Abstract
Bullvalene is a structurally unique dynamic molecule thought to interconvert among 1.2 million degenerate isomers. The incorporation of different chemical substituents onto the bullvalene core should lead to a “shape-shifting” molecule that can interconvert among thousands of discrete structural isomers. Previous NMR spectroscopy and HPLC studies on substituted bullvalenes ascertained the fact that these compounds are dynamic, but they could not attest to whether the molecules are only interconverting among only a few isomers or if a multitude of structures are being accessed. Here we confirm the remarkable shape-shifting property of a tetrasubstituted bullvalene by means of a racemization experiment. We show that a single, though fleeting, chiral, enantioenriched tetrasubstituted bullvalene isomer can spontaneously equilibrate to a racemic population of dynamic compounds. Despite the fact that conversion from one enantiomer of a bullvalene isomer to the other may require dozens or even hundreds of rearrangements and involve many potential pathways, CD spectroscopy and HPLC analysis of different bullvalene populations showed that multiple pathways exist and result in the complete racemization of an initial enantioenriched chiral bullvalene. These oligosubstituted bullvalenes represent a very rare example of an entity that can spontaneously transform itself into different discrete structures using ambient thermal energy. The confirmation that these shape-shifting organic molecules are chemically robust yet structurally dynamic is an important step toward their further use as materials, sensors, and biologically active compounds.
Keywords: cope rearrangement, complexity, interconversion network
Shape-shifting is a coveted and useful ability that has long captivated human imagination. Although typically possessed only by superheroes and mythological creatures, human engineering feats now routinely produce objects with shape-shifting abilities, including the recent construction of a shape-shifting building (http://www.dynamicarchitecture.net/). At the molecular level, select organic molecules are known to possess shape-shifting properties (1–4), albeit usually only between a few distinct states. Examples include cis-trans isomerization and sigma-bond rotations found in molecular motors (5), alkene isomerization as the basis for vision (6), and the ring-chain tautomerization common to carbohydrate monomers (7). Likewise, changes in the structure of large macromolecules such as proteins are often involved in their function and activity (8). This includes, for example, the ability of the prion protein to morph from a benevolent form to a harmful form (9, 10). Shape-shifting has also been recently invoked as a key mechanism for the activity of the human beta-defensin 1 protein involved in the innate immune system (11).
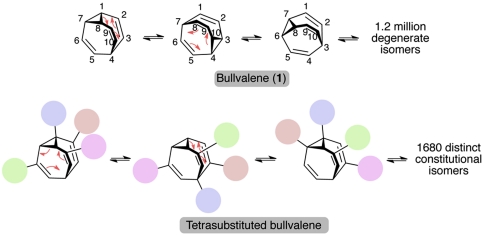
Lacking from the canon of shape-shifting structures is a self-contained small molecule with the ability to transform its shape and properties in response to stimuli or its environment. Inspired by the intense interest in and challenge of shape-shifting, we have explored a class of organic molecules that can spontaneously change their structure through low-barrier molecular rearrangements. Such molecules would find use as adaptable molecules in binding interactions, chemical sensing, and the development of materials with unique properties (12–14). For the synthesis and applications of such molecules, we have built on the pioneering work of Doering and Schröder, who designed bullvalene 1 (Fig. 1) as a small (10 carbons and 10 hydrogens) organic molecule that can spontaneously interconvert among 1.2 million degenerate isomers through strain-assisted Cope rearrangements (15, 16). The replacement of hydrogens on this bullvalene core with substituent groups should result in dynamic, “shape-shifting” organic molecules (17–22) (Fig. 1). We have recently developed a rational synthesis of oligosubstituted bullvalenes that allows for the incorporation of diverse pendant groups (23–25). NMR and HPLC investigations supported our assertion that these molecules are dynamic but did not ascertain if the structures are true shape-shifting molecules that freely rearrange their pendant groups or merely oscillate between a few discrete states without accessing the many other theoretically possible structural isomers.
Fig. 1.
Incorporation of substituents onto bullvalene leads to a shape-shifting molecule as it alters the arrangements of its groups around the core of the molecule.
In this paper we document the experimental demonstration that oligosubstituted bullvalenes are chemically stable but topologically dynamic organic molecules by the preparation and racemization of a chiral, enantioenriched bullvalene. This study presents an unusual example of a chiral object that can undergo racemization even though the interconversion between enantiomers may require dozens or even hundreds of discrete steps and many different pathways are possible. We show that not all of these isomerization pathways are equally facile, and racemization via some may be energetically prohibited. Despite this, accessible pathways exist for the racemization of the initial chiral, enantioenriched bullvalene isomer.
Results
In order to study the racemization of a chiral bullvalene, we sought to prepare an enantioenriched oligosubstituted bullvalene and monitor its optical activity by means of CD spectroscopy. Any single bullvalene isomer has the opportunity to spontaneously undergo one of the three possible Cope rearrangements. In this manner, the initial enantioenriched bullvalene isomer will eventually transform into a population consisting of different isomers through successive rearrangements. If racemization does not occur, the bullvalene sample should maintain CD activity. On the other hand, if racemization does occur, we would expect to observe no signal in the sample’s CD spectrum. Entropic considerations mandate that racemization would eventually occur provided that the molecules do not undergo decomposition, so successful racemization would also be an indication of robustness. It would also provide a qualitative measure of the time needed to reach population equilibrium. However, we could not anticipate whether this process would be fast or if extensive periods of time would be required for isomer equilibration. It is also possible that the activation energy for many of the rearrangements exceeds the available energy at room temperature. This would result in a limited dynamic population of isomers that may not be completely racemized.
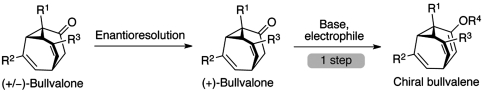
The preparation of a single enantiomer of a single isomer of a dynamic molecule and the assessment of its enantiopurity presents a daunting synthetic and analytical challenge. At the outset of our study, we did not consider it feasible to isolate a single bullvalene isomer from the dynamic population of shape-shifting structures or determine its enantiopurity by HPLC or GC on chiral columns. Therefore, we needed to identify an immediate precursor to a single, chiral oligosubstituted bullvalene isomer that could be prepared as a discrete, static, and enantioenriched structure. Our previous approaches to oligosubstituted bullvalene synthesis involved a precursor that was meso (23) and therefore not suitable for the preparation of a chiral bullvalene. Considering all the desirable properties of an appropriate molecule for this study, we decided to develop a route to synthesize a static bullvalone with three different substituents (Fig. 2). The enantioresolution of this racemic bullvalone, followed by the trapping of its enolate, would provide access to a chiral bullvalene with four different substituents in a single step (Fig. 2). In order to use the racemization of the bullvalene population as an observable indication of the dynamics of the bullvalene, it is important that virtually all of the discreet isomers are chiral. The bullvalene population that may result from the rearrangement of the initially formed tetrasubstituted bullvalene consists of a theoretical 1,680 discrete structural isomers. All of these are chiral except for 24 meso isomers. This molecular design and experimental strategy also has the advantage of providing access to both enantiomers of the starting chiral bullvalene.
Fig. 2.
Plan for the synthesis of a chiral bullvalene.
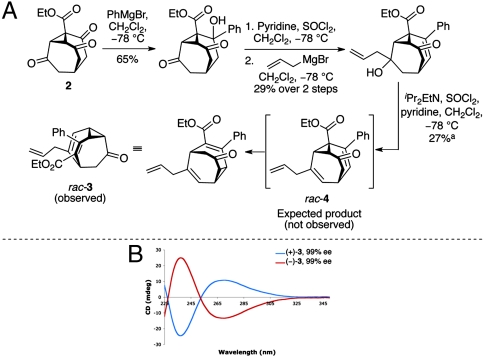
Our synthesis of a racemic, static bullvalone was achieved by the addition of phenyl Grignard reagent to the previously described triketone 2 (23) followed by elimination, allylation, and further elimination to give chiral bullvalone 3 (Fig. 3). The structure of the resulting product was determined through extensive 2D-NMR experiments to be rearranged isomer 3, rather than the expected product 4, a consequence of working with shape-shifting molecules and their immediate precursors. Bullvalone 3 proved to be a racemic, static compound that was resolved by chiral HPLC to give two enantiomers that were optically active and gave mirror image CD spectra.
Fig. 3.
(A) Synthesis of chiral bullvalone 3. aYield after preparative HPLC purification. (B) CD spectra of enantiomers (+)-3 and (−)-3.
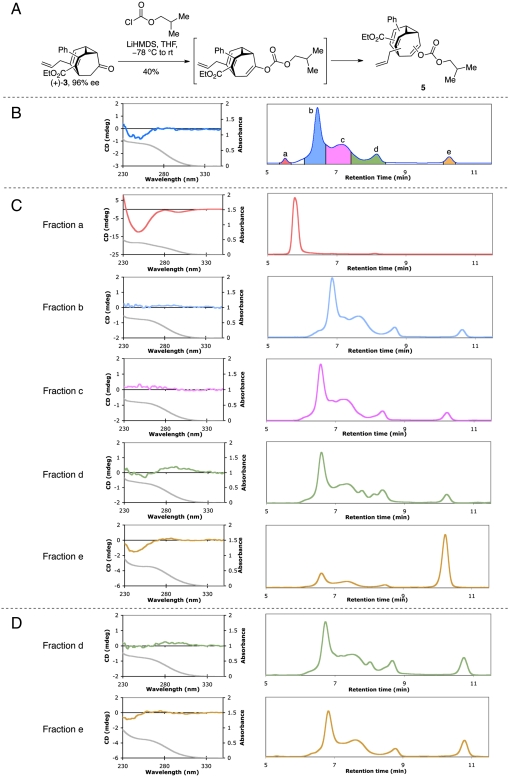
One of the isolated bullvalone enantiomers, (+)-3, was converted to a dynamic bullvalene through enolate formation and trapping to give enol carbonate 5 (Fig. 4A), which we expected to be a dynamic molecule that intercoverts among up to 1,680 discrete isomers. Rough purification of the products of this reaction gave a mixture of molecules that exhibited a residual CD signal of the same sign as the starting bullvalone enantiomer (Fig. 4B). This material was subjected to normal-phase preparative HPLC and a chiral impurity was found (fraction a in Fig. 4B) that displayed a CD spectrum consistent with that of the material analyzed prior to HPLC purification (Fig. 4C). The remainder of the HPLC trace was characterized by a complex set of peaks of similar profile to a prior study from our group on the chromatographic analysis of oligosubstituted bullvalenes (25). Four dynamic bullvalene fractions (b–e) were collected from the HPLC separation and individually analyzed by CD spectroscopy and analytical HPLC (Fig. 4C).
Fig. 4.
Racemization experiment. (A) Preparation of bullvalene. (B) CD Spectrum (Left) and HPLC trace (Right) of column chromatography purified bullvalene 5. Different colors represent fractions collected from preparative HPLC separation. (C) CD spectra and reinjection HPLC traces of fractions a-e 10–12 h after the reaction. (D) CD spectra and reinjection HPLC traces of fractions d and e 24 h and 31 h after the reaction, respectively. Curve shown in gray below each DC spectrum depicts the UV absorption of the corresponding CD sample.
If the chiral bullvalene underwent complete racemization, we would expect each fraction collected from the preparative HPLC to (i) display no detectable CD signal and (ii) give the exact same HPLC profile as every other fraction upon subsequent analysis. For fractions b and c, this proved to be the case. However, fraction d showed a residual CD signal and a slightly different HPLC profile when analyzed 10 h after the reaction. After 24 h, the CD signal of this fraction had disappeared and its HPLC trace became normal (Fig. 4D). In contrast, fraction e maintained a strong CD signal 10 h after the reaction, and HPLC analysis at that time revealed a higher than usual proportion of fraction e (Fig. 4C). This indicates the molecules contained in fraction e are dynamic but equilibrate more slowly than those contained in fractions b–d. The residual CD signal of fraction e gradually decreased with a concomitant normalization of the HPLC profile* (Fig. 4D). This series of experiments was conducted using both enantiomers of the starting bullvalone on two different CD spectrometers with identical results, except for the sign of the CD signal of the static chiral impurity and of the short-lived residual CD signal in fractions d and e.
Discussion
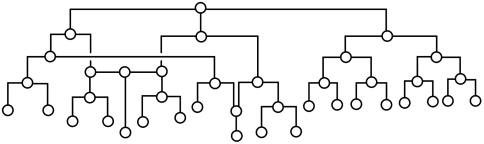
The rearrangement of bullvalene is a complex subject that has been studied as part of the mathematical discipline of combinatorics and graph theory (26–31). Such a complex, dynamic network resembles those commonly encountered in society and nature (e.g., social networks, transportation networks, metabolic pathways, etc.) (32–34). If only structural rearrangements are considered, the bullvalene network can be represented by a binary tree-like diagram, where the nodes are structural isomers and the lines connecting the nodes are Cope rearrangements (Fig. 5). Such network topology would provide a map of all possible isomers and their interconnections via rearrangements. Lacking from the network shown in Fig. 5, however, is the energy dimension that governs the dynamics of the system. The activation barrier of each rearrangement determines which isomers are accessible as well as the rates of these rearrangements (35). Therefore, out of the three possible rearrangements for each specific bullvalene isomer, the choice of its present and subsequent rearrangements is dependent on the energy barriers encountered. In addition, within its kinetically accessible isomers, the thermodynamic stability of these isomers determines the equilibrium distribution.
Fig. 5.
Binary tree diagram representing a subset of rearrangements of a tetrasubstituted bullvalene. Each bullvalene isomer can undergo three different Cope rearrangements (Dataset S1). Four generations of rearrangements from the initially formed chiral bullvalene isomer from bullvalone 3 are shown. No enantiomeric pairs are found in this subset (see SI Appendix for details); many additional rearrangements are needed to reach the enantiomer of the initial isomer.
The racemization of a chiral bullvalene requires the formation of an equal distribution of enantiomeric partners of all energetically present isomers from a single enantioenriched starting isomer. If the molecule is sufficiently dynamic and chemically robust, entropic considerations mandate that racemization takes place. Unlike epimerization commonly found in tetrahedral carbons or atropisomerization, however, the interconversion of one bullvalene enantiomer to the other may require dozens or even hundreds of Cope rearrangements (see Fig. 5). Should the barrier to one of these rearrangements exceed the available energy at a given temperature, this pathway will be blocked. There exists, however, an unusually large number of potential pathways for the interconversion of any two enantiomers. Our results are most consistent with a system in which the majority of rearrangement pathways are accessible but which possesses some metastable isomers that while dynamic, encounter a region of higher activation energies requiring them to convert first to a different structural isomer before continuing rearrangement. This results in a transient enantioenriched domain with a relatively long half-life, i.e., a dead end on the path to racemization. The fact that racemization eventually occurs for all isomers in the present study confirms that the molecule is dynamic and chemically robust; i.e., degradation of certain isomers to static byproducts does not occur.
Conclusion
Taken together, these observations provide compelling evidence that bullvalene 4 and related compounds we have previously prepared are both exceptionally dynamic and chemically robust. Any initial structure will equilibrate to a population of structural isomers that are kinetically accessible, although the equilibrium population at any moment will reflect the relative energy differences of the many discrete structures. As we continue the deployment of these unique shape-shifting molecules as chemical sensors and probes, this information about chemical robustness, different rates of structural interconversion, and the potential of islands of preferential stability will be crucial to the design of both molecules and applications. Furthermore, we expect that these molecules will have unique spectral and chemical properties that merit further investigations.
Methods
Chiral bullvalone (+)-3 (96.5% enantiomeric excess, 12.0 mg, 0.036 mmol) was dissolved in THF (2.5 mL) and cooled to -78 °C. After 15 min, LiHMDS (1 M in THF/ethyl benzene, 126 μL, 0.126 mmol) and isobutyl chloroformate (46 μL, 0.36 mmol) was added dropwise sequentially at -78 °C. After 1 h, the cooling bath was slowly removed and the reaction was allowed to warm to room temperature (RT). The reaction was quenched after 1 h with 1.5 mL NH4Cl (aq), diluted with water, and poured into EtOAc. The reaction mixture was extracted with EtOAc (3 × 20 mL), washed with brine, dried over Na2SO4, and the solvent was concentrated under reduced pressure. Purification by flash column chromatography (EtOAc:Hex 1∶9) obtained bullvalene 5 (6.2 mg, 40%). The CD spectrum of 5 was recorded in CH2Cl2 (Fig. 4B). To investigate the CD activity further, column purified 5 was subjected to Prep HPLC separation (Alltima Silica column, 250 × 22 mm ID, IPA:Hex 2∶98, 10 mL/ min flow rate), and the fractions highlighted in colors in Fig. 3B were collected separately. The CD spectra and analytical HPLC traces of each fraction were recorded (Fig. 4C). For fractions d and e, which have small residual CD signals, they were concentrated and dried under vacuum. After standing at RT for 24 and 31 h after racemization experiment, the CD spectra and analytical HPLC trace of fractions d and e were recorded again, respectively (Fig. 4D).
For complete experimental procedures and full characterization of bullvalene 5 and its intermediates, see SI Appendix.
Supplementary Material
Acknowledgments.
This work was supported by ETH Grant (ETH-11 10-3) and the David Lucile & Packard Foundation. We thank Feng Gai and François Diederich for providing their CD spectrometers for this study and Ximin Li for early investigations.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108170108/-/DCSupplemental.
*For additional evidence of the racemization of fraction e, see SI Appendix.
References
- 1.Bode JW, Suzuki K. Structural incongruities of Coleophomone natural products: Insights by total synthesis of a semi-synthetic derivative. Tetrahedron Lett. 2003;44:3559–3563. [Google Scholar]
- 2.Nicolaou KC, Montagnon T, Vassilikogiannakis G, Mathison CJN. The total synthesis of Coleophomones B, C, and D. J Am Chem Soc. 2005;127:8872–8888. doi: 10.1021/ja0509984. [DOI] [PubMed] [Google Scholar]
- 3.Thoburn JD, Luttke W, Benedict C, Limbach HH. Indigodiimine: A highly fluxional molecule that tautomerizes via double proton transfers. J Am Chem Soc. 1996;118:12459–12460. [Google Scholar]
- 4.Warren S, Chow A, Fraenkel G, Rajanbabu TV. Axial chirality in 1,4-disubstituted (ZZ)-1,3-Dienes. Surprisingly low energies of activation for the enantiomerization in synthetically useful fluxional molecules. J Am Chem Soc. 2003;125:15402–15410. doi: 10.1021/ja035136m. [DOI] [PubMed] [Google Scholar]
- 5.Feringa BL. The art of building small: From molecular switches to molecular motors. J Org Chem. 2007;72:6635–6652. doi: 10.1021/jo070394d. [DOI] [PubMed] [Google Scholar]
- 6.Schoenlein RW, Peteanu LA, Mathies RA, Shank CV. The 1st step in vision—Femtosecond isomerization of rhodopsin. Science. 1991;254:412–415. doi: 10.1126/science.1925597. [DOI] [PubMed] [Google Scholar]
- 7.Crawford TC, Andrews GC, Faubl H, Chmurny GN. The structure of biologically important carbohydrates—A C-13 nuclear magnetic-resonance study of tautomeric equilibria in several hexulosonic acids and related-compounds. J Am Chem Soc. 1980;102:2220–2225. [Google Scholar]
- 8.Lawrence SH, et al. Shape shifting leads to small-molecule allosteric drug discovery. Chem Biol. 2008;15:586–596. doi: 10.1016/j.chembiol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malaga-Trillo E, et al. Regulation of embryonic cell adhesion by the prion protein. PloS Biol. 2009;7:e55. doi: 10.1371/journal.pbio.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Priola SA, Chesebro B, Caughey B. A view from the top—prion diseases from 10,000 feet. Science. 2003;300:917–919. doi: 10.1126/science.1085920. [DOI] [PubMed] [Google Scholar]
- 11.Schroeder BO, et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature. 2011;469:419–423. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 12.Lehn JM. Dynamic combinatorial chemistry and virtual combinatorial libraries. Chemistry. 1999;5:2455–2463. [Google Scholar]
- 13.Otto S, Severin K. Dynamic combinatorial libraries for the development of synthetic receptors and sensors. Top Curr Chem. 2007;277:267–288. [Google Scholar]
- 14.Rowan SJ, Cantrill SJ, Cousins GRL, Sanders JKM, Stoddart JF. Dynamic covalent chemistry. Angew Chem Int Ed Engl. 2002;41:898–952. doi: 10.1002/1521-3773(20020315)41:6<898::aid-anie898>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Doering WVE, Roth WR. A rapidly reversible degenerate Cope rearrangement bicyclo[5.1.0]octa-2,5-diene. Tetrahedron. 1963;19:715–737. [Google Scholar]
- 16.Schröder G. Preparation and properties of tricyclo[3,3,3,04,6]deca-2,7,9-triene (bullvalene) Angew Chem Int Ed Engl. 1963;2:481–482. [Google Scholar]
- 17.Rebsamen K, Schröder G. Tri-, tetramethylbullalene und hexamethylbibullvalenyl. Chem Ber. 1993;126:1419–1423. [Google Scholar]
- 18.Rottele H, Nikoloff P, Oth JFM, Schröder G. Synthesis and NMR spectroscopy of fluorochloro bullvalene. Chem Ber. 1969;102:3367–3377. [Google Scholar]
- 19.Sarma K, Witt W, Schröder G. Bullvaleno-kronenether—Kronenether mit variablen ringgrößen. Chem Ber. 1983;116:3800–3812. [Google Scholar]
- 20.Sarma K, Witt W, Schröder G. Zur frage der stellungsisomerie bei disubstituierten bullvalenen. Chem Ber. 1986;119:2339–2349. [Google Scholar]
- 21.Schröder G, Oth JFM. Recent chemistry of bullvalene. Angew Chem Int Ed Engl. 1967;6:414–423. [Google Scholar]
- 22.Schröder G, Witt W. Crown ethers with fluctuating ring size (“breathing crown ethers”) Angew Chem Int Ed Engl. 1979;18:311–312. [Google Scholar]
- 23.Lippert AR, Kaeobamrung J, Bode JW. Synthesis of oligosubstituted bullvalones: shapeshifting molecules under basic conditions. J Am Chem Soc. 2006;128:14738–14739. doi: 10.1021/ja063900+. [DOI] [PubMed] [Google Scholar]
- 24.Lippert AR, Keleshian VL, Bode JW. Dynamic supramolecular complexation by shapeshifting organic molecules. Org Biomol Chem. 2009;7:1529–1532. doi: 10.1039/b822585k. [DOI] [PubMed] [Google Scholar]
- 25.Lippert AR, Naganawa A, Keleshian VL, Bode JW. Synthesis of phototrappable shapeshifting molecules for adaptive guest binding. J Am Chem Soc. 2010;132:15790–15799. doi: 10.1021/ja107314p. [DOI] [PubMed] [Google Scholar]
- 26.Balaban AT. Applications of graph theory in chemistry. J Chem Inf Comput Sci. 1985;25:334–343. [Google Scholar]
- 27.Brocas J. The reaction graph of the Cope rearrangement in bullvalene. J Math Chem. 1994;15:389–395. [Google Scholar]
- 28.Klin MH. Group-theoretical approach to the investigation of reaction graphs for highly degenerate rearrangements of chemical compounds. J Math Chem. 1991;7:135–151. [Google Scholar]
- 29.Lloyd EK, Jones GA. Reaction graphs. Acta Appl Math. 1998;52:121–147. [Google Scholar]
- 30.Randic M. On complexity of transitive graphs representing degenerate rearrangements. Croat Chem Acta. 2001;74:683–705. [Google Scholar]
- 31.Randic M, Woodworth WL, Kleiner AF, Hosoya H. Graph generators. J Comput Chem. 1987;8:522–535. [Google Scholar]
- 32.Albert R, Barabási AL. Statistical mechanics of complex networks. Rev Mod Phys. 2002;74:47–97. [Google Scholar]
- 33.Boccaletti S, Latora V, Moreno Y, Chavez M, Hwang DU. Complex networks: structure and dynamics. Phys Rep. 2006;424:175–308. [Google Scholar]
- 34.Strogatz SH. Exploring complex networks. Nature. 2001;410:268–276. doi: 10.1038/35065725. [DOI] [PubMed] [Google Scholar]
- 35.Gimarc BM, Brant AR. Bullvalene: Reaction graphs relating polysubstituted positional isomers. J Chem Inf Comput Sci. 1994;34:1167–1173. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.