Abstract
The Wnt signaling pathway is essential for the development of diverse tissues during embryogenesis. Signal transduction is activated by the binding of Wnt proteins to the type I receptor low-density lipoprotein receptor–related protein 5/6 and the seven-pass transmembrane protein Frizzled (Fzd), which contains a Wnt-binding site in the form of a cysteine-rich domain. Known extracellular antagonists of the Wnt signaling pathway can be subdivided into two broad classes depending on whether they bind primarily to Wnt or to low-density lipoprotein receptor–related protein 5/6. We show that the secreted protein Tsukushi (TSK) functions as a Wnt signaling inhibitor by binding directly to the cysteine-rich domain of Fzd4 with an affinity of 2.3 × 10−10 M and competing with Wnt2b. In the developing chick eye, TSK is expressed in the ciliary/iris epithelium, whereas Wnt2b is expressed in the adjacent anterior rim of the optic vesicle, where it controls the differentiation of peripheral eye structures, such as the ciliary body and iris. TSK overexpression effectively antagonizes Wnt2b signaling in chicken embryonic retinal cells both in vivo and in vitro and represses Wnt-dependent specification of peripheral eye fates. Conversely, targeted inactivation of the TSK gene in mice causes expansion of the ciliary body and up-regulation of Wnt2b and Fzd4 expression in the developing peripheral eye. Thus, we uncover a crucial role for TSK as a Wnt signaling inhibitor that regulates peripheral eye formation.
Keywords: eye development, signaling modulator, small leucine-rich proteoglycan
Wnt signaling is involved in multiple developmental events during embryogenesis and it has also been implicated in adult tissue homeostasis (1, 2). Wnt proteins act on target cells by binding to the Frizzled (Fzd)/low-density lipoprotein receptor–related protein (LRP) complex at the cell surface, which transduces Wnt signals into the target cells (3, 4). Extracellular antagonists of the Wnt signaling pathway can be subdivided into two broad classes: the secreted Fzd-related protein class and the Dikkopf class (5). The members of the secreted Fzd-related protein class bind directly to Wnts and alter their ability to bind to the Wnt receptor complex, whereas members of the Dikkopf family inhibit Wnt signaling by binding to the LRP5/LRP6 component of the Wnt receptor complex. A Wnt inhibitor that specifically binds to the Fzd receptor has not yet been identified, however.
The peripheral rim of the optic cup is a unique region of the developing eye that forms two of the peripheral support tissues, the ciliary body (CB) and the iris (6). These tissues are composed of a nonpigmented inner layer, which is continuous with the retina, and a pigmented outer layer, which is continuous with the retinal pigmented epithelium (7). Cho and Cepko (8) performed gain-of-function analyses in vivo and found that retinal cells exposed to high levels of Wnt signaling are induced to acquire CB and iris cell fates. Thus, Wnt signaling is involved in the differentiation of peripheral eye structures, but the molecular interactions involved are not yet clearly understood (8–12).
We previously described the isolation of Tsukushi (TSK) protein isoforms (13), soluble molecules belonging to the small leucine-rich proteoglycan (SLRP) family (14), and showed that they work as extracellular modulators of pivotal signaling cascades during early embryonic development in chicks and frogs (13, 15–17). We also observed that TSK rescues Xenopus embryos from the hyperdorsalized effects induced by Wnts (Fig. S1). In this study, we show that TSK functions as a novel Wnt signaling inhibitor by competing with Wnt2b for binding to Fzd4. Our biochemical analysis demonstrates direct binding between TSK and Fzd4 with an affinity of 2.3 × 10−10M. Using overexpression assays in chicken embryonic retinal cells, we found that TSK inhibits Wnt2b activity both in vitro and in vivo and represses Wnt2b-dependent induction of peripheral eye character. Conversely, TSK inactivation results in expansion of the CB in mice. Consequently, TSK is an important component of the molecular pathways controlling retinal and peripheral eye development.
Results
Expression of TSK at the Peripheral Chick Retina.
There is substantial evidence that the Wnt signaling pathway controls the development of peripheral eye structures in several animal models (8, 12, 18–20). TSK is expressed in the peripheral region of the developing chick eye at E6 (Fig. S2 A and B). As shown in Fig. S2C, Wnt2b expression is localized to the anteriormost tip of the optic cup, whereas chick TSKB (C-TSKB) (15) is expressed in the adjacent ciliary/iris epithelium (Fig. S2B). Comparing C-TSKB expression with the expression pattern of collagen IX (Fig. S2F), a marker of the neighboring ciliary/iris epithelium (8, 21), shows that C-TSKB expression does not extend into the ciliary epithelium region. Other Wnt ligands, such as Wnt3a and Wnt6, are not transcribed in the developing peripheral eye (Fig. S2G). Fzd1, 3, and 4 are expressed in both the ciliary and the iris epithelia, whereas Fzd5 is not expressed in either of these areas (Fig. S2 D, E, and G). These expression patterns suggest that Wnt2b and C-TSKB could functionally interact with each other and regulate Fzd-dependent signaling in the peripheral eye.
TSK Inhibits Wnt2b Activity in Vitro.
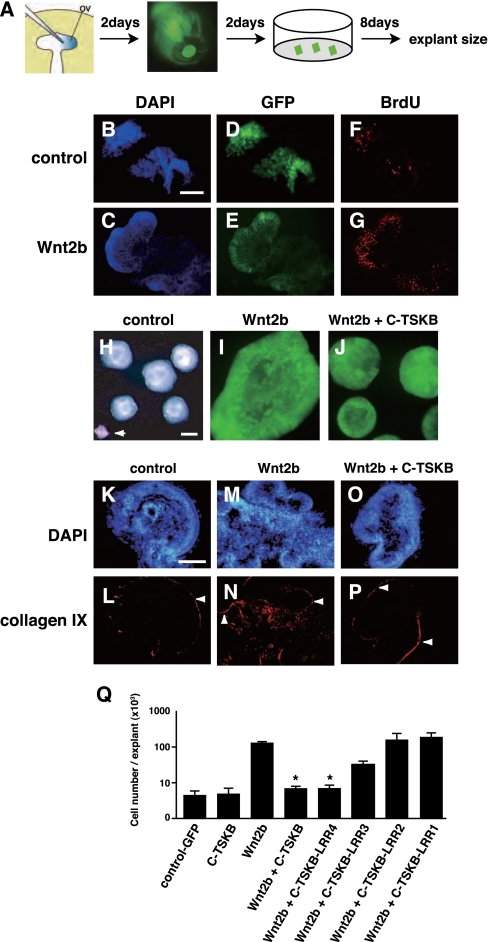
To address whether TSK functions as a Wnt inhibitor, we co-overexpressed C-TSKB with Wnt2b in the chick optic vesicle and observed the effects on the proliferation of retinal explants and the expression of the CB marker collagen IX, both of which are stimulated by Wnt2b (22). We first electroporated the optic vesicles of E1.5 chicken embryos with a Wnt2b-expressing provirus as described previously (22). We then dissected explants of the electroporated retinal tissues (250 × 250 μm2 surface area) from the central region of E5.5 retinas and cultured them in vitro for 8 d (Fig. 1A). Confirming the results of previous studies (22), Wnt2b-expressing retinal explants generated large folded sheets of tissue (Fig. 1I), with high numbers of cells incorporating BrdU (Fig. 1 B–G). Conversely, the coexpression of RCAS:Wnt2b with RCAS:C-TSKB resulted in explants of similar size as the control explants (Fig. 1 H and J). Wnt2b activity also induced ectopic collagen IX expression in retinal explants (Fig. 1 M and N), which was prevented in explants coexpressing Wnt2b and C-TSKB (Fig. 1 O and P). Cell number quantifications (Fig. 1Q) showed that the number of cells in explants coexpressing Wnt2b and C-TSKB (7.2 ± 0.4 × 103; n = 30) was similar to that of control explants (4.3 ± 1.3 × 103; n = 30) and C-TSKB–expressing explants (4.6 ± 1.7 × 103; n = 30), whereas it was exponentially increased in Wnt2b-expressing explants (1.2 ± 0.1 × 105; n = 30) (Fig. 1Q). These effects were not due to changes in cell death (Fig. S3 A and B).
Fig. 1.
TSK inhibits Wnt activity in vitro. (A) Schematic of the experimental procedure. (B–G) BrdU incorporation was increased in the explants expressing Wnt2b. (B and C) DAPI. (D and E) GFP. (F and G) BrdU. (Scale bar: 100 μm.) (H–J) Retinal explants of control (H), Wnt2b- (I), and Wnt2b+C-TSKB–expressing chick retinas (J), which were dissected out at E5.5 and cultured for 8 d in vitro after electroporation at E1.5. The arrow in H points to an explant immediately after dissection. RCAS:GFP was cotransfected to visualize electroporated cells. (K–P) Expression of collagen IX in the explants expressing Wnt2b. (K, M, and O) DAPI. (L, N, and P) Collagen IX. White arrowheads indicate the immunoreactivity of anti-collagen IX antibody on the choroid. (Scale bar: 180 μm.) (Q) Quantification of cell numbers in explants electroporated with the indicated DNA constructs. Note that at least four LRR domains are necessary and sufficient to inhibit the effects of Wnt2b on cell proliferation in vitro. *P < 0.001 (vs. Wnt2b) based on a two-tailed Student's t test. Error bars represent the SD.
We next sought to identify specific subdomains of TSK involved in the inhibition of Wnt activity. For this purpose, we generated Fc-fusion constructs encoding between one and four leucine-rich repeat (LRR) domains from the N terminus (C-TSKB-LRR1-4-Fc), and evaluated their inhibitory function on Wnt2b activity using similar electroporation assays. Only C-TSKB-LRR4-Fc was able to completely inhibit Wnt activity in vitro (C-TSKB-LRR4-Fc: 7.3 ± 0.6 × 103, n = 22; C-TSKB-LRR3-Fc: 3.0 ± 0.7 × 104, n = 32; C-TSKB-LRR2-Fc: 1.4 ± 0.6 × 105, n = 20; C-TSKB-LRR1-Fc: 1.7 ± 0.4 × 105, n = 20) (Fig. 1Q). Thus, it appears that at least four LRR domains of the C-TSKB protein are necessary for robust inhibition of Wnt signaling. To examine the specificity of the inhibition of Wnt2b activity by C-TSKB, we coelectroporated embryos with Wnt2b and either the extracellular domain of LINGO-1 (23), containing 12 LRR domains (LINGO-1-ex), or the secreted proteins Akhirin (24) or Equarin (25), which are not related to the SLRP family. None of these proteins inhibited Wnt activity (Fig. S3C).
TSK Inhibits Wnt2b Activity in Vivo.
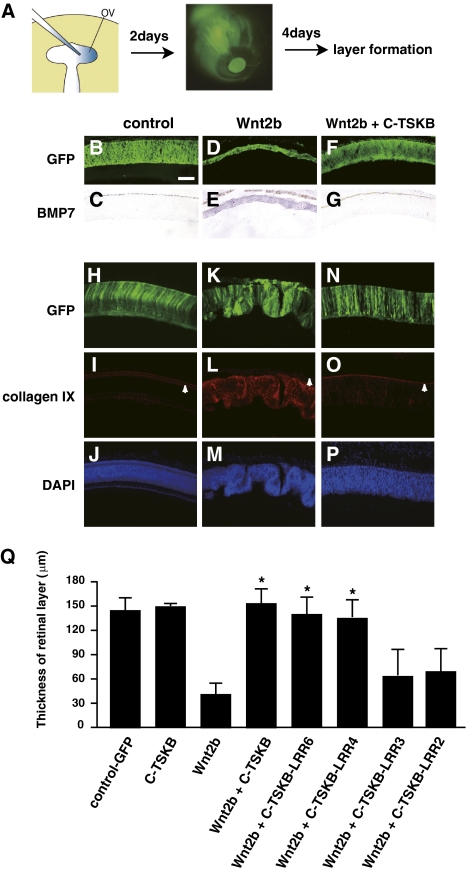
Confirming previous studies (8), overexpression of Wnt2b in the optic vesicle in vivo induced the expression of peripheral eye markers (Fig. 2 C, E, I, and L) and a significant reduction in the number of proliferating cells in Wnt2b-infected regions (Fig. S4 A and B). We used this overexpression assay to examine whether the inhibitory activity of C-TSKB on Wnt signaling could be observed in vivo as well (Fig. 2A). Developing optic vesicles were infected with RCAS:Wnt2b at E1.5 and examined at E7.5. The infected eyes showed thinning and folding of the retinal epithelium at the level of the central retina, up-regulation of the CB markers BMP7 and collagen IX (Fig. 2 D, E, K, L, and M) (8, 21), and down-regulation of β-tubulin III and pax6 expression (Fig. S4 C and D). The average thickness of the RCAS:Wnt2b-infected thinner retina was 40 ± 19 μm (n = 11) (Fig. 2Q). In the presence of both Wnt2b and C-TSKB, the infected retinal tissue failed to show any abnormalities (Fig. 2 F, G, N, O, and P) and was similar to the control retina (Fig. 2 B, C, H, I, and J). The average thickness of control-, RCAS:C-TSKB- and RCAS:Wnt2b+RCAS:C-TSKB-infected retinas was 142 ± 21 μm (n = 8), 147 ± 6 μm (n = 3), and 151 ± 26 μm (n = 7), respectively (Fig. 2Q).
Fig. 2.
TSK inhibits Wnt activity in vivo. (A) Schematic of the experimental procedure. (B–P) Histological sections of central retinal regions from chicken embryos electroporated with the indicated DNA constructs plus RCAS:GFP at E1.5 and incubated for 6 d in vivo. (B, D, F, H, K, and N) GFP expression. (C, E, and G) Expression of BMP7 detected by in situ hybridization. (I, L, and O) Expression of collagen IX detected by immunohistochemistry. White arrowheads indicate the immunoreactivity of anti-collagen IX antibody on the choroid. (J, M, and P) DAPI. Note that the thinning and folding of the retinal epithelium and the ectopic expression of BMP7 and collagen IX caused by Wnt2b overexpression were suppressed by C-TSKB in vivo. (Q) Quantification of the thickness of the retinal layer in retinas electroporated with the indicated DNA constructs. Note that at least four LRR domains are necessary and sufficient to inhibit the effects of Wnt2b in vivo. *P < 0.001 (vs. Wnt2b) based on a two-tailed Student's t test. Error bars represent the SD. (Scale bar: 100 μm.)
We performed the same experiments using mutant forms of C-TSK. The average thickness of C-TSKB-LRR2-Fc–, C-TSKB-LRR3-Fc–, C-TSKB-LRR4-Fc–, and C-TSKB-LRR6-Fc–infected retinas was 72 ± 28 μm (n = 4), 65 ± 33 μm (n = 4), 132 ± 23 μm (n = 4), and 143 ± 22 μm (n = 4), respectively (Fig. 2Q). This indicates that, similar to the in vitro assay shown in Fig. 1, at least four LRR domains are necessary and sufficient to inhibit the effects of Wnt2b in vivo (Fig. 2Q). In the same assay, the infection of RCAS:Wnt2b with RCAS:LINGO-1-ex, RCAS:Akhirin, or RCAS:Equarin failed to inhibit Wnt2b activity (Fig. S4E). Consequently, TSK specifically and efficiently abrogates Wnt2b activity both in vivo and in vitro.
TSK Binds to Fzd4 Directly.
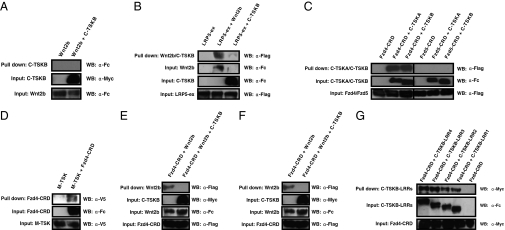
To investigate the possibility of a biochemical interaction between TSK and Wnt signaling molecules, we performed coprecipitation assays by transfecting tagged constructs into COS-7 cells (Fig. S5A). Wnt2b-Fc pulled down both Flag-tagged Fzd4–cysteine-rich domain [CRD; the site of high-affinity Wnt binding (26)] and Flag-tagged Fzd5-CRD (Fig. S5B), in addition to Flag-tagged LRP5-ex (extracellular domain of LRP5) (Fig. 3B). When Myc-His–tagged C-TSKB was coprecipitated with Fc-tagged Wnt2b, precipitation with nickel-chelating resins did not pull down Wnt2b (Fig. 3A). When Fc-tagged C-TSKB was coprecipitated with Flag-tagged LRP5-ex, Flag-tagged Fzd4-CRD, or Flag-tagged Fzd5-CRD, precipitation with protein G resins pulled down only Fzd4-CRD (Fig. 3 B and C). Another isoform of the TSK gene, C-TSKA, which differs in the structure of the C terminus from C-TSKB (15), also pulled down Fzd4-CRD (Fig. 3C).
Fig. 3.
TSK binds to Fzd4 directly. (A) Coprecipitation of C-TSKB-Myc-His and Wnt2b-Fc. (B) Coprecipitation of Wnt2b-Fc or C-TSKB-Fc and LRP5-ex-Flag. (C) Coprecipitation of C-TSKA-Fc or C-TSKB-Fc and Fzd4-CRD-Myc-Flag or Fzd5-CRD-Myc-Flag. (D) Coprecipitation of purified M-TSK-V5-His and Fzd4-CRD-Fc. (E) Competition assay of Wnt2b-Fc and C-TSKB-Myc-His for binding to Fzd4-CRD-Myc-Flag. (F) Competition assay of C-TSKB-Fc and Norrin-3Myc-AP for binding to Fzd4-CRD-Myc-Flag. (G) A single LRR domain is sufficient to bind to Fzd4-CRD.
To exclude the possibility that the detected binding between C-TSKB and Fzd4 is due to nonspecific interactions in the endoplasmic reticulum of transfected cells, we also performed coprecipitation assays with purified proteins. As shown in Fig. 3D, binding between the affinity-purified V5-His–tagged mouse TSK (M-TSK) protein (Fig. S5C) and purified Fc-tagged mouse Fzd4-CRD (M-Fzd4-CRD) protein (Fig. S5D) was detectable as well.
To confirm that TSK binds to the CRD domain of the Fzd4 receptor, we performed competitive binding assays with Fzd4-CRD, C-TSKB, Wnt2b, and Norrin, a highly specific ligand for Fzd4 (27). Fig. 3E shows that the binding of Myc-Flag–tagged Fzd4-CRD with Fc-tagged Wnt2b was inhibited by the addition of the Myc-His–tagged C-TSKB protein, whereas Akhirin and Equarin did not interfere with the interaction of Wnt2b and Fzd4 (Fig. S5 E and F). Furthermore, the binding of Myc-Flag–tagged Fzd4-CRD with Fc-tagged C-TSKB was inhibited by the Myc–alkaline phosphatase (AP)-tagged Norrin protein (Fig. 3F).
We next sought to identify the specific subdomains of TSK involved in Fzd4 binding. We evaluated the binding of different C-terminally truncated constructs of C-TSKB containing a variable number of LRR domains to Fzd4-CRD. Interestingly, Fzd4-CRD bound to all mutant forms of C-TSKB (Fig. 3G), indicating that a single LRR is sufficient for the binding of C-TSKB to Fzd4-CRD.
Fzd4 Is a TSK Receptor.
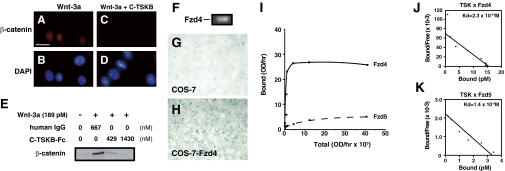
Using immunocytochemistry and immunoblotting, we examined the nuclear translocation of β-catenin after Wnt signaling stimulation in L cells expressing Fzd4 (Fig. 4F) in the presence or absence of TSK (Fig. 4 A–E). As described previously (28), the application of Wnt3a protein to L cells induced the translocation of β-catenin into the nuclei (Fig. 4 A and E), which was not detected when L cells were pretreated with C-TSKB protein (Fig. 4 C and E). This finding is most readily explained by the hypothesis that Fzd4 functions as a receptor for TSK and that TSK inhibits Wnt signaling by competing with Wnt ligands for receptor binding at the cell surface. To further confirm this possibility, we expressed Fzd4 or Fzd5 proteins in COS-7 cells and assayed their binding to TSK-AP, a fusion protein of TSK conjugated with AP. Only Fzd4-expressing COS-7 cells showed specific binding to TSK-AP compared with control COS-7 cells (Fig. 4 G and H). We calculated the dissociation constants (Kd) for TSK-AP binding to Fzd4 and Fzd5 and generated affinity values of 2.3 × 10−10 M and 1.4 × 10−9 M, respectively (Fig. 4 I–K). Moreover, in the presence of Wnt3a protein, the binding of TSK-AP protein to Fzd4 was inhibited (Fig. S6). Taken together, these observations strongly suggest that TSK inhibits Wnt signal transduction by interacting directly with Fzd4 at the cell surface and preventing Wnt2b from binding Fzd4 and thereby stimulating receptor activity.
Fig. 4.
TSK is a Fzd4 ligand. (A–E) Immunocytochemical (A and C), DAPI staining (B and D), and immunoblotting (E) analyses of β-catenin translocation into the nucleus in the presence (C–E) or absence (A, B, and E) of C-TSKB protein. (F) L cells express Fzd4. (G and H) Binding of C-TSKB::AP to control COS-7 cells (G) and Fzd4-expressing COS-7 cells (H). (I–K) Saturation binding (I) and Scatchard analysis of C-TSKB::AP binding to Fzd4- expressing (J) or Fzd5- expressing (K) COS-7 cells. (Scale bar: 20 μm.)
Loss of TSK Results in the Expansion of CB in Vivo.
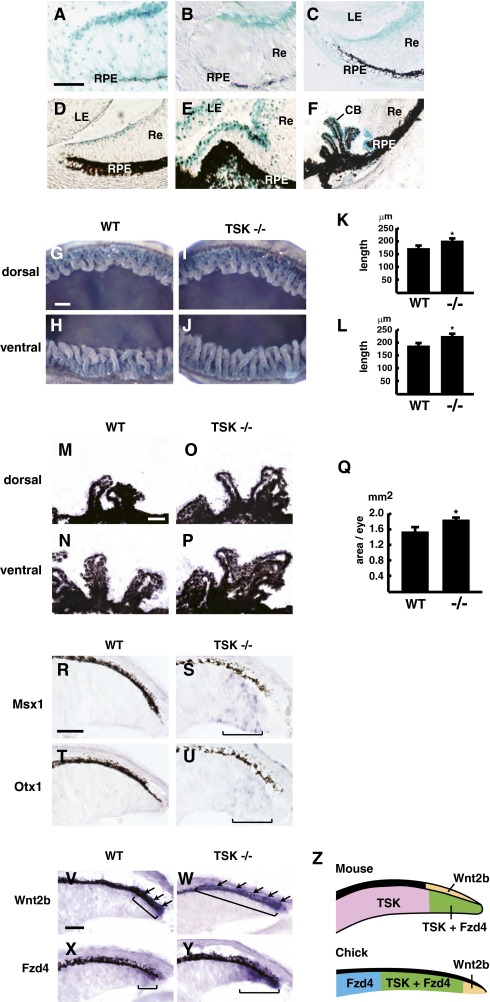
To address the requirement for TSK function in vivo, we generated TSK−/− mice by inserting a lacZ/Neo cassette into the TSK coding exon (29). We examined the expression of the TSK gene in adult TSK+/− eyes and found β-gal activity in the retinal layers, CB, and lens epithelium (Fig. S7 A and B). The expression of TSK-driven β-gal was observed in the prospective CB and continued until adulthood (Fig. 5 A–F). The CB is composed of folds of bilayered epithelium with an inner nonpigmented layer and an outer pigmented layer (30); β-gal activity was present predominantly in the inner nonpigmented layer (Fig. S7 C–E).
Fig. 5.
Expansion of CB in TSK−/− mouse eye. (A–F) Detection of β-gal activity in the developing eye of TSK+/− mice at E11.5 (A), E12.5 (B), E14.5 (C), E15.5 (D), postnatal day 0 (P0) (E), and adult (F). TSK is expressed in the anteriormost tip of the developing optic cup and the mature CB. RPE, retinal pigmented epithelium; Re, retina; LE, lens epithelium. (Scale bars: A, 40 μm; B, 50 μm; C, 70 μm; D, 80 μm; E, 120 μm; F, 200 μm.) (G–J) CB structure of the dorsal (G and I) and ventral (H and J) areas of the eye of an adult TSK−/− (I and J) and WT mouse (G and H). Quantification of the length of the CB in WT and TSK−/− mice. (K) Dorsal area. (L) Ventral area. *P < 0.01 based on a two-tailed Student t test. (M–P) H&E-stained sections of the CB area from representative eyes of TSK−/− mice (O and P) compared with WT (M and N). (Q) Quantification of the size of the CB area in WT and TSK−/− mice. *P < 0.01, two-tailed Student t test. Error bars represent the SD. (Scale bars: G–J, 100 μm; M–P, 50 μm.) (R–U) Expression of Msx1 (R and S) and Otx1 (T and U) in the E12.5 peripheral retina. Note that the expression of Msx1 and Otx1 in TSK−/− mice (bracket in S and U) is induced in the nonpigmented epithelium compared with WT mice (R and T). (Scale bars: 50 μm.) (V–Y) Expression of Wnt2b (V and W) and Fzd4 (X and Y) in the E15.5 peripheral retina. Note that the expression of Wnt2b (arrows) and Fzd4 in TSK−/− mice (W and Y) is expanded in both the pigmented epithelium and the nonpigmented epithelium compared with WT mice (V and X). (Scale bars: 50 μm.) (Z) During early retinal development, Wnt2b (yellow) expressed in the peripheral pigmented epithelium (mouse) and the tip of retina (chick) acts on the nonpigmented cells expressing Fzd4 and TSK (green) to cause proliferation. TSK regulates this effect by inhibiting Wnt2b activity.
To elucidate the role of TSK in CB formation, we performed a morphological analysis of adult eyes from TSK−/− and WT mice by observing the CB structure from the vitreous side. Fig. 5 G and H shows the CB structure in the dorsal and the ventral regions of a WT eye. We found that the ventral CB (Fig. 5H) was larger than the dorsal CB (Fig. 5G). Both the ventral and dorsal CB appeared to be expanded in TSK−/− eyes compared with WT eyes; however, their dorsoventral difference was maintained in the mutant eyes (Fig. 5 I and J). This effect was quantified by measuring the maximal CB length in the dorsal and ventral regions of TSK−/− (n = 12 animals) and WT eyes (n = 10 animals) (Fig. 5 K and L). Measurements of the CB area in histological sections confirmed the expansion of the CB in TSK−/− eyes (n = 10 animals) (Fig. 5 O–Q) compared with WT eyes (n = 8 animals) (Fig. 5 M, N, and Q), Furthermore, immunohistochemical analyses failed to show any difference between adult WT and TSK−/− retinal layering within the inner retina (Fig. S8 A–L). To exclude the possibility that the adjacent lens tissue, which normally expresses TSK, may be involved in the abnormal CB development of TSK−/− mice, we examined the morphology and mitotic activity of the lens and found no significant changes in TSK−/− mice (Fig. S8 M–Q). Bone morphogenetic protein (BMP) signaling is required for the development of the CB, and we previously showed that TSK can work as a BMP antagonist during early embryonic development (13). However, the expression of TGF-β signaling components in the CB was not obviously changed in TSK−/− mice compared with WT animals (Fig. S9A).
We next examined the expression of typical CB markers, Msx1 and Otx1, at E12.5 and detected their ectopic induction in TSK−/− mice retina (Fig. 5 R–U). Normal development of peripheral eye structures is also regulated by Wnt signaling (10–12, 31), with Wnt2b and Fzd4 acting as the primary Wnt ligand and receptor in this context. In situ hybridization analysis of the expression pattern of Wnt2b and Fzd4 at E15.5 eye showed increased and expanded expression of both these genes in the peripheral eye of TSK−/− mice compared with WT mice (Fig. 5 V–Y). Notably, Wnt2b overexpression in chick retinal cells up-regulated Fzd4 expression both in vitro and in vivo, suggesting that Fzd4 up-regulation in TSK−/− eyes is due to enhanced Wnt signaling (Fig. S9C). Furthermore, overexpression of TSK in chick peripheral retina down-regulated the expression of Lef1, which can be used as readout for Wnt signaling activation (10) (Fig. S9B). Thus, TSK inactivation leads to up-regulation of key Wnt signaling components in the developing peripheral eye, similar to what was seen previously after overexpression of an active form of β-catenin (10–12), whereas TSK overexpression has the opposite effects, suggesting that TSK is both necessary and sufficient to modulate Wnt signaling levels during peripheral eye development.
Discussion
The SLRP family encompasses 17 genes, which are subgrouped into five discrete classes based on common structural and functional properties (14). TSK belongs to the group of class IV SLRPs, which includes structurally related chondroadherin (32) and nyctalopin (33), but it shares functional properties with class I SLRPs (34), including their role as BMP inhibitors (13, 15). We have previously reported that TSK interacts with BMP ligands and the BMP antagonist chordin and functions as an inhibitor of BMP signaling during gastrula and neurula stages. In this context, TSK-dependent BMP inhibition is required for germ layer specification, organizer formation, and early ectodermal patterning. However, the BMP antagonistic activity is stronger for the C-TSKA isoform than for C-TSKB, which is the homolog to Xenopus and M-TSK (15). Although BMP signaling has been implicated in the specification of peripheral eye structures, in this context TSK does not seem to have a critical function as a BMP inhibitor, as shown by the normal expression of BMP signaling components in TSK mutant eyes. Instead, we found that TSK regulates β-catenin–dependent Wnt signaling, which plays a crucial role in peripheral eye development. In the peripheral eye, this pathway is activated mainly by the Wnt2b ligand and transduced mainly through the Fzd4 receptor. We show that TSK directly binds to the CRD region of Fzd4 and competes with Wnt2b for binding to the same domain, thereby preventing Wnt activation of β-catenin–dependent signaling.
Our assays in chick retinal cells showed that TSK overexpression can effectively inhibit Wnt signaling both in vivo and in vitro, and in particular, TSK can negate the peripheral eye-inducing effects of Wnt2b overexpression in both conditions. Activation of Wnt signaling also has been shown to affect proliferation of retinal cells; however, there is considerable controversy in the literature with respect to the neurogenic and proliferative effects of the Wnt pathway in the chick retina, with assays performed in vitro (35) and in vivo (8) leading to apparently conflicting results. Kubo et al. (35) demonstrated an increase in the proliferation of Wnt-2b–expressing retinal explants in vitro, whereas Cho and Cepko (8) showed that the level of proliferation in Wnt-2b–infected central retinal areas in vivo was lower than that in the normal control central retina. In this work, we performed the same in vivo and in vitro assays and obtained results similar to those reported previously.
These discrepancies might be explained by the need for additional factors besides Wnt to stimulate proliferation in vivo, or possibly that some other unknown aspects of the in vitro culture environment enhance proliferation in response to Wnt signaling. These data also may reflect experimental differences in the timing, duration, and intensity of Wnt signaling, or regional and temporal differences in the outcome of Wnt signaling in the developing eye. The fact that TSK is able to efficiently abrogate Wnt2b activity in both in vitro and in vivo assays indicates that the differential response of retinal cells to Wnt2b in vitro and in vivo is likely modulated downstream from Fzd4 receptor activation.
Although the currently available data indicate that Wnt signaling is involved in the proliferation of retinal cells in a context-dependent manner, further work is needed to elucidate its precise role in eye development. Nevertheless, the fact that TSK was able to negate the effects of Wnt2b overexpression in both in vitro and in vivo experimental paradigms strongly supports the idea that TSK can function as a Wnt antagonist in the developing eye and especially in the context of peripheral eye differentiation, which was promoted by Wnt2b and repressed by TSK regardless of the experimental conditions applied. The analysis of TSK knockout mice confirmed the requirement for endogenous TSK function in peripheral eye development. Consistent with overexpression assays, TSK mutant eyes showed an enlarged CB. Furthermore, the expression of Wnt2b and Fzd4 was enhanced and expanded in the developing peripheral eye epithelium, indicating that TSK function is also required to restrict their activities at the transcriptional level. Taken together, our results support a model in which TSK expression in the peripheral eye modulates Wnt2b signaling in this region by quenching Fzd4 activation toward more central areas, thereby regulating the size of peripheral eye structures and especially the CB (Fig. 5Z).
Materials and Methods
Detailed information on TSK KO mice, measurement of the length and area of CB, in situ hybridization, immunohistochemistry, chick electroporation and retinal explant culture, immunoprecipitation assays, and AP binding assays is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Shinji Takada, Ritsuko Takada, Yoshiyuki Mochida, Toshihiro Inoue, Fumi Kubo, Jeremy Nathans, Isao Matsuo, Michio Yoshida, and the Developmental Studies Hybridoma Bank for reagents; Bill Harris, Constance L. Cepko, Seo-Hee Cho, Shin-Ichi Aizawa, Tetsuya Taga, Tohru Nakano, Kohji Nishida, Douglas S. Campbell, and Yohei Shinmyo for critical comments; Kumiko Hori and Mihoko IImori for help with the chick embryos; and all members of our laboratories for their valuable help. This work was supported by the Precursory Research for Embryonic Science and Technology program of the Japan Science and Technology Agency (K.O.), the Takeda Science Foundation (K.O.), the Brain Science Foundation (K.O.), and Grants-in-Aid for Scientific Research on Molecular Brain Science from the Ministry of Education Culture, Sports, Science, and Technology of Japan (to K.O. and H.T.), the Italian Ministry of Education, University, and Research program “Rientro dei Cervelli” and a start-up grant from Istituto Pasteur–Fondazione Cenci Bolognetti (to G.L.), a Cancer Research UK Senior Cancer Research Fellowship (to S.-i.O.), a Kumamoto University 21st Century Center of Excellence research grant (to S.K. and H.T.), and a Kumamoto University Global Center of Excellence research grant (to A.I. and H.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100513108/-/DCSupplemental.
References
- 1.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kikuchi A, Yamamoto H, Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 2007;19:659–671. doi: 10.1016/j.cellsig.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 5.Kawano Y, Kypta R. Secreted antagonists of the Wnt signaling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 6.Lamba D, Karl M, Reh T. Neural regeneration and cell replacement: A view from the eye. Cell Stem Cell. 2008;2:538–549. doi: 10.1016/j.stem.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- 8.Cho SH, Cepko CL. Wnt2b/β-catenin–mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development. 2006;133:3167–3177. doi: 10.1242/dev.02474. [DOI] [PubMed] [Google Scholar]
- 9.Trimarchi JM, Cho SH, Cepko CL. Identification of genes expressed preferentially in the developing peripheral margin of the optic cup. Dev Dyn. 2009;238:2327–2329. doi: 10.1002/dvdy.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, et al. Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Dev Biol. 2007;308:54–67. doi: 10.1016/j.ydbio.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Thurig S, Mohamed O, Dufort D, Wallace VA. Mapping canonical Wnt signaling in the developing and adult retina. Invest Ophthalmol Vis Sci. 2006;47:5088–5097. doi: 10.1167/iovs.06-0403. [DOI] [PubMed] [Google Scholar]
- 13.Ohta K, et al. Tsukushi functions as an organizer inducer by inhibition of BMP activity in cooperation with chordin. Dev Cell. 2004;7:347–358. doi: 10.1016/j.devcel.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: From genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohta K, et al. Tsukushi cooperates with VG1 to induce primitive streak and Hensen's node formation in the chick embryo. Development. 2006;133:3777–3786. doi: 10.1242/dev.02579. [DOI] [PubMed] [Google Scholar]
- 16.Kuriyama S, et al. Tsukushi controls ectodermal patterning and neural crest specification in Xenopus by direct regulation of BMP4 and X-delta-1 activity. Development. 2006;133:75–88. doi: 10.1242/dev.02178. [DOI] [PubMed] [Google Scholar]
- 17.Morris SA, Almeida AD, Tanaka H, Ohta K, Ohnuma S. Tsukushi modulates Xnr2, FGF and BMP signaling: Regulation of Xenopus germ layer formation. PLoS ONE. 2007;2:e1004. doi: 10.1371/journal.pone.0001004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Raay TJ, Vetter ML. Wnt/frizzled signaling during vertebrate retinal development. Dev Neurosci. 2004;26:352–358. doi: 10.1159/000082277. [DOI] [PubMed] [Google Scholar]
- 19.Fokina VM, Frolova EI. Expression patterns of Wnt genes during development of an anterior part of the chicken eye. Dev Dyn. 2006;235:496–505. doi: 10.1002/dvdy.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuhrmann S, Stark MR, Heller S. Expression of Frizzled genes in the developing chick eye. Gene Expr Patterns. 2003;3:659–662. doi: 10.1016/s1567-133x(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 21.Dhawan RR, Beebe DC. Differential localization of collagen type IX isoform messenger RNAs during early ocular development. Invest Ophthalmol Vis Sci. 1994;35:470–478. [PubMed] [Google Scholar]
- 22.Kubo F, Takeichi M, Nakagawa S. Wnt2b inhibits differentiation of retinal progenitor cells in the absence of Notch activity by down-regulating the expression of proneural genes. Development. 2005;132:2759–2770. doi: 10.1242/dev.01856. [DOI] [PubMed] [Google Scholar]
- 23.Okafuji T, Tanaka H. Expression pattern of LINGO-1 in the developing nervous system of the chick embryo. Gene Expr Patterns. 2005;6:57–62. doi: 10.1016/j.modgep.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Ahsan M, Ohta K, Kuriyama S, Tanaka H. Novel soluble molecule, Akhirin, is expressed in the embryonic chick eyes and exhibits heterophilic cell-adhesion activity. Dev Dyn. 2005;233:95–104. doi: 10.1002/dvdy.20303. [DOI] [PubMed] [Google Scholar]
- 25.Mu H, et al. Equarin, a novel soluble molecule expressed with polarity at chick embryonic lens equator, is involved in eye formation. Mech Dev. 2003;120:143–155. doi: 10.1016/s0925-4773(02)00423-9. [DOI] [PubMed] [Google Scholar]
- 26.Wu CH, Nusse R. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J Biol Chem. 2002;277:41762–41769. doi: 10.1074/jbc.M207850200. [DOI] [PubMed] [Google Scholar]
- 27.Xu Q, et al. Vascular development in the retina and inner ear: Control by Norrin and Frizzled-4, a high-affinity ligand–receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 28.Shibamoto S, et al. Cytoskeletal reorganization by soluble Wnt-3a protein signaling. Genes Cells. 1998;3:659–670. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- 29.Ito A, et al. Tsukushi is required for anterior commissure formation in mouse brain. Biochem Biophys Res Commun. 2010;402:813–818. doi: 10.1016/j.bbrc.2010.10.127. [DOI] [PubMed] [Google Scholar]
- 30.Morrison JC, Van Buskirk EM, Freddo TF. Anatomy, microcirculation, and ultrastructure of the ciliary body. In: Ritch R, Shields MB, Krupin T, editors. The Glaucomas. 2nd Ed. St. Louis: Mosby; 1996. pp. 75–88. Chap 3. [Google Scholar]
- 31.Mani P, Jarrell A, Myers J, Atit R. Visualizing canonical Wnt signaling during mouse craniofacial development. Dev Dyn. 2010;239:354–363. doi: 10.1002/dvdy.22072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pusch CM, et al. The complete form of X-linked congenital stationary night blindness is caused by mutations in a gene encoding a leucine-rich repeat protein. Nat Genet. 2000;26:324–327. doi: 10.1038/81627. [DOI] [PubMed] [Google Scholar]
- 33.Bech-Hansen NT, et al. Mutations in NYX, encoding the leucine-rich proteoglycan nyctalopin, cause X-linked complete congenital stationary night blindness. Nat Genet. 2000;26:319–323. doi: 10.1038/81619. [DOI] [PubMed] [Google Scholar]
- 34.Chen XD, Fisher LW, Robey PG, Young MF. The small leucine-rich proteoglycan biglycan modulates BMP-4–induced osteoblast differentiation. FASEB J. 2004;18:948–958. doi: 10.1096/fj.03-0899com. [DOI] [PubMed] [Google Scholar]
- 35.Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130:587–598. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.