Habituation, the reduction in an animal's response to the repeated occurrence of an unchanging stimulus, is generally regarded as the simplest form of learning (1). Moreover, it is ubiquitous: every animal with a nervous system seems to possess the capacity for habituation (2). Given these facts, one might expect that habituation would be fairly well understood by modern neurobiologists. In reality, however, our understanding of the cellular mechanisms that underlie habituation is meager at best (3). That habituation remains so poorly grasped, neurobiologically, more than 100 years after the initial scientific accounts of this basic behavioral phenomenon (2) is—or should be—a matter of significant embarrassment for the field of learning and memory. However, two articles in PNAS by Ramaswami and colleagues (4, 5) go some way toward easing the embarrassment. They contain important insights into the cellular and molecular mechanisms of olfactory habituation in Drosophila, insights likely to generalize to other forms of habituation in other species, including mammalian species.
By way of background, the fly's olfactory circuit comprises three levels of neurons (6). Odors are initially detected by olfactory sensory neurons (OSNs), whose cell bodies are in the antennae of the head of the fly. There are ≈1,300 OSNs, and each expresses only 1 of 62 receptor proteins. The axons of the OSNs project to the antennal lobe (AL), where they synapse in glomeruli onto odor-specific projection neurons (PNs), as well as onto local excitatory or inhibitory interneurons (LNs); the LNs make both intraglomerular and interglomerular connections with PNs. Finally, the PNs project to neurons (the Kenyon cells) in the mushroom body, a lobed neuropil in the fly's brain that plays a critical role in associative olfactory learning (7).
To induce short-term olfactory habituation in flies, the investigators exposed flies for 30 min to one of two odors [ethyl butyrate (EB) or CO2] that flies normally find aversive. After this training 30–40 flies were placed together in a Y-maze consisting of two glass tubes joined at their base to an entry tube. (Flies are commonly trained and tested en masse in Drosophila learning experiments.) One arm of the maze contained the training odor (EB or CO2), and the other arm contained air, and the flies were allowed to move into either arm from the entry tube. After 1 min the number of flies in each arm was quantified. Other sets of flies that had not received the habituation training (naïve flies) were tested identically. Flies that had been exposed to an odor more readily entered the arm of the Y-maze containing that odor during the test (avoided the odor less) than did naïve flies, and this reduced avoidance, or habituation, lasted ≈30 min. The olfactory habituation was odor-specific: flies exposed to EB, for example, showed habituation to EB during the testing but exhibited normal avoidance to CO2 (4). The basic protocol for inducing long-term habituation (LTH) was similar to that for short-term habituation (STH), except that flies were given 4 d of exposure to an odor during training. The more prolonged training resulted in habituation that persisted for several days (4).
A priori, there are two likely candidates for a cellular mechanism of habituation: depression of excitatory connections and potentiation of inhibitory connections. Previous cellular work on habituation has largely provided support for the first mechanism (for example, refs. 3 and 8). Having demonstrated both STH and LTH to olfactory stimuli, Ramaswami and colleagues exploited the well-known power of Drosophila genetics to determine which of the two general mechanisms underlay olfactory habituation. First, the investigators showed that both STH and LTH are defective in mutant flies that lack the gene for rutabaga, which encodes a Ca2+-sensitive adenylyl cyclase (9). Using rutabaga mutant flies, they systematically expressed a wild-type rutabaga transgene in different classes of neurons in the Drosophila olfactory circuit to identify the type(s) of neurons in which rutabaga must be expressed for normal habituation. Expression of the wild-type transgene in the LN1 class of local interneurons that use the inhibitory transmitter GABA was sufficient to rescue both STH and LTH in the mutant flies; thus, GABA-mediated inhibition within the AL is critical for olfactory habituation (4). To determine whether GABA-mediated inhibition of the responses of PNs to odors underlies the expression of habituation, a specific GABAA receptor, the resistance to dieldrin (RDL) receptor, was selectively reduced in a cell-specific manner through the use of the Drosophila Gal4-UAS binary expression system (10). The targeted knockdown of RDL receptors in EB-responsive PNs blocked habituation (both STH and LTH) to EB without affecting habituation to CO2, whereas the knockdown of RDL receptors in CO2-responsive PNs blocked habituation to CO2 without affecting habituation to EB. These elegant experiments identified the LN1-to-PN synapse as a critical cellular site of habituation and also showed that olfactory habituation was odor-specific. Last, Ramaswami and colleagues examined whether potentiation of LN1-to-PN inhibitory synaptic transmission was sufficient to induce habituation. A fly line was generated in which a heat-activated, transient receptor potential (TRP) channel was expressed in LN1 cells. The olfactory responses of these flies were measured at a temperature at which the TRP channels would be silent and compared with their olfactory responses at a higher temperature, at which the TRP channels would be active. The temperature elevation caused strong activation of the cation-permeable TRP channel, presumably producing persistent depolarization and firing of the LN1s; the result was a reduction in the responsiveness of the transgenic flies to the odors.
The projections of LN axons within the AL cross glomerular boundaries. The finding that olfactory habituation in Drosophila exhibits odor specificity means that some mechanism exists to ensure that potentiation of the LN1-to-PN synapse during prolonged odor exposure is glomerulus-specific. What might this mechanism be? In the mammalian CNS, synapse-specific potentiation is mediated by N-methyl-d-aspartate-type glutamate receptors (NMDARs) (11). Moreover, the fly's brain also possesses NMDARs (12). To provide evidence that NMDARs in the PNs mediate olfactory habituation, Ramaswami and colleagues expressed a transgene encoding an RNAi construct that targets the NR1 subunit of the NMDAR in either EB-responsive or CO2-responsive PNs; this manipulation blocked habituation in an odor-specific manner in the transgenic flies. The investigators further identified the Drosophila vesicular glutamate transporter DVGLUT in LN1 neurons in the antennal lobe and showed that RNAi-based knockdown of DVGLUT in LN1 neurons blocked both STH and LTH. These results indicate that glutamate is coreleased with GABA from the terminals of LN1 neurons when flies are exposed to odors and point to NMDAR-mediated potentiation of the LN1-to-PN synapse as a mechanism of odor-specific habituation. Interestingly, glutamate cotransmission has also recently been demonstrated to mediate plasticity of inhibitory synapses in the mammalian auditory system (13).
A companion article from Ramaswami's group provides additional insights into olfactory habituation in Drosophila (5). Here they show that Ataxin-2 (Atx2), a protein involved in human spinocerebellar ataxia, is required for LTH. Using genetic methods like those described above, the group found that Atx2 must be present in the PNs for normal LTH, although not for STH. They further show that Atx2 participates in a microRNA-mediated repression of local mRNAs in the dendrites of the PNs, possibly through its association with a specific RNA-induced silencing complex (RISC) pathway. The authors hypothesize that prolonged exposure to an odor somehow dissociates Atx2 from the RISC pathway, thereby triggering the local protein synthesis within the PNs necessary for the cellular changes that mediate LTH, including structural changes in the glomeruli. They suggest that NMDAR-dependent potentiation plays a key role in this process and that the local protein synthesis may induce a retrograde signal from the PNs to the LNs, thereby promoting odor selectivity of LTH. Although the involvement of NMDAR-dependent retrograde signaling in LTH is attractive (14), the authors do not offer any empirical evidence to support this idea. Moreover, it is uncertain what long-term changes in the LNs might be triggered by the putative retrograde signal.
Although the articles by Ramaswami and colleagues demonstrate a critical role for inhibition in olfactory habituation, they do necessarily not rule out other mechanisms. The investigators argue that their results from the experiments on transgenic flies expressing heat-activated TRP channels prove that LN1-to-PN inhibition is sufficient for habitation; but it is possible that some nonspecific effect of the strong activation of the TRP channels within the LN1 cells, rather than, or in addition to, inhibition of the PNs, reduced the responses of flies to the odors. Recent work has implicated down-regulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptors at excitatory synapses in LTH in Caenorhabditis elegans and Aplysia (3, 8). ACh, not glutamate, is the major excitatory transmitter in the insect brain; however, rapid trafficking of nicotinic ACh receptors in the nervous system has been reported (15), and possibly down-regulation of ACh receptors at excitatory synapses in the fly's olfactory system contributes to olfactory habituation. The existence of multiple mechanisms of habituation would be consistent with emerging evidence from studies of habituation in other systems (3, 8, 16, 17).
In summary, the articles by Ramaswami and colleagues provide a fresh perspective on the neurobiology of habituation (Fig. 1). Although GABAergic inhibition has previously been implicated in habituation (17), the sophisticated genetic and cellular manipulations used in the new PNAS articles offer conclusive support for this idea. Furthermore, co-occurrence of NMDAR-dependent potentiation with inhibition at the connection between LNs and PNs represents an elegant solution to the problem of how to derive odor-specific olfactory habituation, given that the projections of LN axons cross glomerular boundaries. Finally—and perhaps most satisfyingly—the work by Ramamawami and colleagues shows that studies of simple forms of learning in humble animals continue to yield major insights into neural function.
Fig. 1.
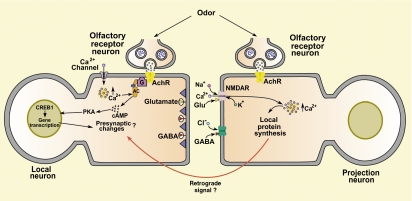
Cellular model of olfactory habituation in Drosophila based on the work of Ramaswami and colleagues (4, 5). Prolonged exposure to an odor produces sustained excitation of olfactory receptor neurons (ORNs), and, in turn, of the local neuron (LN). Repeated firing of the LN activates the Ca2+-sensitive adenylyl cyclase (AC) rutabaga within the LN. When stimulated, the LN coreleases the inhibitory transmitter GABA and the excitatory transmitter glutamate from its terminals. The GABA binds to GABA receptors, and the glutamate binds to NMDARs, in the postsynaptic membrane of the projection neuron (PN). The depolarization of the PN produced by ORN input, together with the released glutamate, opens NMDAR channels, resulting in an influx of Ca2+ into the PN dendrites. The elevated postsynaptic intracellular Ca2+ stimulates local protein synthesis within the PN, and the translational products, possibly together with the elevated intracellular Ca2+, trigger retrograde signaling. In addition, rutabaga activity, possibly in conjunction with the NMDAR-dependent retrograde signal, stimulates the transcription factor cAMP response element binding protein (CREB) in the nucleus of the LN. CREB-dependent transcription produces long-term changes in the LN, including structural changes.
Acknowledgments
I thank Diancai Cai for preparing Fig. 1.
Footnotes
References
- 1.Rankin CH, et al. Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem. 2009;92:135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson RF. Habituation: A history. Neurobiol Learn Mem. 2009;92:127–134. doi: 10.1016/j.nlm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glanzman DL. Habituation in Aplysia: The Cheshire cat of neurobiology. Neurobiol Learn Mem. 2009;92:147–154. doi: 10.1016/j.nlm.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Das S, et al. Plasticity of local GABAergic interneurons drives olfactory habituation. Proc Natl Acad Sci USA. 2011;108:E646–E654. doi: 10.1073/pnas.1106411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCann C, et al. The Ataxin-2 protein is required for microRNA function and synapse-specific long-term olfactory habituation. Proc Natl Acad Sci USA. 2011;108:E655–E662. doi: 10.1073/pnas.1107198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masse NY, Turner GC, Jefferis GS. Olfactory information processing in Drosophila. Curr Biol. 2009;19:R700–R713. doi: 10.1016/j.cub.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Connolly JB, et al. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274:2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- 8.Giles AC, Rankin CH. Behavioral and genetic characterization of habituation using Caenorhabditis elegans. Neurobiol Learn Mem. 2009;92:139–146. doi: 10.1016/j.nlm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Levin LR, et al. The Drosophila learning and memory gene rutabaga encodes a Ca2+/Calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–489. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- 10.Duffy JB. GAL4 system in Drosophila: A fly geneticist's Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 11.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Xia S, et al. NMDA receptors mediate olfactory learning and memory in Drosophila. Curr Biol. 2005;15:603–615. doi: 10.1016/j.cub.2005.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noh J, Seal RP, Garver JA, Edwards RH, Kandler K. Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat Neurosci. 2010;13:232–238. doi: 10.1038/nn.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glanzman DL. Common mechanisms of synaptic plasticity in vertebrates and invertebrates. Curr Biol. 2010;20:R31–R36. doi: 10.1016/j.cub.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Tearle AW, Nai Q, Berg DK. Rapid activity-driven SNARE-dependent trafficking of nicotinic receptors on somatic spines. J Neurosci. 2005;25:1159–1168. doi: 10.1523/JNEUROSCI.3953-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmid S, Brown T, Simons-Weidenmaier N, Weber M, Fendt M. Group III metabotropic glutamate receptors inhibit startle-mediating giant neurons in the caudal pontine reticular nucleus but do not mediate synaptic depression/short-term habituation of startle. J Neurosci. 2010;30:10422–10430. doi: 10.1523/JNEUROSCI.0024-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krasne FB, Teshiba TM. Habituation of an invertebrate escape reflex due to modulation by higher centers rather than local events. Proc Natl Acad Sci USA. 1995;92:3362–3366. doi: 10.1073/pnas.92.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]