereas the deficient diet contained 0.09% LNA, as described previously [12]. The procedures employed in the present study were approved by the Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism (LMS-HK21).
Hippocampal primary culture and fatty acid supplementation
Timed pregnant C57/BL6 mice were obtained from Charles River Laboratories at 16 days of pregnancy and fed with an NIH-31 diet before collecting E18 (embryonic day 18) fetuses. Embryonic neurons were prepared from E18 mouse hippocampi and cultured in Neurobasal™ medium as described previously [11], but using 2% (v/v) B-27 supplement (Gibco), at a density of 30000 cells/cm2. On day 2 after seeding, the cells were treated with DHA complexed with fatty-acid-free BSA (Sigma) (final concentrations of 1 µM and 0.01% respectively), and DEA or fatty acid amides in DMSO. The medium contained 40 µM α-tocopherol (Sigma). In some cases, DHA was dissolved in DMSO and applied to the cell culture medium instead of as a BSA-complexed form. One-half of the growth medium was replaced every 3 days with Neurobasal™ medium with or without fatty acids. For inhibitor studies, 50 µM indomethacin, 10 µM NDGA (nordihydroguaiaretic acid) and 1 µM URB597 (Cayman) in DMSO were added to the E18 hippocampal cultures 1 h prior to the addition of DHA (1 µM). For MS analysis of DEA, an E18 culture at 50000 cells/cm2 density was plated in six-well plates or 10-cm-diameter dishes, supplemented with 1 µM DEA and/or [13C22]DHA for 3 days and the lipids were extracted from both cells and medium as described by Bligh and Dyer [17]. Separately, E18 hippocampi were homogenized in a buffer containing 50 mM Tris/HCl (pH 8.0), 150 mM NaCl and 10 mM CaCl2 and incubated for 1.5 h with 5 µM [13C22]DHA in the presence or absence of 1 µM URB597. Fatty acid analysis from hippocampi was performed by GC as described previously [18].
Immunocytochemistry and evaluation of neurite outgrowth and synapsin puncta formation
Cells were immunostained against MAP2 (microtubule-associated protein 2) and synapsin-1 using a procedure described previously [19]. For nuclear staining, 0.06% DAPI (4′,6-diamidino-2-phenylindole; Sigma) was used. Images were collected using an inverted motorized IX81 Olympus microscope, and neurite length and synapsin puncta were quantified using Metamorph software (Molecular Devices). To minimize bias, neurons were evaluated blindly without knowledge of sample identity. At random, 5–6 fields/well were chosen and only non-clustered neurons were evaluated to ensure the precision of the measurements. From each well, 120 neurons were evaluated at ×20 magnification for total neurite length/neuron, number of branches and the number of synapsin puncta/neuron. A total of 20 neurons were analysed from each well for the number of synapsin puncta/10 µm neurite length at ×60 magnification. Data were obtained from triplicate wells and the experiments were repeated at least three times.
RT (reverse transcription)–PCR assay
Total RNA from mouse hippocampal cultures was isolated after supplementing the cells with DHA (1 µM) or DEA (0.1 µM) for 12 h, using TRIzol® reagent (Invitrogen). Isolated RNA (1 µg) was used for first-strand cDNA synthesis by using 200 units of SuperScript® III reverse transcriptase (Moloney murine leukaemia virus reverse transcriptase; Invitrogen), as described in the manufacturer’s protocol. The newly synthesized cDNA was used for PCR amplification by using the following primers: synapsin-1 (GenBank® accession number NM_001110780, Mus musculus) forward, 5′-CAGGGTCAAGGCCGCCAGTC-3′ (1708–1727, 20 nt) and reverse, 5′-CACATCCTGGCTGGGTTTCTG-3′ (2044–2064, 21 nt, product size 356 bp); and CPT1 (carnitine palmitoyltransferase 1) (GenBank® accession number NM_013495, M. musculus) forward, 5′-GCGAGTCCCTCCAGCTGGCTTATC-3′ (256–279, 24 nt) and reverse, 5′-CTCCAGGTACCTGCTCACGGTATC-3′ (628–651, 24 bp, product size 395 bp). The housekeeping gene primers G3PDH (glyceraldehyde-3-phosphate dehydrogenase; 450 bp PCR product) and β-actin (540 bp PCR product) were from Clontech. After PCR amplification, samples were run on a 1.5% agarose gel and photographed.
Western blotting
A 10–15 µg amount of protein was loaded in each lane. After SDS/PAGE, proteins were blotted on to PVDF membranes (Amersham Pharmacia Biotech). The membranes were blocked for 15 min in TBS (Tris-buffered saline; 20 mM Tris/HCl, pH 7.4, and 150 mM NaCl) containing 5%(w/v) non-fat dried skimmed milk powder and 0.1% Tween 20 (Bio-Rad Laboratories). Incubation with primary and secondary antibodies was performed in TBS containing 5%(w/v) non-fat dried skimmed milk powder overnight and for 2 h respectively. Subsequently, membranes were washed in TBS containing 0.1% Tween 20. The primary antibodies used were rabbit monoclonal anti-synapsin-1 (1:1000 dilution, Sigma), rabbit monoclonal GluR1 (glutamate receptor 1; 1:500 dilution, Abcam), goat monoclonal anti-β-actin (1:200 dilution), rabbit polyclonal anti-LPL (lipoprotein lipase) (H-53; 1:200 dilution), goat polyclonal anti-CPT1 (N-17, 1:200 dilution) (all from Santa Cruz Biotechnology) and mouse monoclonal anti-NR2B [NMDA (N-methyl-d-aspartate) receptor subunit 2B] (1:1000 dilution, NeuroMab). Secondary antibodies were horseradish peroxidase-conjugated anti-mouse, anti-goat and anti-rabbit antibodies (1:2000 dilution, Santa Cruz Biotechnology). The signal was detected using an ECL (enhanced chemiluminescence) kit (Thermo Fisher Scientific) and analysed using a Kodak Gel Logic 440 imaging system.
Electrophysiology
For evaluating the synaptic function of the cultured neurons, synaptic currents were measured in the conventional whole-cell voltage-clamp mode using a glass pipette electrode (3–5 MΩ) at a holding potential of −60 mV as described previously [12]. To isolate GABA-sPSCs {GABA (γ-amino-butyric acid)ergic sPSCs [spontaneous PSCs (postsynaptic currents)]}, NBQX (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium, 5 µM) and APV (d-2-amino-5-phosphonopentanoic acid, 50 µM) were locally applied to the target neurons using a multibarrelled array of square glass applicators on a Perfusion Fast-Step system (Warner Instruments). For measurement of Glu-sPSCs (glutamatergic sPSCs), bicuculline (20 µM) was superfused on to the neuron using the same application system. TTX (tetrodotoxin, 1 µM) superfused to block action potentials in the presynaptic neurons did not alter the amplitude and frequency of PSCs, indicating that the detected sPSCs were miniature PSCs. The spontaneous synaptic currents were detected using MiniAnalysis software (Synaptosoft) with a threshold detection of 20 pA and manual detection for smaller events (down to 10 pA).
Synthesis of DEA, d4-DEA and DHA-amide (N-docosahexaenoylamide)
DEA and d4-DEA were synthesized by a procedure similar to that described by Devane et. al. [20] for AEA (arachidonylethanolamine, also called anandamide). In brief, docosahexaenoyl chloride (Nu-Chek Prep) was reacted with a 10-fold molar excess of either ethanolamine or d4-ethanolamine (Sigma) in dried methylene chloride under an Ar atmosphere at 0°C for 15 min. The product was washed with water several times to remove excess ethanolamine. The solvent was evaporated under nitrogen and the product dissolved in a stock solution of methanol and quantified by transmethylation with BF3/methanol (Sigma) followed by GC as described previously [18]. DHA-amide was prepared using the procedure reported for oleamide synthesis [21] with slight modification by bubbling anhydrous ammonia gas (Air Liquide) through a solution of docosahexaenoyl chloride in dried methylene chloride for 15 min at room temperature (25°C). The solvent was evaporated, yielding an oily residue that contained both DHA-amide and unwanted DHA. The oil was run over a reverse-phase HPLC column using a methanol/H2O/hexane solvent system, and the DHA-amide was collected and re-extracted into chloroform/methanol as described in [17], dried under nitrogen and resuspended in methanol. The product was characterized by ESI (electrospray ionization)–LC (liquid chromatography)–MS/MS (tandem MS), compared with an authentic oleamide standard (Sigma), and quantified by GC-FAME (fatty acid methyl ester) analysis as for DEA and d4-DEA. The purity of all final products was also confirmed using ESI–LC–MS in both the positive and negative ionization modes.
MS analysis of DHA metabolites
Cells, culture medium and tissue homogenate were extracted as described previously [17] in the presence of internal standards including d4-AEA and d4-DEA. Organic layers were collected, dried under nitrogen and reconstituted with methanol. HPLC–ESI–MS/MS analysis was performed using a Finnigan TSQ Quantum mass spectrometer, which was equipped with an ESI source. The separation was achieved using a BDS Hypersil C18 Pioneer column (2.1×50 mm; Thermo Electron) as described previously [22]. For quantification, the mass spectrometer was operated in the MRM (multiple reaction monitoring) mode. CID (collision-induced dissociation) was performed using argon as the collision gas at 1.5 mTorr (1 Torr = 0.133 kPa) with the relative collision energy set at 15 V. The following specific transitions were monitored: m/z 348→62 for AEA; m/z 352→66 for d4-AEA; m/z 372→62 for DEA; m/z 376→66 for d4-DEA; m/z 394→62 for [13C22]DEA; m/z 328→293 and 311 for DHA-amide; and m/z 350→315 and 333 for [13C22]DHA-amide.
Statistical analysis
All values are means ± S.D. unless specified. Statistical analysis was performed using unpaired Student’s t tests unless specified; *P < 0.05, **P < 0.01 and ***P < 0.001. Significant differences between groups were determined by post-hoc Tukey’s HSD (Honestly Significant Difference) tests. Different alphabetical letters indicate significant differences at P < 0.05, unless otherwise specified.
RESULTS
A fatty acid amide form is involved in DHA-induced neurite growth and synaptogenesis
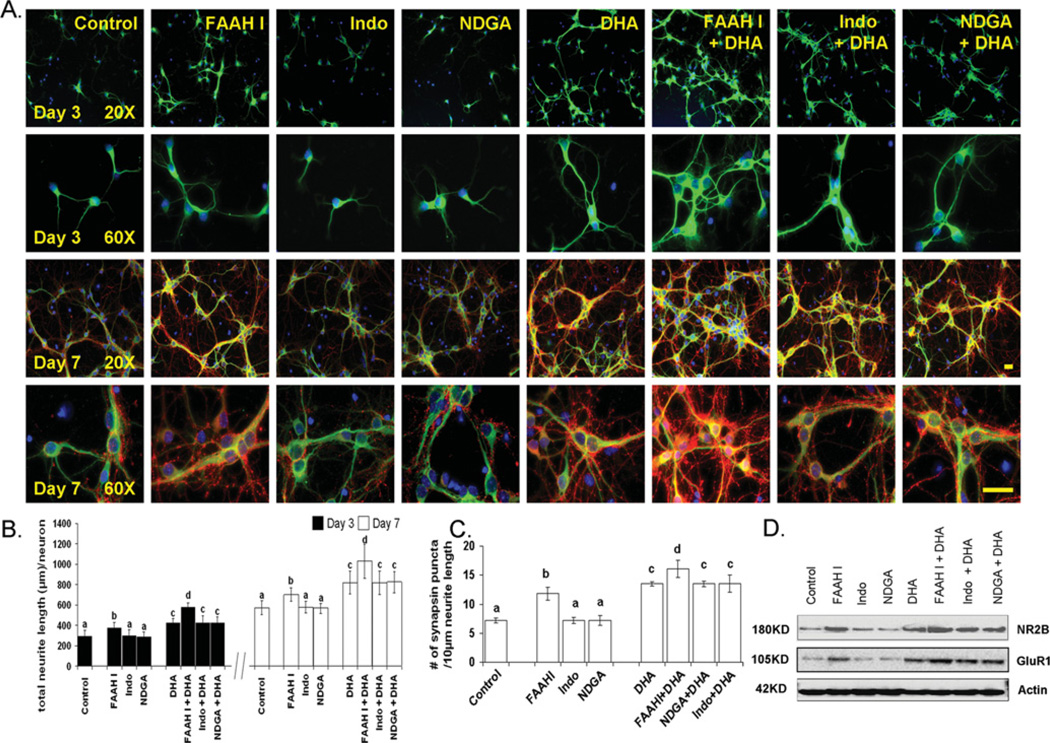
We have previously demonstrated that DHA uniquely promotes neurite growth, synaptogenesis and synaptic protein expression, including synapsins and glutamate receptor subunits, in E18 hippocampal neuronal cultures, and enhances excitatory synaptic function [12]. To understand the molecular mechanism for the observed effect of DHA, we investigated the possible involvement of DHA metabolism. E18 hippocampal neuronal cultures were treated with URB597, NDGA or indomethacin, inhibitors of FAAH (fatty acid amide hydrolase), lipoxygenase or cyclooxygenase respectively. As reported previously [12], hippocampal neurons supplemented with DHA showed significantly increased neurite outgrowth and improved synaptogenesis evaluated by the number of synapsin puncta normalized per given neurite length (Figure 1). Although NDGA or indomethacin showed little effect, inhibition of FAAH by the selective inhibitor URB597 promoted neurite growth (Figures 1A and 1B) and synaptogenesis (Figures 1A and 1C). The inclusion of URB597 in the DHA-supplemented culture further promoted neurite development and synaptogenesis. The protein expression of glutamate receptors (probed with NR2B and GluR1), which have been shown to be elevated with DHA treatment [12], was increased by FAAHI (FAAH inhibitor) treatment (Figure 1D). These results indicated that an amide form of DHA metabolites is involved in the DHA-promoted neurite growth, synaptogenesis and synaptic protein expression. Therefore subsequent experiments were performed to determine whether an amide derivative of DHA is produced in the hippocampal cultures that also shows bioactivity for the neuronal development observed with DHA.
Figure 1. Effect of inhibitors of fatty acid metabolism on hippocampal development.
Although indomethacin (Indo, 50 µM) and NDGA (10 µM) exerted no effects, inhibition of FAAH using URB597 (1 µM, FAAHI) enhanced neurite growth at 3 and 7 DIV (days in in vitro culture) (B) as well as synapsin puncta formation (C) and NMDA receptor expression at 7 DIV (D). (A) Representative photomicrographs of E18 mouse hippocampal neurons after culturing for 7 days in the presence of various inhibitors: MAP2 (a neuron-marker protein) green; synapsin-1, red; and DAPI (for nuclei), blue. Scale bars, 30 µm. (B and C) Quantitative changes in neurite growth (B) and synaptogenesis evaluated by the number of synapsin puncta/10 µm of neurite (C). Statistical analysis was performed by post-hoc Tukey’s HSD tests at the significance level of P < 0.05. Different alphabetical letters indicate statistically significant differences. (D) Western blot analysis for NR2B expression.
DHA is actively metabolized to DEA in developing hippocampi
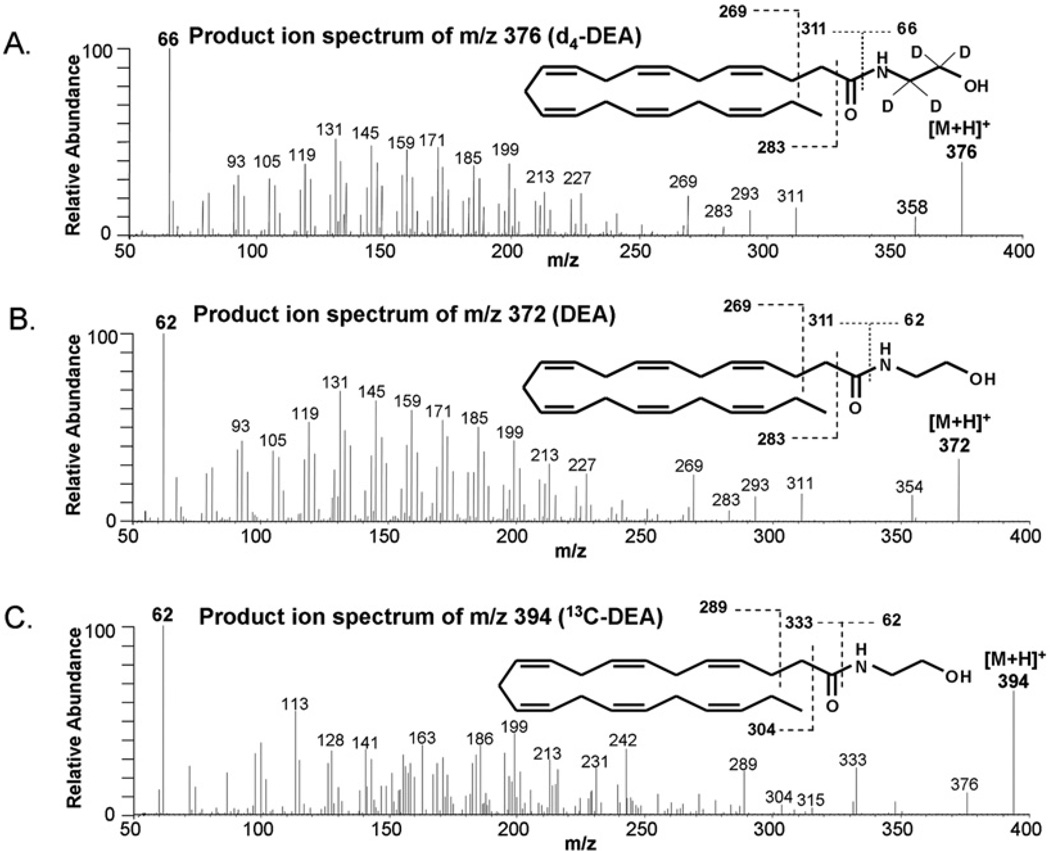
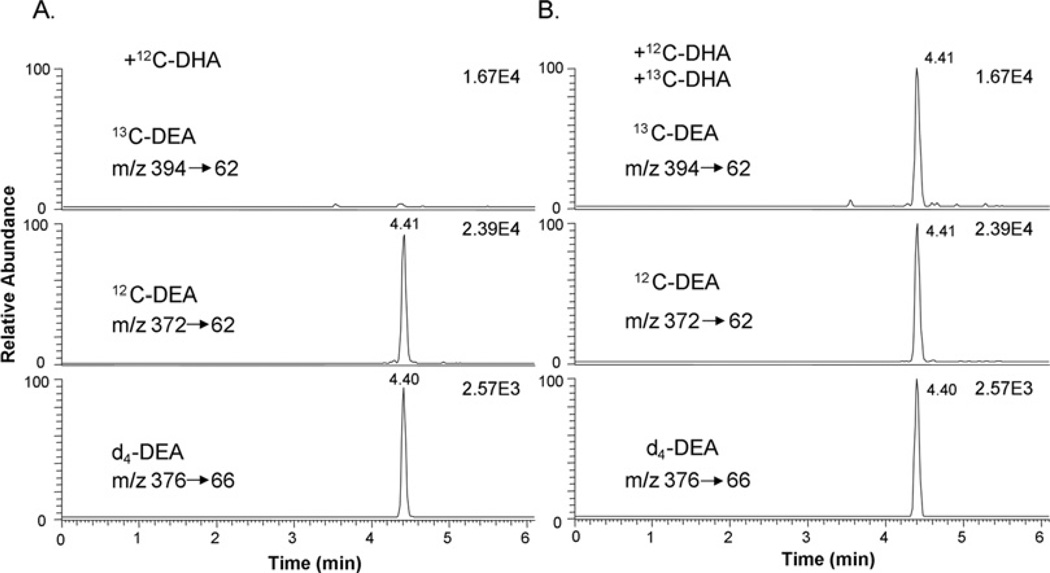
To examine DHA metabolism in the hippocampal neuronal culture, we monitored transformation of DHA and uniformly labelled [13C22]DHA by MS (Figures 2 and 3). When the culture was incubated with unlabelled DHA or [13C22]DHA, formation of a metabolite was detected at m/z 372 or 394 respectively. The MS fragmentation pattern (Figure 2) revealed the identity of the metabolite as DEA, a DHA-derived structural analogue of AEA, a well known endocannabinoid. The chromatographic retention time of DEA and [13C22]DHA produced was identical with the authentic standard, as indicated by the selected MRM of a specific mass transition from [M+H]+ to an ethanolamine fragment (m/z 62) (Figure 3). Although the cultures supplemented with DHA alone showed no trace of DEA production in the [13C22]DEA ion channel (Figure 3A), inclusion of [13C22]DHA in the culture clearly showed the production of [13C22]DEA (Figure 3B).
Figure 2. Biotransformation of DHA into DEA in hippocampal neuronal cultures.
MS/MS spectra from [M+H]+ of 50 fmol of d4-labelled DEA internal standard (A), DEA standard (B) or 13C22[DEA] produced in E18 hippocampal neuronal cultures after supplementation with 1 µM [13C22]DHA (C) for 3 days. The characteristic fragmentation pattern indicated the production of [13C22]DEA in the hippocampal culture. The MS/MS spectrum of DEA produced from DHA in the culture was virtually identical with that of the standard DEA.
Figure 3. DEA production in hippocampal neuronal cultures identified by MRM.
The MRM chromatograms using characteristic mass transitions from [M+H]+ to the ethanolaminemoiety at m/z 62 confirmed the production of DEA in E18 hippocampal cultures after supplementation with 1 µM DHA (A) or 1 µM each of DHA and [13C22]DHA (B) for 3 days.
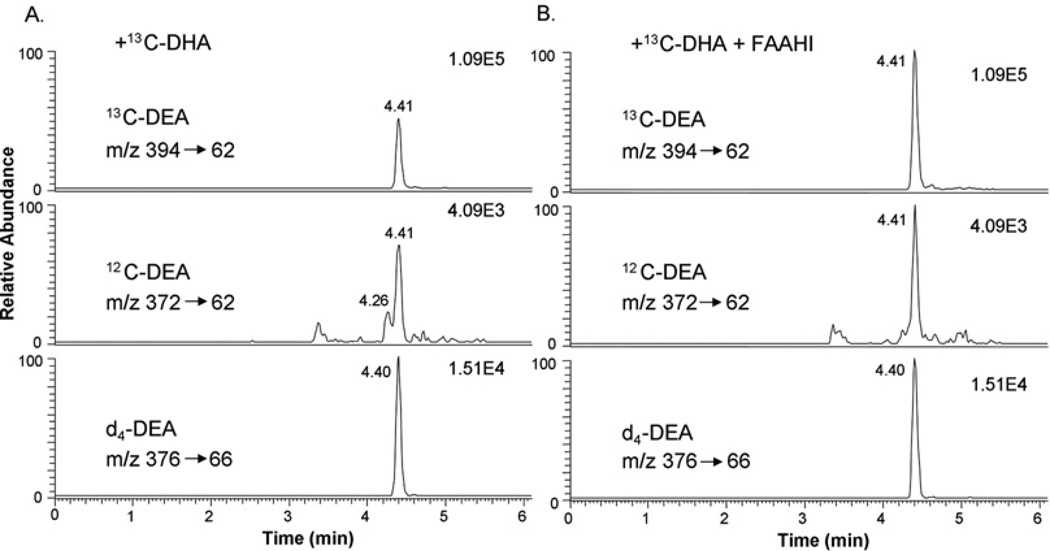
The MRM approach also allowed quantitative determination of DEA with high specificity in the presence of the 2H-labelled internal standard. Supplementation of the E18 hippocampal neuronal culture (6×104 cells/cm2 in a 10-cm-diameter dish) with 1 µM DHA for 3 days increased the DEA level from 55 ± 6 to 313 ± 30 fmol. Inhibiting hydrolysis of DEA using URB597 substantially increased the DEA content for both the control and the DHA-supplemented culture (350 ± 41 and 5560 ± 125 fmol respectively), indicating that FAAH was active in the culture. Similarly, the homogenate of E18 hippocampi actively converted [13C22]DHA into [13C22]DEA, the level of which was increased further in the presence of URB597 (Table 1 and Figure 4). In addition, the production of DEA from endogenous DHA was slightly, but significantly, increased in the presence of URB597.
Table 1. Effects of FAAHI on DEA production by E18 hippocampal homogenates.
E18 hippocampi were homogenized and incubated with 5 µM [13C22]DHA for 1.5 h in the presence or absence of 1 µM URB597 (FAAHI), and the DEA and [13C22]DEA levels were determined by MS/MS as shown in Figure 4. DEA was produced from the endogenous DHA. The data are expressed as fmol/E18 hippocampus and are representative of triplicate experiments that were repeated three times.
| DEA production (fmol/E18 hippocampus) | ||
|---|---|---|
| Treatment | DEA | [13C22]DEA |
| −FAAHI | 12 ± 0.8 | 166 ± 20.0 |
| +FAAHI | 15 ± 1.1* | 399 ± 39.5** |
P < 0.05 and **P < 0.01 compared with the −FAAHI group for the DEA or [13C22]DEA production.
Figure 4. Effects of FAAHI on DEA production by hippocampal homogenate.
The production of [13C22]DEA from exogenously added 5 µM [13C22]DHA by the E18 hippocampal homogenate (A) was increased when 1 µM URB597 (FAAHI) was included in the incubation mixture (B), as indicated by the MRM chromatograms. DEA produced from endogenous DHA also increased in the presence of FAAHI.
DHA-amide is another class of DHA derivatives that can be affected by the inhibition of FAAH. To test the possible involvement of this molecule, the production of DHA-amide in the hippocampal cell culture was first examined after supplementation with 1 µM DHA. The authentic standard oleamide produced characteristic fragments at m/z 265 and 247, presumably representing [M+H–NH3]+ and [265–H2O]+ respectively (Supplementary Figure S1A at http://www.BiochemJ.org/bj/435/bj4350327add.htm). The DHA-amide standard prepared by chemical synthesis [21] produced similar characteristic fragments at m/z 311 and 293 (Figure S1A). Monitoring the MRM transition from [M+H]+ to the characteristic fragment ions showed the chromatographic peaks at 4.54 and 4.72 min for the DHA-amide and oleamide standards respectively (Figure S1B). Nevertheless, no MRM chromatographic peaks were detected for DHA-amide at the corresponding retention time when the hippocampal neuronal cell culture was supplemented with 1 µM each of DHA and [13C22]DHA (Figure S1C), with the current detection limit of the sub-femtomolar range. From the same culture, DEA production was clearly indicated, suggesting that DHA-amide is not a major amide derivative of DHA produced in developing hippocampi. In addition, treatment of the E18 hippocampal culture with 1 µM DHA-amide had no significant effects on neurite outgrowth and synaptogenesis (Supplementary Figure S2 at http://www.BiochemJ.org/bj/435/bj4350327add.htm), suggesting that DHA-amide may not mediate the effects of DHA on hippocampal development.
DEA promotes neurite growth and synaptogenesis
Active transformation of DHA to DEA (Figure 2), together with the positive effect of FAAHI on neurite development and synaptogenesis shown in Figure 1, suggested that DEA is a potential mediator for the DHA-promoted neurite growth and synaptogenesis in hippocampal neuronal cultures. To test this proposition, E18 hippocampal cultures were supplemented with DEA and neuronal development was examined in comparison with AEA, which has been implicated in neurodevelopment (Figure 5). Indeed, DEA dose-dependently increased neurite growth after 3 or 7 days of supplementation (Figures 5A and 5B). Significant increases in the number of synapsin puncta normalized per 10 µm of neurite (Figure 5C) or per neuron (Figure 5D) were also observed in DEA-treated neurons after 7 days, suggesting that synaptogenesis had been promoted. DEA was effective at concentrations as low as 0.1 µM in promoting neurite growth and synaptogenesis as well as synapsin protein expression (Figure 5E), whereas supplementation of the neurons with 0.1 µM DHA exerted no measurable effects (results not shown). AEA did not promote neurite growth, synaptogenesis or synapsin expression at 0.1–1 µM and showed a slight effect at concentrations only above 1.5 µM (Figures 5B–5E).
Figure 5. Effect of DEA on hippocampal neurite growth and synaptogenesis.
Effect of DEA on neurite growth (B), synaptogenesis (C) and synapsin expression (D and E). Representative photomicrographs are shown for MAP2 immunofluorescence in green, synapsin in red and DAPI in blue (A); scale bars, 30 µm. DEA at 0.1 µM promoted neurite development, synaptogenesis and synapsin expression evaluated by the total neurite length/neuron and Western blotting at 7 DIV (days in in vitro culture). Statistical analysis was performed by post-hoc Tukey’s HSD tests (different letters indicate significant difference at P < 0.05).
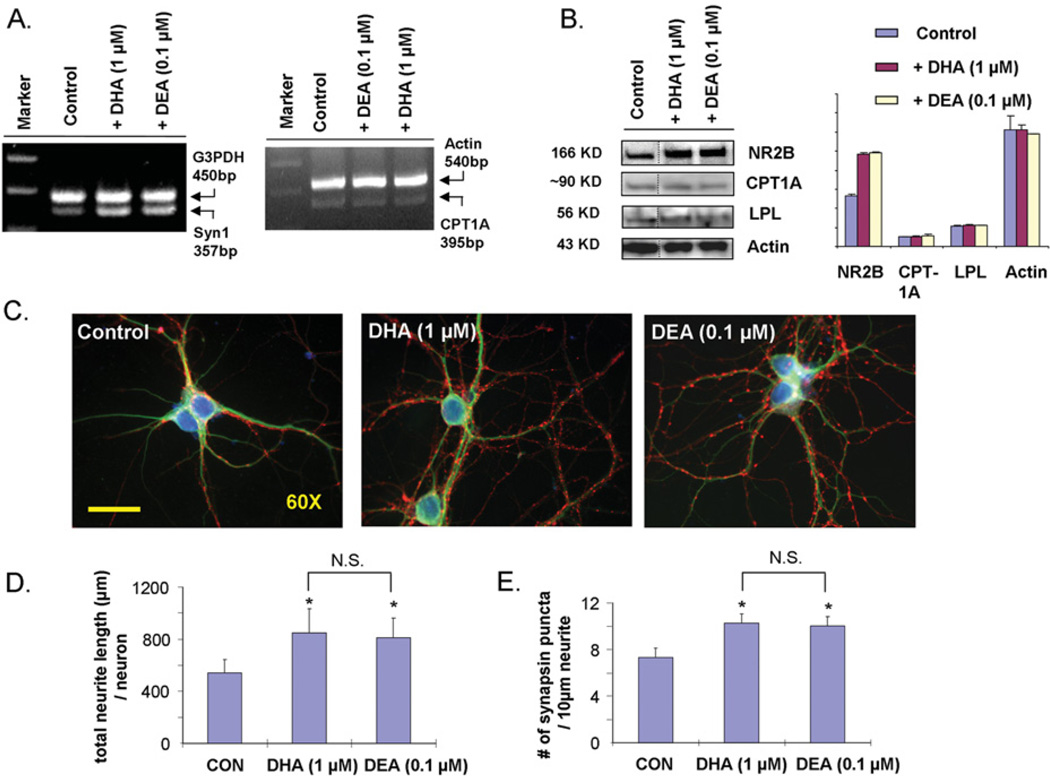
Since both DEA and DHA promote hippocampal development, their effects on synaptic protein expression, neurite outgrowth and synaptogenesis were compared under the same conditions (Figure 6). The treatment of hippocampal neurons with DHA at 1 µM and DEA at 0.1 µM increased the expression of synapsin and NR2B to a similar extent, whereas neither treatment changed the expression of the PPARα (peroxisome-proliferator-activated receptor α) downstream targets CPT1A or LPL (Figures 6A and 6B). In addition, DEA at 0.1 µM promoted neurite growth (Figures 6C and 6D) and synaptogenesis (Figures 6C and 6E) to a similar extent observed with 1 µM DHA. Although the cellular concentration of DEA at any given moment cannot be accurately determined, 0.1 µM is estimated to be in the attainable range when cells were incubated with 1 µM DHA. The DEA level after 3 days of 1 µM DHA supplementation reached up to 5.6 pmol/4.7×106 cells when its hydrolysis was inhibited by URB597, corresponding to a cellular concentration of approx. 240 nM on the basis of an estimated cell volume of 5 pl/cell [23]. These results strongly suggested that the observed DHA-induced neurite growth, synaptogenesis and synaptic protein expression was mediated, at least in part, through the metabolism of DHA to DEA.
Figure 6. Comparison of synaptic protein expression, hippocampal neurite outgrowth and synaptogenesis induced by DHA and DEA.
Expression of synapsin (A) and NR2B (B) in hippocampal neurons supplemented with 1 µM DHA or 0.1 µM DEA, showing comparable increases in synapsin and NR2B expression without altering the expression of the PPARα downstream targets CPT1A and LPL. The mRNA (A) or protein levels (B) were determined by RT–PCR or Western blot analysis at 12 h or 7 days after supplementation respectively. (C) Representative photomicrographs of E18 mouse hippocampal neurons after culturing for 7 days in the presence of 1 µM DHA or 0.1 µM DEA: MAP2, green; synapsin-1, red; and DAPI, blue. Scale bar, 30 µm. Hippocampal neurite development (D) and synaptogenesis (E) induced by DHA or DEA at 7 DIV (days in in vitro culture), also showing comparable effects at these concentrations. *P < 0.05 compared with control; N.S., not significant. CON, control.
DEA enhances glutamatergic synaptic activity
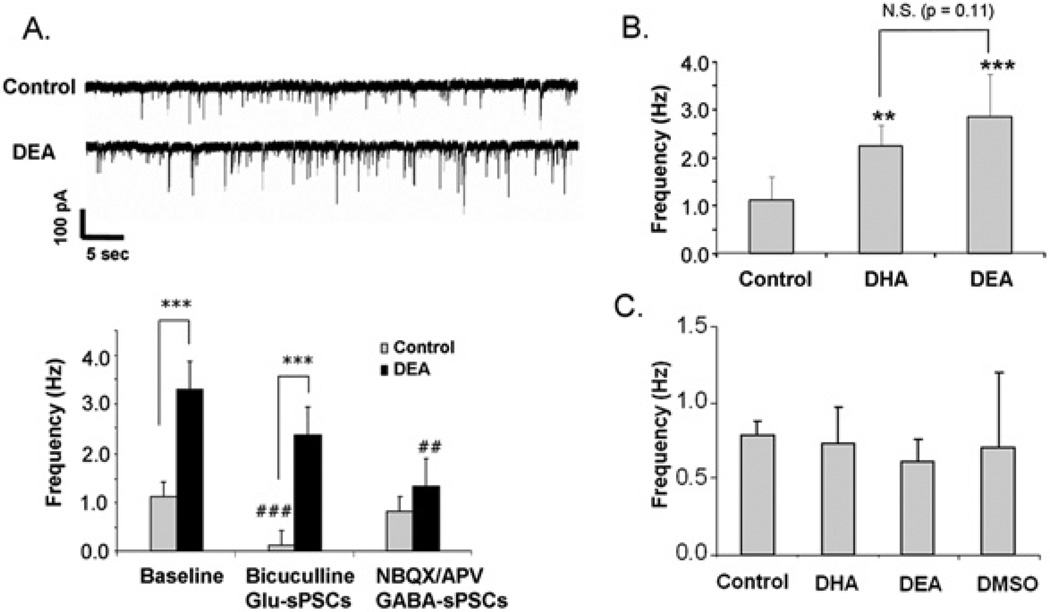
Previously, glutamatergic synaptic transmission in hippocampal neurons has been shown to be promoted by DHA supplementation [12]. We observed that the treatment of neurons with 0.1 µM DEA for 10 days also enhanced sPSCs (Figure 7). The sPSC frequency, which represents the number of active synapses and relative levels of presynaptic release, was significantly greater in the DEA-treated neurons in comparison with the control neurons (1.13 ± 0.48 and 3.29 ± 1.72 Hz for control and DEA-treated neurons respectively; P = 0.001) (Figure 7A), indicating that DEA promoted synaptogenesis. As with DHA, the enhanced synaptic activity was derived predominantly from glutamatergic synapses, since applying NBQX and APV (2-amino-5-phosphonovaleric acid) to block both AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid) and NMDA receptors abolished the observed enhancement (Figure 7A). In contrast, blocking GABAA receptors with bicuculline decreased transmission under control conditions, but had no significant effects in the DEA-treated cultures. The antagonist-based assignment for either glutamatergic or GABAergic synaptic activity was further supported by the analysis of decay constants. The neurons in the culture showed average decay time constants of 6.1 ± 4.1 or 23.2 ± 6.1 ms (n = 15, P < 0.001) in the presence of bicuculline or NBQX/APV respectively. This observation was consistent with previously reported faster kinetics of glutamatergic PSCs in comparison with GABAergic PSCs with decay constants of 3.01 ± 0.28 and 19.05 ± 1.98 ms respectively [24]. The hippocampal neurons supplemented with 0.1 µM DEA or 1 µM DHA enhanced sPSCs to a similar extent (Figure 7B). Acute application of DEA or DHA to the target neurons had no significant effects on sPSCs (Figure 7C), suggesting that neurite growth, synaptogenesis and synaptic protein expression induced by the treatment with DEA during development is most probably responsible for the enhanced synaptic activity.
Figure 7. Effects of DEA on synaptic activity.
(A) sPSCs, including Glu-sPSC and GABA-sPSC components, in hippocampal neurons cultured with or without 0.1 µM DEA for 10 days. DEA-supplemented neurons exhibited increased frequency of sPSCs. The frequency of sPSCs in unsupplemented control neurons was decreased significantly by bicuculline, whereas NBQX/APV decreased the frequency in the DEA-supplemented neurons. Upper panel: representative sPSC traces obtained from control and DEA-treated neurons. Paired Student’s t tests were performed against the baseline value of each group (##P < 0.01, ###P < 0.001) or between indicated groups (***P < 0.001). (B) sPSCs in hippocampal neurons supplemented with 1 µM DHA or 0.1 µM DEA for 10 days. Paired Student’s t tests were performed against the control value (**P < 0.01, ***P < 0.001). (C) No effects of acute application of DHA or DEA on synaptic activity. Error bars represent S.E.M. (n = 5). The data represent three independent experiments. N.S., not significant.
Endogenous DEA levels in fetal hippocampi are affected by maternal n−3 fatty acid intake
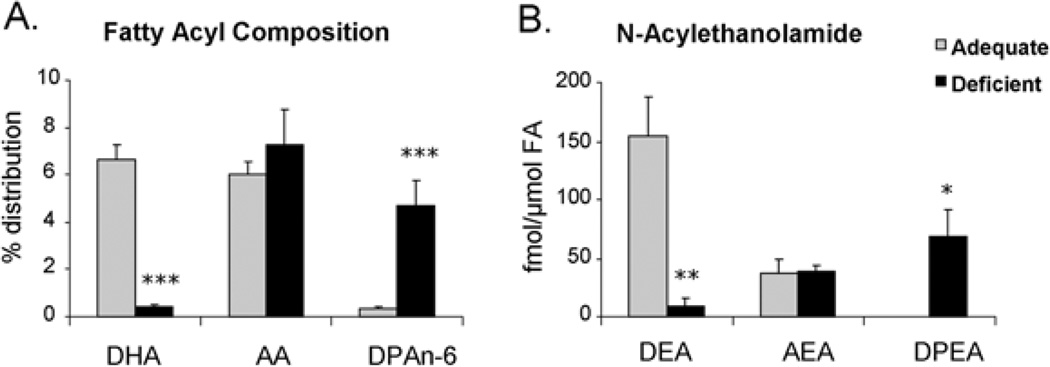
Maternal dietary n−3 fatty acid depletion has been shown to deplete DHA in fetal and offspring hippocampi, leading to inhibited hippocampal neurite outgrowth and synaptogenesis in in vitro culture, and impaired long-term potentiation in the offspring hippocampus [12]. To test the in vivo implications of DEA formation in hippocampal neuronal development, we examined whether endogenous DEA levels can be lowered by inducing prenatal n−3 fatty acid deficiency. In E18 hippocampi obtained from n−3 fatty acid-adequate dams, the DEA level (155 ± 35 fmol/µmol of total fatty acids or 11.5 ± 2.3 fmol/hippocampus) was significantly higher than AEA (44 ± 3 fmol/µmol of total fatty acids or 4.4 ± 0.8 fmol/hippocampus) despite the fact that DHA (6.7 ± 0.7%) and AA (arachidonic acid, C20:4,n−6;6.1 ± 0.5%) contents were comparable (Figure 8). When pregnant mice were fed with an n−3 fatty acid-deficient diet, the DEA level decreased significantly (to 9 ± 7 fmol/µmol of fatty acid or 0.6 ± 0.4 fmol/hippocampus; P = 0.008), with concomitant reduction in the DHA content (to 0.5 ± 0.1%). Neither AA nor AEA content was significantly affected by the deficient diet, but DPAn-6 (docosapentaenoic acid, C22:5,n−6) was increased from 0.4 ± 0.1 to 4.7 ± 1.0% with a concomitant increase of DPEA (N-docosapentaenoylethanolamine), the DPA analogue of AEA and DEA, from a non-detectable level to 69.2 ± 22.8 fmol/µmol of fatty acid or 4.5 ± 1.7 fmol/hippocampus. These results indicated that DEA production is an endogenous mechanism that can be modulated by the DHA status, supporting in vivo significance of DEA in DHA-induced hippocampal neuronal development.
Figure 8. Effects of maternal depletion of dietary n−3 fatty acids on DEA levels in fetal hippocampi at E18.
Fatty acyl composition (A) and N-acylethanolamine levels (B) in E18 hippocampi, indicating significant decreases in DHA and DEA after n−3 fatty acid depletion without altering AA or AEA contents. *P < 0.05, **P < 0.01 and ***P < 0.001. FA, total fatty acids.
DISCUSSION
In the present study, we demonstrate that metabolism of DHA to DEA is a significant mechanism for hippocampal development. Neurite outgrowth, synaptogenesis and the expression of synapsins and glutamate receptors, which are known to be important for synaptic transmission and glutamatergic synaptic activity, were promoted by DEA treatment in developing hippocampal neurons. DEA effects on hippocampal development and glutamatergic synaptic activity are similar to those previously demonstrated with DHA [12], suggesting that DEA is, at least in part, responsible for hippocampal development and function promoted by DHA.
The involvement of an FAAH-sensitive form of DHA was apparent in DHA-induced hippocampal development, at least for the neurite growth, synaptogenesis and expression of some synaptic proteins (Figure 1). The metabolism of DHA to DEA was found to be active in developing hippocampi, and the biological activity of DEA was similar in nature to, but significantly higher than, that of DHA, strongly suggesting that DEA is a principal component for the observed DHA effects. Specific inhibitors of DEA biosynthesis may further confirm whether DEA mediates DHA effects on hippocampal development and function, when such inhibitors become available. The biochemical mechanisms for DEA synthesis are not clear at present. It is possible that similar biosynthetic pathways for producing AEA via NAPE (N-arachidonyl phosphatidylethanolamine) [25] may also be involved for DEA formation.
The hippocampal development in non-supplemented neurons was also sensitive to the FAAHI URB597 (Figure 1). In addition to DEA, N-oleoylethanolamide at a rather high concentration has been shown to promote neurite growth through PPARα in a fatty acid-depleted experimental condition [26], suggesting that there are multiple mechanisms supporting the basic requirements for neurite growth, synaptogenesis and synaptic protein expression. The DEA- and DHA-dependent synaptic protein expression demonstrated in the present study may involve the activation of distinctive transcription factors that have yet to be identified. Nevertheless, involvement of PPARα is unlikely, as the expression of PPARα downstream target proteins, CPT1A or LPL, was not altered by DHA or DEA (Figure 6).
Previous studies have indicated that NPD1 (neuroprotectin D1, also called 10,17S-docosatriene), a DHA metabolite formed by lipoxygenation, can exert a neuroprotective function, particularly under pathophysiological conditions such as ischaemia [16]. It has been also reported that 17-hydroxy-DHA, which is thought to be the immediate precursor of NPD1, is produced in ischaemic brains [27]. For hippocampal neurite growth and synaptogenesis, we found that DEA, an N-acylated ethanolamide, is a key active metabolite of DHA. The DEA content in the fetal hippocampi decreased with the reduction of DHA through maternal dietary depletion of n−3 fatty acids (Figure 8), which is consistent with the previous report that the DEA content in the pig brain can be increased by dietary inclusion of DHA [28]. As the DEA level correlates with the DHA status, provision of DHA to the developing brain, as well as its metabolism to DEA, is important for neuronal development in offspring. In addition to ethanolamine, DHA has been shown to be N-acylated to amino acids or neurotransmitters in the brain [29], although their biological activity has yet to be determined.
The DEA-induced hippocampal synaptogenesis paralleled the enhancement of synaptic activity. The synaptic activity increase due to the DEA treatment was derived mostly from glutamatergic activity, as has been observed in DHA-treated neurons [12]. Acute applications of DEA showed no effects on synaptic activity (Figure 7), suggesting that availability of DHA-derived DEA during development for neurite growth, synaptogenesis and synaptic protein expression is an important aspect for enhanced synaptic activity. In this regard, DHA metabolism to DEA is a key mechanism leading to enhanced synaptic function through promoted hippocampal neurodevelopment.
It is well established that the endocannabinoid AEA exerts its biological effects principally through binding to G-protein-coupled CB (cannabinoid) receptors [30]. It has been demonstrated that activation of CB1 receptors promotes hippocampal neurogenesis in both the embryonic and adult hippocampus [31]. Although a possible role of DEA in CB1-mediated signalling cannot be ruled out, involvement of CB1 in the DEA-induced hippocampal development is unlikely. It has been reported that DEA binding to CB1 is substantially weaker than AEA [32]. Despite its higher binding capability as a natural ligand for CB1, AEA showed minimal effects on hippocampal development in comparison with DEA (Figure 5). Moreover, the AEA content observed in E18 hippocampi was significantly lower in comparison with DEA (Figure 8), further supporting the unlikely involvement of a CB1-mediated mechanism. Our present results appear to be in line with the previous finding that CB1 activation by AEA does not promote but inhibits neuronal progenitor cell differentiation [33].
It has been reported that DHA is an endogenous ligand to RXR (retinoid X receptor) [34]. It has been also shown that DHA can activate PPARγ, particularly as oxidized forms [35]. At present, it is not clear whether DEA can also activate RXR or PPARγ. Nevertheless, DHA and DEA appear to target the same transcriptional activity, since DHA and DEA promote the expression of similar specific synaptic proteins. Since DEA is produced from DHA and DEA is significantly more effective than DHA for synaptic protein expression, the involvement of DEA in DHA-promoted hippocampal synaptic protein expression is strongly suggested. It appears possible that the effective DEA level can be reached in the hippocampal neuronal culture even from low micromolar concentrations of DHA, further supporting the role of DEA as an active component in DHA-mediated transcriptional activation for neuronal differentiation and specific synaptic protein expression.
In conclusion, the present study demonstrates that the metabolism of DHA to DEA is a novel and significant mechanism for hippocampal neuronal development and function. DEA produced from DHA promotes neurite development, synaptogenesis and expression of synapsins and glutamate receptors, which in turn leads to improved synaptic transmission. Since DEA is derived from DHA, compromised DEA-dependent hippocampal development may be an underlying mechanism for the learning disability and memory deficit associated with neural DHA depletion due to dietary deficiencies of n−3 fatty acids. More importantly, the DEA-dependent mechanism may offer new molecular targets for the regulation of hippocampal neuronal development and function.
Supplementary Material
ACKNOWLEDGEMENT
We thank Dr Arthur Spector for helpful comments on the manuscript.
FUNDING
This work was supported by the Intramural Research Program of the National Institute of Alcohol Abuse and Alcoholism, National Institutes of Health.
Abbreviations used
- AA
arachidonic acid
- AEA
arachidonylethanolamine
- CB receptor
cannabinoid receptor
- CPT1
carnitine palmitoyltransferase 1
- DAPI
4′,6-diamidino-2-phenylindole
- DEA
N-docoxahexaenoylethanolamide
- DHA
docosahexaenoic acid
- DHA-amide
N-docosahexaenoylamide
- DPA
docosapentaenoic acid
- E18
embryonic day 18
- ESI
electrospray ionization
- FAAH
fatty acid amide hydrolase
- FAAHI
FAAH inhibitor
- FAME
fatty acid methyl ester
- GABA
γ-aminobutyric acid
- HSD
Honestly Significant Difference
- LC
liquid chromatography
- LNA
linolenic acid
- LPL
lipoprotein lipase
- MAP2
microtubule-associated protein 2
- MRM
multiple reaction monitoring
- MS/MS
tandem MS
- NBQX
2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium
- NDGA
nordihydroguaiaretic acid
- NMDA
N-methyl-d-aspartate
- NPD1
neuroprotectin D1
- NR2B
NMDA receptor subunit 2B
- PPAR
peroxisome-proliferator-activated receptor
- PSC
postsynaptic current
- RT
reverse transcription
- RXR
retinoid X receptor
- sPSC
spontaneous PSC
- GABA-sPSC
GABAergic sPSC
- Glu-sPSC
glutamatergic sPSC
- TBS
Tris-buffered saline
Footnotes
AUTHOR CONTRIBUTION
Hee-Yong Kim conceived the idea, designed the experiments, analysed the data and wrote the paper. Hyun-Seuk Moon and Dehua Cao performed the cell biology experiments and analysed the data. Jeongrim Lee and Karl Kevala performed the MS analysis. Sangbeom Jun and David Lovinger performed the electrophysiology experiments. Mohammed Akbar and Bill Huang carried out some of the Western blotting and RT–PCR experiments. Karl Kevala and Bill Huang synthesized d0- and d4-DEA and DHA-amide.
REFERENCES
- 1.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 2.Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M. Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet. 1998;352:688–691. doi: 10.1016/s0140-6736(97)11374-5. [DOI] [PubMed] [Google Scholar]
- 3.Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev. Med. Child Neurol. 2000;42:174–181. doi: 10.1017/s0012162200000311. [DOI] [PubMed] [Google Scholar]
- 4.Gamoh S, Hashimoto M, Sugioka K, Shahdat Hossain M, Hata N, Misawa Y, Masumura S. Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience. 1999;93:237–241. doi: 10.1016/s0306-4522(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 5.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n−3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics. 2003;111:e39–e44. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 6.Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369:578–585. doi: 10.1016/S0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 7.Moriguchi T, Greiner RS, Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. J. Neurochem. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 8.Lim SY, Hoshiba J, Moriguchi T, Salem N., Jr n−3 fatty acid deficiency induced by a modified artificial rearing method leads to poorer performance in spatial learning tasks. Pediatr. Res. 2005;58:741–748. doi: 10.1203/01.PDR.0000180547.46725.CC. [DOI] [PubMed] [Google Scholar]
- 9.Vancassel S, Aid S, Pifferi F, Morice E, Nosten-Bertrand M, Chalon S, Lavialle M. Cerebral asymmetry and behavioral lateralization in rats chronically lacking n − 3 polyunsaturated fatty acids. Biol. Psychiatry. 2005;58:805–811. doi: 10.1016/j.biopsych.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 10.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J. Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- 12.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J. Neurochem. 2009;111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He C, Qu X, Cui L, Wang J, Kang JX. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc. Natl. Acad. Sci. U.S.A. 2009;106:11370–11375. doi: 10.1073/pnas.0904835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robson LG, Dyall S, Sidloff D, Michael-Titus AT. Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurones throughout development and in aged animals. Neurobiol. Aging. 2010;31:678–687. doi: 10.1016/j.neurobiolaging.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Serhan CN. Novel chemical mediators in the resolution of inflammation: resolvins and protectins. Anesthesiol. Clin. 2006;24:341–364. doi: 10.1016/j.atc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- 17.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 18.Wen Z, Kim HY. Alterations in hippocampal phospholipid profile by prenatal exposure to ethanol. J. Neurochem. 2004;89:1368–1377. doi: 10.1111/j.1471-4159.2004.02433.x. [DOI] [PubMed] [Google Scholar]
- 19.Calderon F, Kim HY. Role of RXR in neurite outgrowth induced by docosahexaenoic acid. Prostaglandins Leukotrienes Essent. Fatty Acids. 2007;77:227–232. doi: 10.1016/j.plefa.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 21.Xu MZ, Lee WS, Kim MJ, Park DS, Yu H, Tian GR, Jeong TS, Park HY. Acyl-CoA: cholesterol acyltransferase inhibitory activities of fatty acid amides isolated from Mylabris phalerate. Pallas. Bioorg. Med. Chem. Lett. 2004;14:4277–4280. doi: 10.1016/j.bmcl.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G. A biosynthetic pathway for anandamide. Proc. Natl. Acad. Sci. U.S.A. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korchev YE, Gorelik J, Lab MJ, Sviderskaya EV, Johnston CL, Coombes CR, Vodyanoy I, Edwards CR. Cell volume measurement using scanning ion conductance microscopy. Biophys. J. 2000;78:451–457. doi: 10.1016/S0006-3495(00)76607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barazangi N, Role LW. Nicotine-induced enhancement of glutamatergic and GABAergic synaptic transmission in the mouse amygdala. J. Neurophysiol. 2001;86:463–474. doi: 10.1152/jn.2001.86.1.463. [DOI] [PubMed] [Google Scholar]
- 25.Astarita G, Ahmed F, Piomelli D. Identification of biosynthetic precursors for the endocannabinoid anandamide in the rat brain. J. Lipid Res. 2008;49:48–57. doi: 10.1194/jlr.M700354-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Bento-Abreu A, Tabernero A, Medina JM. Peroxisome proliferator-activated receptor-α is required for the neurotrophic effect of oleic acid in neurons. J. Neurochem. 2007;103:871–881. doi: 10.1111/j.1471-4159.2007.04807.x. [DOI] [PubMed] [Google Scholar]
- 27.Farias SE, Basselin M, Chang L, Heidenreich KA, Rapoport SI, Murphy RC. Formation of eicosanoids, E2/D2 isoprostanes, and docosanoids following decapitation-induced ischemia, measured in high-energy-microwaved rat brain. J. Lipid Res. 2008;49:1990–2000. doi: 10.1194/jlr.M800200-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger A, Crozier G, Bisogno T, Cavaliere P, Innis S, Di Marzo V. Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6402–6406. doi: 10.1073/pnas.101119098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan B, O’Dell DK, Yu YW, Monn MF, Hughes HV, Burstein S, Walker JM. Identification of endogenous acyl amino acids based on a targeted lipidomics approach. J. Lipid Res. 2010;51:112–119. doi: 10.1194/jlr.M900198-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 31.Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J. Clin. Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheskin T, Hanus L, Slager J, Vogel Z, Mechoulam R. Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J. Med. Chem. 1997;40:659–667. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- 33.Rueda D, Navarro B, Martinez-Serrano A, Guzman M, Galve-Roperh I. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J. Biol. Chem. 2002;277:46645–46650. doi: 10.1074/jbc.M206590200. [DOI] [PubMed] [Google Scholar]
- 34.de Urquiza AM, Liu S, Sjoberg M, Zetterstrom RH, Griffiths W, Sjovall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, Itoh T, Abe D, Shimizu M, Kanda T, Koyama T, Nishikawa M, Tamai T, Ooizumi H, Yamada S. Identification of putative metabolites of docosahexaenoic acid as potent PPARγ agonists and antidiabetic agents. Bioorg. Med. Chem. Lett. 2005;15:517–522. doi: 10.1016/j.bmcl.2004.11.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.