Abstract
The estrogen receptor (ER) is a ligand-dependent transcription factor containing two transcriptional activation domains. AF-1 is in the N terminus of the receptor protein and AF-2 activity is dependent on helix 12 of the C-terminal ligand-binding domain. Two point mutations of leucines 543 and 544 to alanines (L543A, L544A) in helix 12 minimized estrogen-dependent transcriptional activation and reversed the activity of the estrogen antagonists ICI182780 (ICI) and tamoxifen (TAM) into agonists in a similar manner that TAM activated WT ERα through AF-1 activation. To evaluate the physiological role of AF-1 and AF-2 for the tissue-selective function of TAM, we generated an AF-2–mutated ERα knock-in (AF2ERKI) mouse model. AF2ERKI homozygote female mice have hypoplastic uterine tissue and rudimentary mammary glands similar to ERα-KO mice. Female mice were infertile as a result of anovulation from hemorrhagic cystic ovaries and elevated serum LH and E2 levels, although the mutant ERα protein is expressed in the AF2ERKI model. The AF2ERKI phenotype suggests that AF-1 is not activated independently, even with high serum E2 levels. ICI and TAM induced uterotropic and ER-mediated gene responses in ovariectomized AF2ERKI female mice in the same manner as in TAM- and E2-treated WT mice. In contrast, ICI and TAM did not act as agonists to regulate negative feedback of serum LH or stimulate pituitary prolactin gene expression in a different manner than TAM- or E2-treated WT mice. The functionality of the mutant ERα in the pituitary appears to be different from that in the uterus, indicating that ERα uses AF-1 differently in the uterus and the pituitary for TAM action.
Estrogen regulates gene transcription via the estrogen receptors (ERs) α and β, which are ligand-dependent transcription factors. Transcriptional activation is mediated by AF-1 in the N-terminal domain and AF-2 in the C-terminal ligand-binding domain (LBD). ER ligands bind to the LBD and induce a conformational change of this LBD domain to modulate transcriptional activation. A portion of the AF-2 domain resides in helix 12 and plays a crucial role in determining interactions with coactivators and corepressors for transcriptional regulation influencing respective agonist or antagonist effects of the ligand (1, 2). Helix 12 has conserved hydrophobic amino acids between species. The mouse ERα residues L543 and L544 are correlated to the L539 and L540 residues of human ERα helix 12. The mutation of these residues in mouse and human ERα has been reported to have similar properties (3–9). Despite these amino acid mutations, binding to estrogen-responsive DNA sequences and estradiol (E2) is unaffected (3, 6); however, transcription activity is markedly lower in the presence of E2 compared with WT ERα because of the failure to recruit the p160 transcriptional coactivators (4). The mutation of these residues has been shown to convert the antiestrogens, including ICI164384, RU54876, and tamoxifen (TAM), into agonists (5, 9). The AF-1 region is required for a transcriptionally active configuration of this mutant with antagonists (5, 9). TAM is a well known selective ER modulator (SERM) that is a partial antagonist/weak agonist for ERα WT (10, 11). Several reports proposed that the N-terminal AF-1 of ERα WT is required for TAM-mediated partial activity and that may be related to the cell type specific functionality of TAM (12). However, it is still not entirely clear how TAM manifests agonist activities through ERα WT in different tissues. We focused on the L543A and L544A mutations in the ERα AF-2 domain (AF2ER) to evaluate the ERα AF-1 and AF-2 functions in vivo and the SERM functionality in the tissues.
The ERα-KO (αERKO) mouse is an established model for evaluating ERα function in vivo. The αERKO does not express functional ERα protein as a result of a genetic modification of Esr1 (13, 14). The αERKO mouse model has revealed various physiological functions involving ERα (15). However, this model cannot discern the selective functionality of the ERα AF-1 or AF-2 in ERα-mediated physiological responses in vivo because no receptor protein is expressed. The aim of this study was to evaluate the physiological function of the ERα AF-1 and AF-2 in vivo. We developed a knock-in mouse model with the AF2ER mutations (AF2ERKI). In the AF2ERKI mice, we can definitively determine that the mutation of helix 12 inactivates the ERα-mediated response to endogenous estrogens, making the AF2ERKI mice comparable to the αERKO mice. Our present studies confirmed that estrogen-induced AF-2 activation is critical for regulating female reproductive tissue hormone responses and AF-1 is not activated independently without AF-2. In addition, this report shows that the antiestrogens ICI182780 (ICI) or TAM can be shown to act as agonists in vivo involving AF2ER mutations. Our in vitro studies indicated the AF-1 activity of ERα is involved in ICI-mediated AF2ER activation and that activation is similar to the condition of TAM-mediated ERα WT transactivation. Therefore, the in vivo observation of ICI-mediated regulation suggests the ERα AF-1 mediates physiological functions in certain tissues and may represent tissue specific SERM functions.
Results
Properties of the L543A, L544A Mutant ERα.
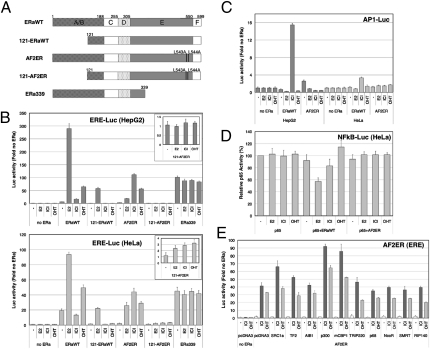
We demonstrated the differential functionality of the AF2ER (L543A, L544A mutated ERα) on the ERα-mediated transcription regulation with in vitro transient transfection assays using two different ERα-negative cell lines (HepG2 and HeLa cells; Fig. 1 and Fig. S1). First, we assessed estrogen response element (ERE)-mediated transcription activity. As expected, E2 produced strong activation of the ERE reporter with WT ERα. In contrast, the antiestrogen ICI was inactive with WT ERα but activated the ERE-mediated transcription of AF2ER in both cell lines. ICI-mediated AF2ER activation was more potent than E2 activity. This activation was not observed in the N-terminal truncated AF2ER (121-AF2ER)-transfected HepG2 cells, but very weak activity was observed in HeLa cells (Fig. 1B), indicating that the AF2ER mutant has minimized the AF-2 function and AF-1 is necessary for ICI-mediated AF2ER activation. Furthermore, to confirm the level of ERα AF-1 activity in these cells, C-terminal truncated ERα (ERa339), which activates ERE-mediated transcription irrespective of the presence or absence of ligand, was cotransfected with the ERE reporter. Coincidentally, the level of ERa339-mediated activation in these cells is similar to the level of ICI-mediated AF2ER activity (Fig. 1B), which suggests that the ICI-dependent AF2ER activity is derived from AF-1. Second, to assess AF2ER-mediated DNA-tethered transactivation, we tested AF2ER activity with an AP-1 reporter. ICI activated the AP-1–mediated transcription through WT ERα as previously reported (16). However, AF2ER did not activate the AP-1–mediated transcription with any ligands [E2, ICI, and 4-hydroxy-TAM (OHT); Fig. 1C]. Furthermore, we assessed the transrepression activity of AF2ER in NF-κB–dependent gene activation. AF2ER did not repress the NF-κB–mediated transcription (Fig. 1D). These results suggest that AF2ER is likely to predominantly regulate the ERE-mediated transcription. OHT, a known ERα AF-1–activating partial antagonist, weakly activated WT ERα and activated AF2ER, but to a lesser extent than ICI (Fig. 1B). Therefore, we used ICI for the further experiments. To determine if coregulators for WT ERα were involved in the ICI agonist activity of the AF2ER, expression vectors for coregulators were cotransfected, and p300, CREB binding protein (CBP), and SRC1a were found to increase the ICI-mediated AF2ER activity in HeLa and HepG2 cells (Fig. 1E and Fig. S1A, Top). We also determined the effect of coregulators on the OHT agonistic activity of WT ERα. Surprisingly, the profile of coregulators’ effect on OHT-bound WT ERα shows an almost identical pattern to ICI-liganded AF2ER (Fig. S1A, Middle, and Fig. S1B, Top). In this condition, however, the profile of coregulators effect on E2-bound WT ERα is different from OHT-bound WT ERα (Fig. S1A, Bottom, and Fig. S1B, Bottom). The p300/CBP coactivators also increased ERa339-mediated ERE activation regardless of the presence or absence of ICI, but did not enhance 121-AF2ER–mediated activity (Fig. S1 C and D). SRC1a slightly enhanced 121-AF2ER activation but did not alter ERa339 transactivation (Fig. S1 C and D). Furthermore, we found an additive effect of p300/CBP together with SRC1a on the ICI-dependent AF2ER activation that was observed in ERa339- but not 121-AF2ER–mediated activation. A similar activation profile was observed for OHT-mediated WT ERα activation (Fig. S1 E and F). These results suggest that ICI liganded AF2ER is likely to mimic the OHT-mediated WT ERα activation.
Fig. 1.
Transcription function of AF2ER (L543A, L544A mutated ERα). (A) Schematic illustration of the ERα mutants. (B) HepG2 or HeLa cells were transfected with the reporter gene (3xERE-TATA-luc) and expression vectors for WT or mutated receptors were maintained with or without ligands. (C) HepG2 or HeLa cells were transfected with the reporter gene (7xAP1-TATA-luc) and expression vectors for WT or AF2ER. The cells were maintained with or without ligands. The luciferase activities for the each treatment were represented as fold change for the empty expression vector, pcDNA3 (no ERα). (D) Transrepression function of WT ERα and AF2ER. HeLa cells were transfected with the NF-κB reporter gene (3xMHC-luc), and expression vectors for p65/RelA and WT ERα or AF2ER were maintained with or without ligands. The luciferase activities were expressed relative to p65 activity in the absence of ligand (100%). (E) The effect of cofactors on ICI- or OHT-dependent AF2ER activation. HeLa cells were transfected with the 3xERE reporter gene and expression vectors for cofactors and AF2ER. The cells were maintained with or without ligands (100 nM E2, ICI, or OHT were used for treatments). Luciferase activity is represented as the mean ± SD of three independent experiments.
Generation of AF2ERKI Mice.
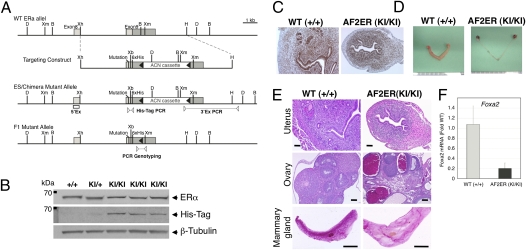
To assess the effect of loss of AF-2 function in vivo, we generated AF2ERKI mice through homologous recombination in mouse ES cells. The construct targeted the ninth exon of mouse Esr1, which contains helix 12, possessing the AF-2 region of the LBD as well as the stop codon. Leucines 543 and 544 were mutated to alanines, and a 6xHis-tag was added to the C-terminal end of the mouse ERα protein (Fig. 2A, SI Materials and Methods, and Fig. S2). The expression of AF2ER mutant protein in the AF2ERKI mice was evaluated by Western blot analysis of uterine tissues by using the anti-mouse ERα antibody and anti–His-tag antibody. The uterine tissue was collected from AF2ERKI homozygote (AF2ERKI/KI), heterozygote (AF2ERKI/+), and WT female mice. As shown in Fig. 2B, we detected a 66-kDa signal using the anti ERα antibody. After stripping, the membrane was probed with the anti–His-tag antibody. A 66-kDa signal was detected in the AF2ERKI/KI but not in WT, and a faint signal was detected in the AF2ERKI/+. Furthermore, we performed immunohistochemistry using the AF2ERKI/KI and WT female uteri. As shown in Fig. 2C, anti-ERα antibody–derived signal was detected in all the tissue compartments of the uterus, including the endometrial epithelial cells and stromal cells of both genotypes. Taken together, these results indicate that the mutant AF2ER protein is being expressed in the mice.
Fig. 2.
Targeting strategy and confirmation of L543A, L544A ERα knock-in mutation. (A) Schematic illustration of the targeting strategy used to introduce mutation. Diagrams show the WT ERα locus, targeting construct, targeted mutant allele in the ES cells/chimera mice, and F1 mutant allele after ACN cassette self-excision. The targeting construct contained ERα exon 9 (light gray boxes show coding sequence and dark gray box shows 3′ UTR), the L543A, L544A mutations (“mutation”), an extra XbaI site (Xb), and a 6xHis-tag epitope (6xHis). The ACN cassette was flanked at the 5′ and 3′ ends by loxP sites (closed arrowheads). Open box suggests the position of 5′ external probe for Southern blot (5′Ex), and pairs of open arrowheads suggest PCR primer sets for 3′ external PCR (3′Ex PCR), His-tag PCR, and PCR genotyping. D, DrdI; Xm, XmnI; B, BamHI; Xh, XhoI; H, HindIII. (B) Representative results of Western blot probed for the ERα, His-tagged ERα (His-Tag), and β-tubulin in the 8-wk-old individual mouse uterus are shown. β-Tubulin was used as a loading control. +/+, WT; KI/+, heterozygote; KI/KI, homozygote. (C) Uterine ERα immunohistochemistry of 8-wk-old representative mice. (D) Morphology of AF2ERKI female reproductive organs in the 8-wk-old representative mice. (E) Histology of 8-wk-old representative AF2ERKI female mice. Uterine (Top) and ovarian (Middle) tissue H&E staining from WT (Left) and AF2ERKI homozygote (Right) mice. (Scale bar: 100 μm.) Mammary gland (Bottom) whole-mount Carmine alum staining from 8-wk-old representative mice. (Scale bar: 1 cm.) (F) The mRNA level of Foxa2 was quantified by real-time PCR. The mRNA levels were represented as fold change for the WT. Values are presented as mean ± SD.
Phenotype of AF2ERKI Female Is Similar to That of αERKO Female.
Continuous breeding studies indicated that AF2ERKI/KI female mice were infertile. We evaluated the estrous cycle of WT, AF2ERKI/+, and AF2ERKI/KI female mice by daily vaginal lavage for nine consecutive days. WT and AF2ERKI/+ female mice showed a normal estrous cycle. On the contrary, the AF2ERKI/KI female mice showed no estrous cycle (Fig. S3). Another endpoint linked to reproductive cyclicity and responsiveness is serum luteinizing hormone (LH) levels. The serum LH level of AF2ERKI/KI (5.39 ± 2.33 ng/mL) was elevated fourfold vs. WT (1.29 ± 2.12 ng/mL), and the serum E2 level of AF2ERKI/KI (96.8 ± 10.1 pg/mL) was twofold higher than WT (44.2 ± 9.43 pg/mL). These hormone levels were similar to those from a pool of αERKO serum from several female mice (LH, 5.78 ng/mL; E2, 87.6 pg/mL). Such elevated LH levels suggested that the negative feedback of the hypothalamic–pituitary–gonadal axis in AF2ERKI/KI is disrupted, as has been reported in the αERKO animals (17), consistent with the infertility. Female reproductive tract organs from WT and AF2ERKI/KI mice were analyzed for morphological and histological parameters. AF2ERKI/KI uteri were significantly smaller and more hypoplastic than those of their WT littermates (Fig. 2D). AF2ERKI/KI uterine tissues possess luminal epithelium, but fewer glandular structures were evident compared with WT uteri (Fig. 2E, Top). We evaluated the expression level of uterine Foxa2, a gene that is implicated in uterine gland development (18). The level of uterine Foxa2 mRNA was significantly lower in AF2ERKI/KI than WT (Fig. 2F), which is consistent with less glandular morphology. The ovaries from the AF2ERKI/KI female mice show cystic and hemorrhagic follicles, reminiscent of the αERKO ovarian phenotype. A few primary follicles can be seen, but no corpora lutea were observed in AF2ERKI/KI ovaries (Fig. 2E, Middle). Mammary gland whole-mounts were analyzed for evidence of ductal proliferation and differentiation. In 8-wk-old WT mice, ductal trees extended past the lymph node and had enlarged terminal end buds. AF2ERKI/KI mammary glands never developed beyond a rudimentary epithelial ductal tree (Fig. 2E, Bottom), similar to αERKO females. Taken together, these results clearly suggest that estrogen-dependent AF-2–mediated transactivation of ERα is essential for developing and maintaining female reproductive functions.
Antiestrogens ICI and TAM Induce Uterotropic Responses in AF2ERKI Homozygote.
Based on our in vitro studies, to assess the functionality of AF2ER in vivo, ovariectomized (OVX) AF2ERKI/KI female mice were injected in a 3-d bioassay with vehicle, ICI, TAM, or E2, and uteri were collected 24 h after the last treatment. E2 and TAM significantly induced the uterine growth of WT mice, and ICI treatment was ineffective. In contrast, ICI and TAM treatment increased the uterine wet weight of AF2ERKI/KI mice (Fig. 3 A and B and Fig. S4). We detected the incorporation of 5-ethynyl-2′-deoxyuridine (EdU) into newly synthesized DNA in the ICI- and TAM-treated AF2ERKI/KI uterine endometrial epithelial cells (Fig. 3C), suggesting that ICI or TAM induces the proliferation of AF2ERKI/KI endometrial epithelial cells. Furthermore, we evaluated the regulation of some uterine estrogen-responsive genes—lactotransferrin (Ltf) (19), insulin-like growth factor 1 (Igf1) (20), and cytochrome c oxidase subunit VIIa polypeptide 2-like (Cox7a2l) (21)—by quantitative PCR. As shown in Fig. 3D, the expression of these genes was regulated by E2 and TAM in the WT uterus and not by ICI treatment. In contrast, the expression of those genes was regulated by ICI and TAM in the AF2ERKI/KI uterus but not by E2. These results indicate that the functionality of the AF2ER mutant in the in vivo AF2ERKI mouse model is similar to the in vitro findings. We also assessed the AF2ER function in the receptive uterus model. In the receptive uterus, estrogen induces stromal cell but not epithelial cell proliferation (22). We detected that ICI increased proliferation of stromal cells but not epithelial cells in the AF2ERKI/KI uterus, as did E2 in WT (Fig. S5).
Fig. 3.
Assessment of AF2ER function in the AF2ERKI mouse uterus. (A) Uterine wet weight after vehicle (Veh), ICI (2 mg/kg), TAM (2 mg/kg), E2 10 μg/kg, or E2 2 mg/kg treatments for three consecutive days. Values are presented as mean ± SEM. (B) Uterine histology after vehicle, ICI, TAM, or E2 (10 μg/kg) treatments for three consecutive days in representative mice. (C) ICI and TAM induce the proliferation of endometrial epithelial cells in AF2ERKI uterus. Uterine EdU incorporation was analyzed. Hoechst was used as a counterstain to visualize tissue. (D) The mRNA levels of Ltf, Igf1, and Cox7a2l genes were quantified by real-time PCR. The mRNA levels were represented as fold change vs. vehicle treatment of WT. Values are shown as mean ± SD; *P < 0.05 vs. vehicle in each genotype. (E) Representative results of Western blots probed for ERα and β-tubulin from the vehicle- and ICI-treated individual mouse uteri are shown. The ERα level was normalized to the level of β-tubulin in each sample and presented as fold change vs. vehicle in each group.
One of the major antagonistic effects of ICI has been shown to result from loss of ERα protein in vivo and in vitro, which involves a proteasome-mediated proteolysis of ERα (23, 24). As ICI was an agonist in AF2ERKI/KI, we examined the effect of ICI on the level of AF2ER protein by Western blot. The level of AF2ER protein was not reduced, whereas the level of WT ERα was markedly reduced, after ICI treatment (Fig. 3E). It appears that, in contrast to WT, the pure antiestrogen does not accelerate the turnover and loss of the AF2ER protein.
ICI and TAM Do Not Regulate Pituitary Gene Expression in AF2ERKI Homozygote.
It is known that ovariectomy releases negative feedback, resulting in increased serum LH level. Additionally, E2 replacement down-regulates serum LH level. As expected, serum LH levels were high in the vehicle-treated OVX WT female mice and were decreased by E2 treatment to 25% of the vehicle level. This level was similar to that seen in intact (i.e., non-OVX) WT female serum (Fig. 4A). TAM treatment of OVX WT female mice also down-regulated serum LH level, similar to E2 treatment. In contrast, the serum LH level of AF2ERKI/KI was not regulated by ICI, TAM, or E2 treatment (Fig. 4A). To correlate serum LH levels to pituitary gene regulation, we analyzed the expression of the LH β-subunit gene (Lhb). The level of Lhb mRNA in the OVX WT pituitary was reduced by E2 or TAM treatment. However, the expression of Lhb was not changed by ICI, TAM, or E2 in the AF2ERKI/KI pituitary (Fig. 4B). The basal level of Lhb expression in the AF2ERKI/KI pituitary was 1.5 times higher than in WT and was similar to that in αERKO female mice (17, 25). We also analyzed the regulation of the prolactin gene (Prl), a well known estrogen-responsive gene in the pituitary (26) (Fig. 4C). The basal level of Prl expression in the AF2ERKI/KI pituitary was 20% lower than in WT and was similar to that in the αERKO pituitary (17, 25). The expression of the Prl gene was increased by E2 but not TAM in the WT pituitary, as opposed to Lhb gene regulation. In the AF2ERKI/KI pituitary, the Prl gene expression was not regulated by ICI, TAM, or E2. Because Pit1 is a key regulator for Prl expression, we measured the Pit1 mRNA level and found no differences between genotypes (Fig. 4D). Lack of pituitary responsiveness of the AF2ERKI to ICI or TAM raised the question whether AF2ER protein was expressed in the AF2ERKI/KI pituitary. AF2ER protein expression was confirmed by Western blot in the AF2ERKI/KI mouse pituitary. Although the level of AF2ER protein in the AF2ERKI/KI pituitary appeared lower than in WT, the ratio of uterine and pituitary receptor protein levels is similar between WT and AF2ERKI/KI mice. Furthermore, the molecular sizes of the uterine and pituitary AF2ERs were identical at 66 kDa (Fig. 4E). These results indicate tissue-specific differences in gene-regulation functions of the ERα AF-1 in the pituitary compared with the uterus.
Fig. 4.
Functional difference of AF2ER in the AF2ERKI mouse pituitary. (A) Serum LH levels were determined after vehicle (Veh), ICI (2 mg/kg), TAM (2 mg/kg), or E2 (10 μg/kg) treatments for three consecutive days. Values are presented as mean ± SEM; *P < 0.05 vs. vehicle in each genotype. The pooled serum from the non-OVX WT and αERKO female mice was used as reference. (B) The mRNA level of Lhb was quantified by real-time PCR. (C) The mRNA level of Prl was quantified by real-time PCR. The mRNA levels are presented as fold change for vehicle of WT. Values are presented as mean ± SD; *P < 0.05 vs. vehicle in each genotype. (D) The mRNA level of Pit1 was quantified by real-time PCR. The mRNA levels are presented as fold change vs. WT. Values are presented as mean ± SD. (E) Representative results of Western blots probed for the ERα, His-tagged ERα, and β-tubulin from WT and AF2ER homozygote individual mouse uteri and pituitaries are shown.
Discussion
In the present study, we report the initial generation and characterization of AF2ERKI mice, which have L543A and L544A mutations in the AF-2 region of helix 12 of the LBD. First, we demonstrated the properties of this mutation by using HepG2 and HeLa cells (Fig. 1 and Fig. S1). We suggested here that the ICI activity of the AF2ER mutant is mediated through the N-terminal AF-1 predominantly, as the AF-1–truncated AF2ER mutant (i.e., 121-AF2ER) reduces the ICI-mediated activation, and the level of ICI-mediated AF2ER activity is coincident with the level of AF-2–truncated mutant (i.e., ERa339)–mediated activation in each cell type. However, we were not able to exclude the possibility of AF-2 contribution from ICI-mediated AF2ER activation, because 121-AF2ER in the HeLa cells was weakly activated by ICI. Furthermore, we found that the p300/CBP and SRC1a cooperatively activate the ICI-dependent AF2ER activation in the HeLa cells, and it was observed in AF-1- but not AF-2–mediated activation. Interestingly, p300/CBP increased AF-1 activity but not AF-2 activity. On the contrary, SRC1a increased ICI-dependent AF-2 activity modestly, but not that of AF-1. These results may suggest that the ICI-liganded AF2ER induces modest activation of AF-2 and may involve enhancing the AF-1 activity in such cells or tissues. It may explain the differential tissue response of AF2ER with ICI. We also tested the OHT-mediated WT ERα activation to compare ICI-mediated AF2ER activation because the OHT is known as a WT ERα AF-1–activating SERM (27). In our experimental condition, the activation functions of coregulators for E2 or OHT bound WT ERα were different. This result may suggest that OHT-bound WT ERα makes a different coactivator interface for E2-bound WT ERα (28). Interestingly, the profile of coactivators’ effect on ICI-mediated AF2ER activation is almost identical to that of OHT-mediated WT ERα activation. These results suggest that the ICI-liganded AF2ER is mimicking the OHT-bound WT receptor. To analyze the involvement of coregulators in the ICI-dependent AF2ER activation of various tissues, it will be helpful to understand AF-1–dependent tissue-selective functionality of SERMs.
We confirmed that the AF2ERKI mouse expresses the AF2ER mutant ERα protein in the uterus (Fig. 2) and pituitary (Fig. 4) by identifying the His-tag incorporated into the knock-in allele, using an anti–His-tag antibody. Even though the in vitro analysis indicated weak activation of AF2ER by E2, the phenotype described herein of the AF2ERKI female is similar to that of the αERKO female (15), indicating that the mutation of AF-2 is unable to mediate physiological responses to E2. We also confirmed that treatment with a pharmacological level of E2 (2 mg/kg) does not induce the uterotropic growth in the AF2ERKI/KI female (Fig. 3 and Fig. S4). These results clearly suggested that the AF-1 function is not activated by E2 independently. Our preliminary results suggest that growth factors (IGF-1 or EGF) are unable to activate uterine proliferation, specifically DNA synthesis, as reflected by lack of incorporation of the deoxyuridine analogue EdU in the AF2ERKI/KI female (Fig. S6A). This result is different from those in a similar mouse model in which the ERα AF-2 is mutated: the ENERKI mouse. The ERα in the ENERKI mouse is mutated in the ligand-binding pocket (G525L), is unable to bind E2, and exhibits reduced E2-dependent transcription activation while maintaining helix 12 structure (29). In the ENERKI homozygous female, IGF-1 induced the proliferation of uterine endometrial epithelial cells without E2, as reflected by an increase in the general cell cycle marker Ki67. However, progression to S-phase as reflected by DNA synthesis was not evaluated in the ENERKI mouse. These differences between AF2ERKI and ENERKI may suggest that helix 12 also contributes toward regulating the growth factor-mediated ERα activation.
It has been suggested from in vitro cell studies that the differential functionality of AF-1 in different cell types and on different gene promoters is an explanation for the mechanism of SERM action (12, 30). We demonstrated here that TAM regulated uterine responses (Fig. 3) and serum LH level but not pituitary Prl gene expression in the OVX WT female (Fig. 4). On the contrary, ICI or TAM regulated uterine responses in the OVX AF2ERKI female, like the response seen in TAM-treated WT female mice (Fig. 3); however, it had no response in the pituitary (Fig. 4). Differential tissue actions of ERα have been proposed as being dependent on the ERα isoforms ERα66 and ERα46 (31). ERα66 is the full-length ERα (harboring AF-1 and AF-2) and ERα46 is the shorter AF-1–deficient isoform. In this report, we confirmed that the full-length ERα is expressed in the uterus and pituitary by using the anti-ERα antibody and anti–His-tag antibody (Fig. 4). This result clearly suggests that the differences in the TAM-mediated transcriptional activity of the ERα in the uterus and pituitary are results of the differential AF-1 functionality, not differential isoform action.
The TAM responses in WT female pituitary suggest that TAM affects the pituitary functions differently than E2. A study that used the ERα−/AA (NERKI) mouse, which lacks ERE-dependent transactivation but retains ERE-independent activity (32), identified that the negative feedback action of estrogen on LH is mediated by ERE-independent mechanisms and that Prl gene regulation is mediated by ERE-dependent mechanisms (33). Taken together, these findings suggested that TAM affects ERE-independent responses (serum LH regulation) but not ERE-dependent actions (Prl gene regulation) in the pituitary. This result implies that the ERα AF-1 is not functioning to regulate Prl gene expression. Neither ICI nor TAM were able to regulate any responses in AF2ERKI female pituitary (Fig. 4). Our in vitro results indicated that the AF2ER mutant regulates ERE-mediated but not non–ERE-mediated transcription (Fig. 1). These findings suggest that our results are consistent with previous reports and the AF2ERKI mouse model is able to distinguish the differential TAM functionality in tissues in vivo.
Recently, Börjesso et al. reported generation of AF-1 truncated mouse (ERαAF-10) and helix 12 (AF-2) truncated mouse (ERαAF-20) models to evaluate the in vivo functionality of ERα AF-1 and AF-2 (34). The report used a loss-of-function approach of truncated mutants for comparison with WT ERα and observed varied E2 responses in different tissues of the ERαAF-10, but no responses to E2 in the ERαAF-20 tissues, similar to αERKO mice lacking the receptor protein (34), indicating that ERα AF-1 function is tissue dependent and AF-2 is required for the estrogenic responses. Our AF2ERKI phenotypes and differential stimulated tissue activities with ICI or TAM would be consistent with those observations and allow assignment of a differential activity of female neuroendocrine and reproductive tract tissues. Our preliminary results suggested that raloxifene, a new-generation SERM, does not activate AF2ER function in vitro (Fig. S6B) and in vivo uterine response (Fig. S6C). Our results so far suggest that AF2ERKI mice can experimentally demonstrate tissue selective function and response of ERα AF-1 in estrogen target tissues for evaluating tissue-selective SERM functions and identifying the beneficial actions of SERMs as potential hormonal therapeutic agents.
Materials and Methods
Cell Culture and Luciferase Assay.
Cells were cultured in phenol red-free medium supplemented with 10% charcoal-stripped FBS for transient transfection. The cells were transfected for 6 h, then changed to fresh media supplemented with 100 nM of E2, ICI, or OHT. Luciferase assay was performed 18 h after treatments. Details are described in SI Materials and Methods.
Generation of Knock-In Animals.
A targeting vector containing mouse ERα exon 9 with an additive XbaI site in exon 9, an18-bp 6xHis-tag epitope sequence, the L543A and L544A mutations, and the ACN cassette was used for the selection of ES cells. The targeted ES clones were injected into C57BL/6 blastocysts to generate chimeric mice. Male chimeras were bred to C57BL/6 female mice to establish germline transmission, and the resulting heterozygous mice were interbred. Further details are described in SI Materials and Methods.
ER Ligand Treatments.
OVX WT or AF2ERKI female mice (n = 6 per group) were injected s.c. daily for 3 d with 10 μg/kg or 2 mg/kg E2 dissolved in sesame oil, intraperitoneally with 2 mg/kg ICI, or 2 mg/kg TAM dissolved in DMSO. DMSO (50 μL) was used as vehicle in all experiments. The tissues and serum were collected 24 h after the last injection. All procedures involving animals were conducted in accordance with National Institutes of Health guidelines and were in compliance with a National Institute of Environmental Health Sciences-approved animal protocol.
Quantitative PCR.
For quantitative PCR, tissues were homogenized and total RNA was extracted. The RNA was reverse-transcribed, and then real-time PCR was performed. The primer sets are described in Table S1. Samples were analyzed in triplicate, and the 18s rRNA was used as an internal control for all experiments.
Statistical Analysis.
Statistical analysis was performed with two-way ANOVA with GraphPad Prism software (GraphPad), and a P value lower than 0.05 was considered statistically significant. Other methods are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Geoffrey Greene for the gift of the ACN cassette plasmid, Sandra Burkett (Mouse Cancer Genetics Program/National Cancer Institute) for conducting the FISH analysis, and David Monroy and members of the National Institute of Environmental Health Sciences Comparative Medicine Branch staff for animal care. We also thank Drs. Karina Rodriguez, Joy Winuthayanon, and April Binder for the hormone analysis; Sylvia Hewitt for critical reading of the manuscript; and other members of the Receptor Biology Group for helpful discussions. We also value the early contributions of Trisha Castranio and Dr. Deborah Swope to this project. This study was funded by Z01ES70065 from the Division of Intramural Research of the National Institute of Environmental Health (to K.S.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109180108/-/DCSupplemental.
References
- 1.Brzozowski AM, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 2.Wu YL, et al. Structural basis for an unexpected mode of SERM-mediated ER antagonism. Mol Cell. 2005;18:413–424. doi: 10.1016/j.molcel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Danielian PS, White R, Lees JA, Parker MG. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992;11:1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valentine JE, Kalkhoven E, White R, Hoare S, Parker MG. Mutations in the estrogen receptor ligand binding domain discriminate between hormone-dependent transactivation and transrepression. J Biol Chem. 2000;275:25322–25329. doi: 10.1074/jbc.M002497200. [DOI] [PubMed] [Google Scholar]
- 5.Mahfoudi A, Roulet E, Dauvois S, Parker MG, Wahli W. Specific mutations in the estrogen receptor change the properties of antiestrogens to full agonists. Proc Natl Acad Sci USA. 1995;92:4206–4210. doi: 10.1073/pnas.92.10.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ince BA, Zhuang Y, Wrenn CK, Shapiro DJ, Katzenellenbogen BS. Powerful dominant negative mutants of the human estrogen receptor. J Biol Chem. 1993;268:14026–14032. [PubMed] [Google Scholar]
- 7.Ince BA, Schodin DJ, Shapiro DJ, Katzenellenbogen BS. Repression of endogenous estrogen receptor activity in MCF-7 human breast cancer cells by dominant negative estrogen receptors. Endocrinology. 1995;136:3194–3199. doi: 10.1210/endo.136.8.7628351. [DOI] [PubMed] [Google Scholar]
- 8.Schodin DJ, Zhuang Y, Shapiro DJ, Katzenellenbogen BS. Analysis of mechanisms that determine dominant negative estrogen receptor effectiveness. J Biol Chem. 1995;270:31163–31171. doi: 10.1074/jbc.270.52.31163. [DOI] [PubMed] [Google Scholar]
- 9.Montano MM, Ekena K, Krueger KD, Keller AL, Katzenellenbogen BS. Human estrogen receptor ligand activity inversion mutants: receptors that interpret antiestrogens as estrogens and estrogens as antiestrogens and discriminate among different antiestrogens. Mol Endocrinol. 1996;10:230–242. doi: 10.1210/mend.10.3.8833652. [DOI] [PubMed] [Google Scholar]
- 10.McDonnell DP. The Molecular Pharmacology of SERMs. Trends Endocrinol Metab. 1999;10:301–311. doi: 10.1016/s1043-2760(99)00177-0. [DOI] [PubMed] [Google Scholar]
- 11.Smith CL, Nawaz Z, O'Malley BW. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol. 1997;11:657–666. doi: 10.1210/mend.11.6.0009. [DOI] [PubMed] [Google Scholar]
- 12.Berry M, Metzger D, Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990;9:2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubahn DB, et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewitt SC, et al. Biological and biochemical consequences of global deletion of exon 3 from the ER alpha gene. FASEB J. 2010;24:4660–4667. doi: 10.1096/fj.10-163428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 16.Jakacka M, et al. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem. 2001;276:13615–13621. doi: 10.1074/jbc.M008384200. [DOI] [PubMed] [Google Scholar]
- 17.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 18.Jeong JW, et al. Foxa2 is essential for mouse endometrial gland development and fertility. Biol Reprod. 2010;83:396–403. doi: 10.1095/biolreprod.109.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MO, Liu Y, Zhang XK. A retinoic acid response element that overlaps an estrogen response element mediates multihormonal sensitivity in transcriptional activation of the lactoferrin gene. Mol Cell Biol. 1995;15:4194–4207. doi: 10.1128/mcb.15.8.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewitt SC, Li Y, Li L, Korach KS. Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. J Biol Chem. 2010;285:2676–2685. doi: 10.1074/jbc.M109.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourdeau V, et al. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol. 2004;18:1411–1427. doi: 10.1210/me.2003-0441. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien JE, et al. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem. 2006;281:26683–26692. doi: 10.1074/jbc.M601522200. [DOI] [PubMed] [Google Scholar]
- 23.Gibson MK, et al. The mechanism of ICI 164,384 antiestrogenicity involves rapid loss of estrogen receptor in uterine tissue. Endocrinology. 1991;129:2000–2010. doi: 10.1210/endo-129-4-2000. [DOI] [PubMed] [Google Scholar]
- 24.Dauvois S, Danielian PS, White R, Parker MG. Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proc Natl Acad Sci USA. 1992;89:4037–4041. doi: 10.1073/pnas.89.9.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scully KM, et al. Role of estrogen receptor-alpha in the anterior pituitary gland. Mol Endocrinol. 1997;11:674–681. doi: 10.1210/mend.11.6.0019. [DOI] [PubMed] [Google Scholar]
- 26.Maurer RA, Notides AC. Identification of an estrogen-responsive element from the 5′-flanking region of the rat prolactin gene. Mol Cell Biol. 1987;7:4247–4254. doi: 10.1128/mcb.7.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McInerney EM, Katzenellenbogen BS. Different regions in activation function-1 of the human estrogen receptor required for antiestrogen- and estradiol-dependent transcription activation. J Biol Chem. 1996;271:24172–24178. doi: 10.1074/jbc.271.39.24172. [DOI] [PubMed] [Google Scholar]
- 28.McDonnell DP, Clemm DL, Hermann T, Goldman ME, Pike JW. Analysis of estrogen receptor function in vitro reveals three distinct classes of antiestrogens. Mol Endocrinol. 1995;9:659–669. doi: 10.1210/mend.9.6.8592512. [DOI] [PubMed] [Google Scholar]
- 29.Sinkevicius KW, et al. An estrogen receptor-alpha knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology. 2008;149:2970–2979. doi: 10.1210/en.2007-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danielian PS, White R, Hoare SA, Fawell SE, Parker MG. Identification of residues in the estrogen receptor that confer differential sensitivity to estrogen and hydroxytamoxifen. Mol Endocrinol. 1993;7:232–240. doi: 10.1210/mend.7.2.8469236. [DOI] [PubMed] [Google Scholar]
- 31.Penot G, et al. The human estrogen receptor-alpha isoform hERalpha46 antagonizes the proliferative influence of hERalpha66 in MCF7 breast cancer cells. Endocrinology. 2005;146:5474–5484. doi: 10.1210/en.2005-0866. [DOI] [PubMed] [Google Scholar]
- 32.Jakacka M, et al. An estrogen receptor (ER)alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol. 2002;16:2188–2201. doi: 10.1210/me.2001-0174. [DOI] [PubMed] [Google Scholar]
- 33.Glidewell-Kenney C, et al. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA. 2007;104:8173–8177. doi: 10.1073/pnas.0611514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Börjesson AE, et al. Roles of transactivating functions 1 and 2 of estrogen receptor-alpha in bone. Proc Natl Acad Sci USA. 2011;108:6288–6293. doi: 10.1073/pnas.1100454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.